Kansas Geological Survey, Bulletin 211, Part 4, p. 17-22
The optimum firing range (based on firing shrinkage and porosity measurements), thermal expansion, and transverse modulus of rupture were determined for mixtures of volcanic ash (Norton County) and bentonite (Phillips County) which had been pressed and sintered. The clay and clay-ash mixtures used throughout this study mature in the vicinity of 1100° C. The mean linear coefficient of expansion varies from approximately 40 X 10-7 inches/inch/°C for a 80 percent (by weight) ash body to around 61 X 10-7 inches/inch/°C for a 100 percent bentonitic body. The transverse modules of rupture vary from very weak strengths of 300 psi for underfired samples to several thousand psi for mature samples. Increasing the bentonite to ash ratio increases both the strength and thermal expansion of the bodies. Bentonite bar specimens sintered at 1100°C possessed a strength of over 6,000 psi.
Small specimens of these materials can be pressed and then heated to 1100°C in one hour without apparent cracking. Such bodies have potential for brick and especially tile.
Considerable amounts of volcanic ash and bentonite clay (formed by the weathering of volcanic ash) are present in Kansas, both materials have limited specialized uses. This study was undertaken to determine whether, after firing such bodies have suitable properties to be used for brick and/or tile production. The results using volcanic ash from Calvert, Kansas, in Norton County and bentonite from drill hole Adee #2 in adjacent Phillips County are given in this paper.
Bentonite (-200 mesh) and volcanic ash to bentonite ratios of 0.25, 0.67, 1.5 and 4.0 were weighed into jars and mixed dry by rolling for one-half hour. One-quarter inch diameter pellets of these mixtures were formed using a steel pellet die and pressing at 10,000 psi on a Carver press. Pellets of each mixture were placed directly under thermocouples in a Glo-Bar heated gradient furnace and were fired using a three and one-half hour rise (at which time the hottest portion of the furnace reached 1200°C) and then soaked or held at temperature for 30 minutes. Afterwards, diameters were measured and the percent shrinkage was calculated and used to determine the approximate maturing range.
In order to measure the transverse strength and expansion after firing it was necessary first to press bar specimens (1 cm x 10 cm x 0.5 cm thickness) of the above mixtures. Because the high percentage ash mixtures could not be pressed in the dry state, five percent (by weight) water was added to all mixtures as a drying agent. The mixtures were placed in sealed containers for several days to insure a homogeneous moisture content prior to pressing. Bars were formed in a steel bar mold by pressing at 5,000 psi on the Carver press. The bars were fired in a Glo-Bar kiln at desired temperatures and times. After firing, the percent linear shrinkage and apparent porosity (ASTM procedures) were determined. The mean linear thermal expansion coefficient was derived from the fused silica dilatometer technique while the transverse modulus was obtained using the minimum load rate (10 pounds per second) on a Dillon Strength Tester.
Table 1 lists the chemical analyses of the bentonite and volcanic ash used throughout the study. Both of these materials are considered Pliocene in age. A comparison of the oxide contents indicates that portions of alkali and silica were removed during the weathering of ash to bentonite, which in turn enriched the clay in alumina. The major fluxing components in these materials are the alkali and iron oxides. The clay contains four percent less alkali but four percent more iron relative to the ash. Based on the chemical analyses, one might predict some similarity in the physical properties of sintered mixtures in this system.
Table 1--Chemical Analyses of Bentonite (Phillips County: Sec. 11, T. 1 S., R 18 W.) and Volcanic Ash (Norton County: Sec. 25, T. 2 S., R. 22 W.).
| Bentonite Adee #2 |
Volcanic Ash NNV #1 |
|
|---|---|---|
| SiO2 | 61.6 | 72.7 |
| Al2O3 | 15.7 | 11.2 |
| Fe2O3 | 6.1 | 2.0 |
| TiO2 | 0.6 | 0.4 |
| CaO | 1.0 | 1.4 |
| MgO | 1.3 | 0.1 |
| K2O | 3.6 | 5.6 |
| Na2O | 0.3 | 2.2 |
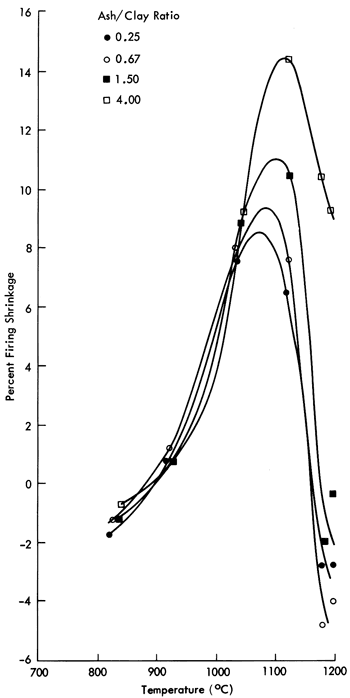
The percent shrinkage from the gradient firings as a function of the sintering temperature and composition is given in Table 2 while Figure 1 plots these results. The significant feature derived from this data is that the optimum firing (maturing) temperature, marked by the maximum shrinkage, increases only slightly with increasing ash content and all compositions mature in the vicinity of 1100°C. This firing behavior is not unexpected in view of the chemical similarity between the bentonite clay and volcanic ash. The percent firing shrinkage also increases with increasing ash content. As shown in Figure 1, the maturing range of these bodies is relatively narrow; this presents a drawback to the use of these bodies for industrial applications.
Table 2--Percent Shrinkage of Pellets as a Function of Temperature (°C) and Composition. Negative values indicate expansion relative to initial length. This expansion is often observed in clay-containing bodies fired at low temperatures. Negative values at high temperatures are due to bloating that occurs during overfiring.
| Approximate Firing Temp. |
Ash/Clay Ratio | |||
|---|---|---|---|---|
| 1/4 | 2/3 | 3/2 | 4/1 | |
| 1195 | -2.8 | -4.0 | 0.4 | 9.2 |
| 1180 | -2.8 | -4.8 | -2.0 | 10.4 |
| 1125 | 6.4 | 7.6 | 10.4 | 14.4 |
| 1040 | 7.6 | 8.0 | 8.8 | 9.2 |
| 925 | 0.8 | 1.2 | 0.8 | 0.8 |
| 830 | -1.6 | -1.2 | -1.2 | -0.8 |
Figure 1--Percent shrinkage as a function of firing temperature for pellets of volcanic ash-bentonite mixtures subjected to gradient firings.

X-ray diffraction analysis of bar specimens of these mixtures fired to maturity indicates that little crystalline material remains: the bars are essentially a glassy phase containing very small amounts of a-quartz. The amount of crystalline quartz present decreases with increasing ash content due to the glassy nature of the volcanic ash.
Table 3 lists the percent linear firing shrinkage and percent apparent porosity of bar specimens, used for subsequent thermal expansion and transverse strength measurements, as a function of the sintering temperature, sintering time, and composition. The percent linear firing shrinkage (LFS) was calculated as:
% LFS = [(LD = LF) / LD] X 100
where LD and LF represent dry and fired lengths respectively. The percent apparent porosity (AP) was calculated as:
%AP = [(Wsatd - WD) / (Wsatd - WsusD)] X 100
where WD, Wsatd, and Wsus signify the dry, saturated, and suspended weights respectively. The latter two weights were obtained from bars which had been soaked overnight in water and then boiled four hours in accordance with ASTM testing procedures.
Table 3--Percent Linear Firing Shrinkage (LFS) and Percent Apparent Porosity (AP) of Bar Specimens as a Function of Sintering Temperature (°C), Time (Hours), and Composition.
| Sintering Temp./Time |
Ash/Clay Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/4 | 2/3 | 3/2 | 4/1 | Dry 1/4 | ||||||
| LFS | AP | LFS | AP | LFS | AP | LFS | AP | LFS | AP | |
| 1000/4 | 7.1 | 15.8 | 7.2 | 19.1 | 6.8 | 25.2 | 6.7 | 27.0 | 6.6 | 27.0 |
| 1050/4 | 10.5 | 6.2 | 10.7 | 7.7 | 11.0 | 12.8 | 11.9 | 18.0 | 10.6 | 13.4 |
| 1100/4 | 12.2 | 1.7 | 13.0 | 1.8 | 15.0 | 2.0 | 18.3 | 1.8 | 14.6 | 0.8 |
| 1050/8 | 10.5 | 3.1 | 11.0 | 6.9 | 12.5 | 8.3 | ||||
| 1075/4 | 11.4 | 1.5 | 11.7 | 2.7 | 13.4 | 5.2 | ||||
| 1075/10 | 12.3 | 0.9 | 13.4 | 15.1 | ||||||
Table 3 shows that as one approaches maturity for a given composition, the sintering temperature and/or sintering time increases the linear shrinkage and decreases the apparent porosity. Note that the mature specimens for a given composition show the maximum shrinkage and relatively zero porosity.
The thermal expansion of these bodies are similar in their general nature. Table 4 lists some typical expansion coefficients of these materials. The coefficient, specifically the aggregate mean linear thermal expansion coefficient, am is expressed in units of inches/inch/°C and is calculated from the expression:
am = ΔL / L0ΔT
where ΔL is the change in length of the bar specimen during heating, L0 is the initial length of the bar (at room temperature) and ΔT is the change in temperature or temperature range over which the change in length is measured. Table 4 shows that for a given composition, little variation in am occurs as a function of the sintering temperature. The coefficient for a given composition is slightly larger when the body is sintered at 1100°C compared to 1000°C because the lower temperature results in more porosity and therefore probably less grain to grain contact.
Table 4--Mean Linear Thermal Expansion Coefficient (am X 10+6 in./in./°C) for Bentonite-Volcanic Mixtures. The actual expansion, for example for bentonite, is 6.06 x 10-6 in./in./°C. The am values have been multiplied by 106 for ease of presenting the data.
| Sintering Temp./Time |
Ash/Clay Ratio |
Pure Bentonite |
1/4 | 2/3 | 3/2 | 4/1 | Dry 1/4 |
|---|---|---|---|---|---|---|---|
| 1000/4 | 5.39 | 4.83 | 4.17 | 4.06 | 5.11 | ||
| 1050/4 | 5.78 | 5.22 | 4.44 | 4.17 | 5.68 | ||
| 1100/4 | 6.06 | 5.78 | 5.33 | 4.67 | 4.39 | 5.61 | |
| 1050/8 | 6.06 | 5.44 | 4.67 | ||||
| 1075/4 | 6.11 | 5.56 | 4.72 | ||||
| 1075/10 | 5.56 | 5.17 | 4.22 |
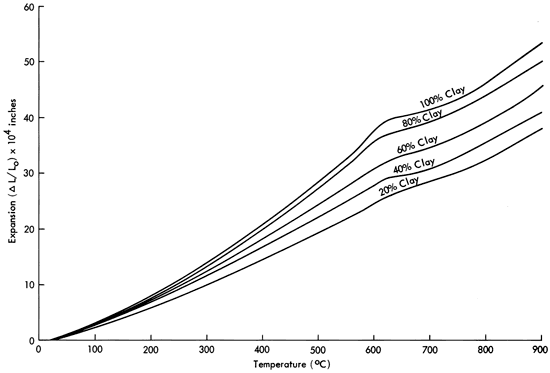
Increasing the clay-to-ash ratio increases the thermal expansion of these materials. Figures 2 and 3 illustrate typical expansion curves for bar specimens previously sintered at 1000° and 1100°C for four hours respectively. From room temperature to 925°C, the expansion coefficient of mature bodies varies from around 40 X 10-6 for a 20 percent by weight clay body to around 60 X 10-6 for the pure bentonite. The variation in expansion as a function of composition is relatively small for this binary mixture system, again presumably due to the chemical similarity of the two (alkali aluminosilicates).
Figure 2--Thermal expansion of bentonite-volcanic ash mixtures after sintering at 1000°C for four hours.
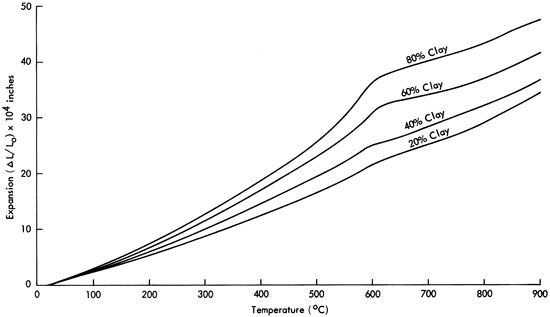
Figure 3--Thermal expansion of bentonite-volcanic ash mixtures after sintering at 1100°C for four hours.
The inflections on the expansion curves in Figures 2 and 3 in the vicinity of 600°C are due to the α↔β quartz transition. These inflections and volume changes are quite small compared to a typical quartz sample such as that shown for a chert specimen in Figure 4. All sintered clay-ash compositions contain a small amount of α-quartz, as measured by x-ray diffraction, and the amount of quartz decreases with in- creasing ash content. As a result, at temperatures below 600°C the coefficient of expansion decreases with increasing ash content because (see Figure 4) the expansion of α-quartz is relatively large. The small amount of α-quartz and consequent small volume change resulting from the α↔β quartz inversion would be beneficial in making brick or tile because volume changes produced by crystallographic transitions can cause cracking.
Figure 4--Comparison of the thermal expansion of bentonite (sintered at 1100°C, four hours) and chert (α-quartz).
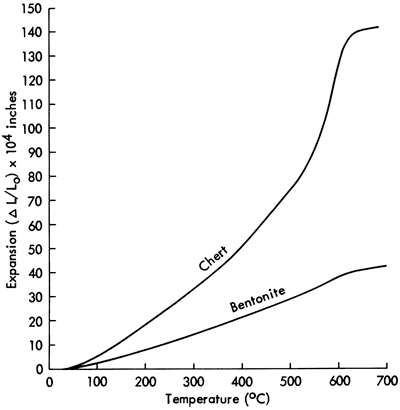
The thermal expansion curves determined at the K.G.S. of another bentonite after sintering show the presence of significant amounts of cristobalite. This form of silica is undesirable in ceramics in large quantities because it undergoes a large volume change at low temperatures and may cause cracking in the ceramic product. By contrast, the bentonite used in this study appears ideal.
The transverse modulus of rupture, MR is expressed in units of pounds per square inch (psi) and for bar specimens is calculated from the formula:
MR = 3PL / 2bd2
where P is the load (exerted perpendicularly to the long axis of the bar) required to break the bar, L is the span of the specimen under stress and b and d are the breadth (width) and depth (thickness) respectively of the bar.
The strength of these bodies is dependent upon the composition, firing temperature, and firing time. Table 5 summarizes the modulus of rupture values for these materials. Each value represents the average of six specimens. The strength increases with an increase of bentonite concentration and an increase in firing temperature between 1000° and 1100°C. Longer soaking times also improve the strength. Up to the maturing temperature the effect of increased temperature and/or soaking time is to produce a denser or less porous body resulting from increased sintering (time and temperature dependent).
Table 5--Transverse Modulus of Rupture (psi) as a Function of the Sintering Temperature (°C), Time (Hours), and the Composition.
| Sintering Temp./Time |
Ash to Clay Ratio |
Pure Bentonite |
1/4 | 2/3 | 3/2 | 4/1 | Dry 1/4 |
|---|---|---|---|---|---|---|---|
| 1000/4 | 1520 | 1130 | 420 | 270 | 320 | ||
| 1050/4 | 2780 | 1950 | 1740 | 1620 | 1850 | ||
| 1100/4 | 6400 | 4700 | 3650 | 3020 | 1850 | 4420 | |
| 1050/8 | 3780 | 3550 | 2310 | ||||
| 1075/4 | 4720 | 3480 | 2550 | ||||
| 1075/10 | 5350 | 3180 | 2930 |
Although the sintering mechanism is outside the scope of this work, one can assume that sintering is primarily accomplished by the development of a liquid or glassy phase. The lack of components with high vapor pressures, e.g., alkali halides, suggests that vapor transport plays an insignificant role during the sintering of these materials.
The question arises as to why the strength for a given firing temperature and time increases with increasing clay content. Since all compositions fired around 1100°C have negligible apparent porosity, one can say that the increase in strength with increasing bentonite is due to one or more of the following reasons: (1) an increased strength of the glassy matrix, (2) fewer sealed pores or a more favorable sealed pore distribution, and (3) a possible development of small amounts of intertwined mullite needles. It is likely that (1) and/or (2) are the major factors giving rise to the increased strength since mullite was not detected in x-ray diffraction patterns.
The crushing or compressive strengths of well-sintered. specimens in this study could not be determined. Attempts to crush were unsuccessful, indicating their strengths were greater than 60,000 psi, the maximum measurable values on the Dillon Strength Testing Instrument.
Several rod specimens of the clay-ash mixtures were extruded. The non-plastic ash prevented the drying cracks which often occur when pure bentonite bodies are extruded.
In general, extrusion produces greenware of a higher density than dry pressing. The water addition necessary for extrusion acts somewhat like a lubricant in that it imparts flexibility to the body and allows particles to slide past one another and form a denser arrangement. Thus, in theory, extrusion should allow one to obtain maturity at a lower firing temperature and/or a shorter soak period.
The bodies in this system behaved in the predicted manner: extruded clay-ash mixtures matured around 1070°C with a three-hour soak, whereas extruded specimens fired at 1100°C (maturing range for dry pressed specimens) were slightly bloated and, hence, overfired. The bloated samples had a slightly lower transverse strength than samples fired at 1070°C.
Perhaps the most significant feature of this test was the strength values obtained from extruded rods and dry-pressed bars. The modulus of rupture for a cylindrical rod is calculated from the formula:
MR = 8PL / πD3
where P is the load required to break the rod, L is the span under stress and D is the rod diameter. For a three-inch span the formula becomes
MR = 7.64P / D3
The modulus of rupture obtained from rods of the 60/40 clay to ash ratio bodies when fired at 1065°C/3 hours approached 5,000 psi. This value represents a striking improvement over the 3650 psi value obtained from dry-pressed bars of the 60/40 mixture fired at 1100°C (see Table 5).
An examination of the firing properties, thermal expansion and strength has been completed for a bentonite clay (Phillips County, Adee #2)-volcanic ash (Calvert, Ks. in Norton County) binary system. The system served as an excellent example of classical ceramic behavior.
For all dry-pressed compositions, as one obtains maturity, one observes a maximum firing shrinkage and zero apparent porosity. From 1000°C to the optimum firing temperature, 1100°C, one observes increases in strength and thermal expansion for a given composition. In a given thermal treatment, an increase in ash content reduces both the strength and thermal expansion. Pure bentonite gives a strength value of over 6,000 psi and a mean linear thermal expansion coefficient of 6 X 10-6 in./in./°C. By contrast, an 80 percent (by weight) ash content at maturity gives a strength value of only 1850 psi and an expansion coefficient of 4.4 X 10-6 in./in./°C.
All bodies in this system have relatively narrow firing ranges that would require close control of kiln temperatures. However, these bodies can be subjected to rapid firing in an electric shuttle kiln lined with refractory fiber which represents a substantial savings in time and production costs. The adequate strength, moderate expansion, and rapid firing give this system potential for brick and especially floor tile production because the latter can be rapidly pressed with little or no binder and being thin, can be fired quite rapidly.
In conclusion, bodies in this system appear to have potential for use in a tile production facility, possibly in northwestern Kansas where substantial reserves of ash and bentonite are located.
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version updated June 18, 2010. Original publication date April 1978.
URL=http://www.kgs.ku.edu/Publications/Bulletins/211_4/grisafe.html