Kansas Geological Survey, Bulletin 204, pt. 1, originally published in 1972
Originally published in 1972 as part of Kansas Geological Survey Bulletin 204, pt. 1, p. 3-10. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version of the complete bulletin (15 MB) is also available.
Organic geochemical studies of a widespread Pennsylvanian black shale (the Excello) indicate the portion of the shale present in the Midcontinent contains greater amounts of extractable hydrocarbons and has much higher hydrocarbon yields and higher saturate-to-aromatic hydrocarbon ratios than does the Excello present in the Illinois basin, which contains greater amounts of organic carbon.
These differences suggest that the Excello deposited in the Illinois basin incorporated greater amounts of terrestrial plant debris, although the predominant source of organic material in both areas is believed to have been marine plankton.
This interpretation is valid only if the organic material incorporated in the Excello Shale in both the Illinois basin and the Midcontinent has been subjected to maturation processes with similar thermal histories. The distribution of intermediate and heavy n-paraffins in the saturated hydrocarbon fractions of samples from both areas suggests maturation effects are quite similar in both the Midcontinent and the Illinois basin.
The purpose of this investigation is to determine the nature and distribution of the extractable hydrocarbons present in a widespread Pennsylvanian black shale (the Excello) of the Midcontinent and the Illinois basin.
Although the bulk of the organic material present in ancient sediments is apparently in the form of a complex high molecular weight polymer (kerogen), the results of studies of the extractable hydrocarbons may reflect not only hydrocarbon generation and maturation processes, e.g., Philippi (1965), but also primary source variations in the type of organic material (i.e., marine vs. terrestrial) originally incorporated in the sediment. Previous organic-geochemical studies by Baker (1962) and Baker et al. (1969) of the Midcontinent Cherokee cyclothems indicate that Pennsylvanian rock units composed predominantly of terrestrial plant debris (coals) can be characterized by low hydrocarbon yields and have low saturate-to-aromatic hydrocarbon ratios, while rock units incorporating organic material derived predominantly from marine plankton (marine shales and limestones) have much higher hydrocarbon yields and tend to display higher saturate-to-aromatic ratios. As all these rock units have been subjected to equivalent thermal histories, the differences in hydrocarbon yields among various lithologic units must reflect primary organic source variations.
Presumably the organic material present in Pennsylvanian sediments in both the Midcontinent and the Illinois basin has been subjected to post-depositional maturation processes with similar thermal intensities; therefore regional studies of the extractable hydrocarbons present in the Excello Shale should indicate whether or not differing proportions of marine and terrestrial organic material were originally incorporated in the shale.
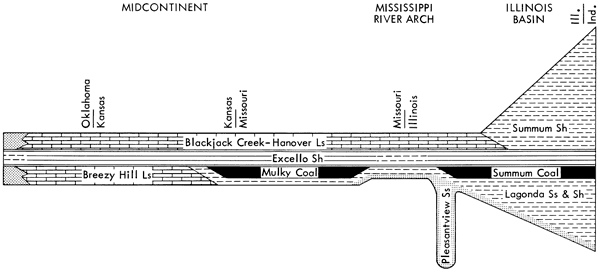
The regional stratigraphic relationships of the Excello Shale are well known; the unit, which lies at the top of the Cherokee Group of the Des Moines Series of the Midcontinent, has been mapped as a member of the Summum cyclothem by Weiner (1961), Gamble (1967), and Wanless (1969). As is schematically shown in Figure 1, the Excello overlies marine carbonates (the Breezy Hill Limestone), gray mudstones, and coals (the Mulky and Summum) progressively from Oklahoma and Kansas to Indiana. It is in turn overlain by normal marine shales and carbonates of the basal Marmaton Group (the Blackjack Creek-Hanover limestones) over most of the Midcontinent and portions of the Illinois basin, but is overlain by a deltaic sequence (the Summum Shale) over most of the Illinois basin.
Figure 1--Generalized lateral variations of Summum cyclothem.

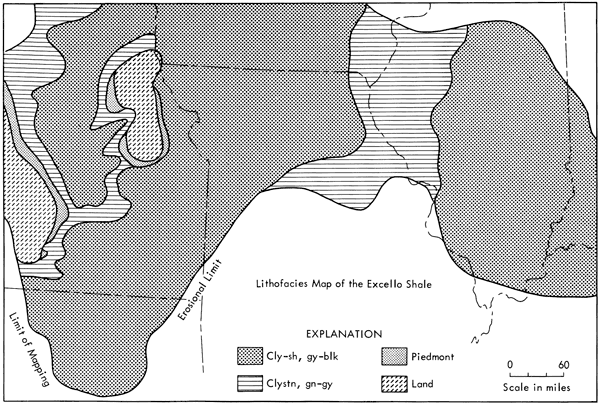
The lithologic characteristics of the Excello have been described by James (1970); the unit averages 4 feet in thickness and generally consists of three major lithologic zones: a thin basal shell coquina, a thick middle zone of phosphatic dark gray-black clay-shale, and a thin upper zone of lighter colored mottled and burrowed claystones which grades into the overlying calcareous normal marine gray claystones and carbonates. As is shown in Figure 2, the Excello Shale, despite erosion from structural highs, covers an area of well over 100,000 square miles and grades laterally into more oxidized greenish-gray facies as it onlaps structural highs such as the Ozark uplift and the Mississippi River arch. The gross physical characteristics of the thinly laminated, organic-rich Excello Shale are amazingly uniform throughout the Midcontinent and the Illinois basin.
Figure 2--Lithofacies map of Excello Shale in Midcontinent and Illinois basin. Modified from Weiner (1961) and Gamble (1968).
The environmental conditions which allowed the accumulation and preservation of organic material in the Excello have been interpreted by James (1970) and are summarized as follows:
The organic-geochemical procedures used in this study were based on methods of hydrocarbon extraction and separation described by Ferguson (1962).
All samples prepared for analysis were first jaw crushed to 1/4-inch pieces and then passed once through a hammer mill (Metals Disintegrating Co., "MikroPulverizer"). [Note: Mention of companies or products is not to be considered an endorsement.] The resultant median particle size ranged from 8 to 16 microns.
Organic carbon was determined by combustion of an acid-treated portion of each sample in a Leco induction furnace with the resultant CO2 being measured volumetrically in a Leco carbon analyzer.
"Hydrocarbon" extracts were obtained from the pulverized rock samples in Soxhlet extractors using redistilled reagent grade benzene as the solvent. Original sample weights ranged from 10 to 750 g. Depending on the estimated sample size needed to obtain approximately 150 mg of residue, the samples were extracted with either 750 ml or 2000 ml of benzene for 24 hours; cycle time was adjusted to 20 minutes. The benzene extracts were concentrated by distilling under partial vacuum at 40° C using a Buchler flash-evaporator. The extracts were isolated in a nitrogen evaporator by drying to constant weight (within 2.0 mg) in tared 2-dram vials. If necessary, the residues were dissolved in 100 ml of benzene, aliquoted, and redried to yield a final residue weight ranging from 120 to 170 mg.
The sediment extract was separated into a "saturated hydrocarbon" fraction and an "aromatic hydrocarbon" fraction by elution chromatography. The chromatographic columns (8 mm I.D. by 26 cm) were packed with two layers of silica gel slurried in cyclohexane; 13 cm of code 950 gel, used as a bottom layer to effect the analytical separation, were overlain by 13 cm code 70 gel (pulverized and sieved to produce a 40 to 60 mesh fraction) used to provide adsorption capacity for very large molecules. The sediment extracts were loaded on the columns with 2 ml of cyclohexane and were successively eluted with 30 ml cyclohexane and 30 ml benzene. The saturate and aromatic fractions collected were isolated by means of the rotary and nitrogen evaporators.
The analytical data obtained and calculated from the organic carbon determinations and the extraction and chromatographic procedures include: (1) organic carbon weight percent, (2) "saturated hydrocarbons" in ppm, (3) "aromatic hydrocarbons" in ppm, (4) "total hydrocarbons" in ppm (summation of 2 and 3), (5) the ratio of saturate-to-aromatic hydrocarbons, and (6) the ratio of total hydrocarbons-to-organic carbon (hydrocarbon yield) in each rock sample.
The molecular size distribution of the intermediate and heavy (C13-C33) n-paraffins present in the saturated hydrocarbon fractions was determined by gas-liquid chromatography. The total alkane fractions were analyzed with a programmed temperature run from 115° C to 265° C at 6°/minute (held isothermally at 115° for 2 minutes and at 265° for 12 minutes) on a Perkin-Elmer Model 990 Gas Chromatograph equipped with dual flame ionization detectors and 8' x 1/8" copper columns coated with SE-30 silicone gum. Peak identities were determined by a series of co-injections with reference n-paraffin standards. All patterns were obtained by injecting a 4 µl of sample (solvent/extract = 1 ml hexane per 20 mg extract) and attenuating by a factor of 200. Comparison of the patterns from the total alkane fractions with patterns obtained by urea adduction and molecular sieving indicated resolution was such that the branched and cyclic compounds did not interfere with the peaks from the straight chain normal paraffins. No identities of the iso-paraffins and cyclo-paraffins were determined.
It has not been possible to establish the accuracy of the results of the procedure for the determination of sediment hydrocarbons because there are no suitable standards available. However, the procedures utilized in this investigation parallel those used by Baker (1962) and Baker et al. (1969), so that the data obtained in each of these investigations may be directly compared. Ferguson (1962) discussed the problems of making these measurements and presented precision data for the extraction and elution chromatographic procedures; the analytical errors (expressed as coefficient of variation) for the organic carbon analyses and the hydrocarbon determinations are expected to be less than 5 percent and 10 percent, respectively, at the concentration levels present in the Excello. [Note: Coefficient of variation (V) = (S/X) x 100%, where S = the standard deviation, X = the mean.]
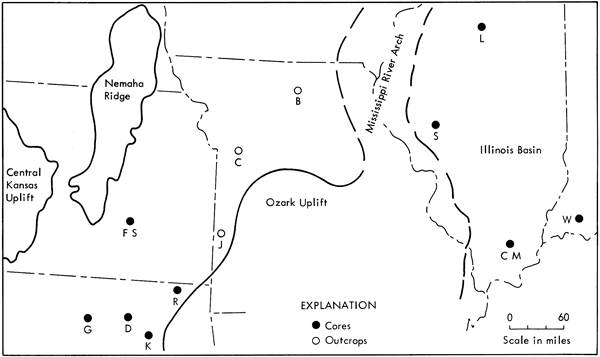
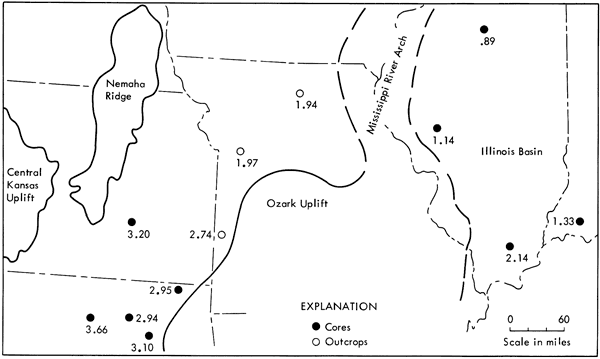
Composite samples of the Excello from 12 widespread localities were prepared for analysis to provide regional data: 5 from core sections in the Midcontinent which overlie marine limestone, 3 from outcrop sections in the eastern portion of the Midcontinent which overlie coal, and 4 from core sections in the Illinois basin which also overlie coal. The distribution of these samples in the Midcontinent and the Illinois basin with respect to Middle Pennsylvanian structural features is shown in Figure 3.
Figure 3--Middle Pennsylvanian structural features and distribution of samples in Midcontinent and Illinois basin. Sample location index letters refer to samples listed in Table 1.
The results of the organic carbon and hydrocarbon analyses are shown in Table 1. Organic carbon analyses are presented in weight percent; extraction and elution chromatographic data are shown as total hydrocarbons (ppm), saturated-hydrocarbons (ppm), aromatic-hydrocarbons (ppm), and as the ratios of saturated-to-aromatic hydrocarbons and hydrocarbons-to-organic carbon (times 10-2). The relationship between the odd-carbon-numbered normal-paraffins and the even-carbon-numbered normal-paraffins is characterized by the carbon preference index (CPI) value, calculated according to Kvenvolden (1966). [Note: CPI = 1/2 {(Σ conc. odd C17 to C31) / (Σ conc. even C16 to C30) + (Σ conc. odd C17 to C31) / (Σ conc. even C18 to C32)}.] Table 2 summarizes the data by averaging the results obtained from samples in the Midcontinent and the Illinois basin; the data from one coal analysis are included for comparative purposes.
Table 1--Organic composition of Excello Shale.
| Org C % |
HC ppm |
Sat ppm |
Aro ppm |
Sat/Aro | HC/Org C (x10-2) |
CPI | |
|---|---|---|---|---|---|---|---|
| Midcontinent | |||||||
| Gross (G) | 7.5 | 2750 | 1435 | 1315 | 1.09 | 3.66 | 1.02 |
| Ft. Scott (FS) | 6.6 | 2115 | 965 | 1150 | 0.84 | 3.20 | 1.00 |
| Kelly (K) | 11.0 | 3415 | 1450 | 1965 | 0.74 | 3.10 | 1.00 |
| Drummond (D) | 10.4 | 3060 | 1310 | 1750 | 0.75 | 2.94 | 1.03 |
| Rexwinkle (R) | 11.0 | 3250 | 1475 | 1775 | 0.83 | 2.95 | 1.05 |
| Jones Quarry (J) | 15.4 | 4215 | 1600 | 2615 | 0.61 | 2.74 | 1.15 |
| Concordia (C) | 7.8 | 1535 | 615 | 920 | 0.67 | 1.97 | 1.16 |
| Bevier (B) | 12.5 | 2430 | 1000 | 1430 | 0.70 | 1.94 | 1.08 |
| Illinois Basin | |||||||
| Carrier Mill (CM) | 15.2 | 3260 | 1365 | 1895 | 0.72 | 2.14 | 1.15 |
| Warrick (W) | 18.5 | 2455 | 985 | 1470 | 0.67 | 1.33 | 1.08 |
| Sangamon (S) | 17.0 | 1945 | 795 | 1150 | 0.69 | 1.14 | 1.05 |
| LaSalle (L) | 7.0 | 625 | 250 | 375 | 0.67 | 0.89 | 1.13 |
Table 2--Average regional organic composition of Excello Shale.
| Org C % |
HC ppm |
Sat/Aro | HC/Org C (x 10-2) |
CPI | |
|---|---|---|---|---|---|
| Midcontinent | |||||
| 8 samples | 10.3 | 2845 | 0.78 | 2.81 | 1.06 |
| s.d. | 3.0 | 835 | 0.14 | 0.61 | 0.06 |
| Illinois Basin | |||||
| 4 samples | 14.4 | 2070 | 0.69 | 1.38 | 1.10 |
| s.d. | 5.1 | 1060 | 0.02 | 0.44 | 0.05 |
| Warrick Coal | 65.5 | 8890 | 0.30 | 1.35 | |
Statistically, the differences of the mean values for the organic carbon and hydrocarbon contents of the Excello Shale samples in the Midcontinent and the Illinois basin do not appear to be significant; however, the following observations may be made from the data in Tables 1 and 2:
Figure 4--Distribution of saturated-to-aromatic hydrocarbon ratios of Excello Shale in Midcontinent and Illinois basin.
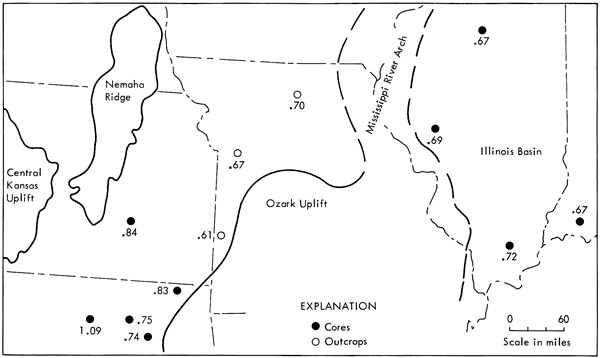
Figure 5--Distribution of hydrocarbon-to-organic carbon ratios (times 10-2) of Excello Shale in Midcontinent and Illinois basin.
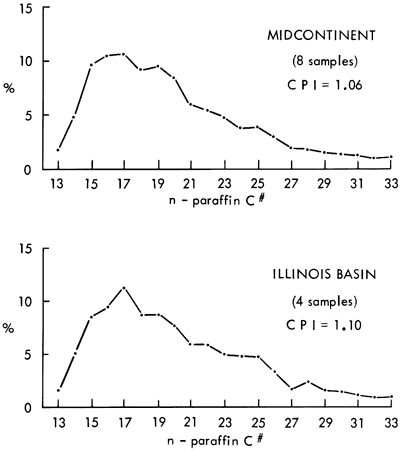
The average molecular weight distributions of the n-paraffins in the Midcontinent and the Illinois basin are illustrated in Figure 6. The n-paraffins occur predominantly in the C15 to C20 range and tail off in a somewhat smooth distribution curve to C33. The overall distribution curves of the n-paraffins are fairly similar throughout the Excello. It should be noted that the extraction and isolation techniques utilized in this study prohibit quantitative recovery of the n-paraffins with molecular weights less than C15.
Figure 6--Average n-paraffin distribution of Excello Shale in Midcontinent and Illinois basin.
The data obtained from this investigation indicate that the Excello Shale contains varying amounts of organic carbon which can be characterized by differences in hydrocarbon yields and, to a limited extent, saturate-to-aromatic ratios. These differences appear to be regional in nature; i.e., the Excello present in the Illinois basin tends to contain greater amounts of organic carbon than does the Excello in the Midcontinent, but has lower hydrocarbon-to-organic carbon ratios and slightly lower saturate-to-aromatic ratios. The differences in the amounts of organic carbon incorporated in the shale reflect source supply, as the relatively large amounts of organic carbon (greater than 6 percent) indicate highly reducing environmental conditions were prevalent in both the Midcontinent and the Illinois basin. The variations in the hydrocarbon yields and the saturate-to-aromatic ratios, however, may be due to (1) differences in the original organic source material (i.e., marine vs. terrestrial) or (2) differences in the maturation history of the Excello in the Midcontinent and the Illinois basin, or a combination of both.
The maturation history of organic material in a sediment is primarily related to the duration and intensity of its thermal history. Two of the more important effects of the maturation process are the generation of additional hydrocarbons in the sediment and the gradual disappearance of the odd-carbon-number predominance in the normal paraffins (straight chain saturated hydrocarbons with the formula CnH2n+2). These changes increase the hydrocarbon-to-orgainc carbon ratio and cause the CPI value to approach unity. The similarities of the CPI values and the distribution curves of the C16-C32 normal paraffins of the Excello indicate that the post-depositional maturation history of the Excello present in the Illinois basin has been essentially equivalent to that of the Excello in the Midcontinent region. Since the thermal history of a sediment is dependent on two factors, geothermal gradient and depth of burial, it seems likely that the organic material in the Excello in both areas should be in the same maturation stage, as the Excello has been subjected to relatively shallow burial depths and as it is not unreasonable to assume that the Midcontinent and the Illinois basin have had similar geothermal gradients since late Paleozoic time.
The interpretation of the results from this study in terms of organic source differences may be aided by first examining the summary of similar data obtained by Baker et al. (1969) on different lithologic units present in the Cherokee cyclothems of the Midcontinent. Such a comparison is warranted since these units were deposited during Middle Pennsylvanian time and hence the original organic source material, whether terrestrial plants or marine algae, presumably had similar components which yielded saturated and aromatic hydrocarbons similar to the hydrocarbons generated from the organic material which contributed to the formation of the Excello Shale.
As is shown in Table 3, the extractable organic material present in the different Cherokee rock types displays a fairly wide range of saturate-to-aromatic ratios and hydrocarbon yields. Limestones, deposited in warm, shallow, oxygenated waters free from terrestrial influx, contain very little organic carbon, but have very high saturate-to-aromatic ratios and hydrocarbon yields. At the other extreme, with very low saturate-to-aromatic and hydrocarbon yields, are the carbon-rich coals which accumulated primarily from the decay of terrestrial plants. The clay-silt shales, with visible carbonized plant remains, have fairly low saturate-to-aromatic ratios and hydrocarbon yields. The calcareous fossiliferous shales, generally deposited in slightly oxidizing marine waters, and the phosphatic black shales, deposited in highly reducing marine environments, have similar saturate-to-aromatic ratios and hydrocarbon yields, despite the fact that the black shales contain approximately ten times the amount of organic carbon that is present in the calcareous shales. Presumably the organic material present in these shales, as in most modern shales, represents the remains of marine plankton somewhat diluted by the remains of terrestrial plants carried to the site of deposition.
Table 3--Organic composition of the Cherokee lithologic units. Data from Baker et al., 1969; published with permission of the Marathon Oil Co.
| Lithology | Org C % |
HC ppm |
Sat/Aro | HC/Org C (x 10-2) |
|---|---|---|---|---|
| Limestone | 0.19 | 423 | 2.1 | 30.8 |
| s.d. | 0.14 | 239 | 0.6 | 33.6 |
| Foss. Shale | 1.17 | 384 | 1.5 | 3.4 |
| s.d. | 0.15 | 78.5 | 0.1 | 1.0 |
| Blk PO4 Shale | 10.85 | 3695 | 1.4 | 3.4 |
| s.d. | 1.04 | 849 | 0.4 | 0.8 |
| Cly-Slt Shale | 1.05 | 211 | 0.9 | 2.1 |
| s.d. | 0.30 | 78.1 | 0.2 | 0.6 |
| Coal | 58.3 | 2648 | 0.4 | 0.5 |
| s.d. | 3.6 | 733 | 0.1 | 0.1 |
While the hydrocarbon yields and the saturate-to-aromatic ratios of the Excello Shale in the Midcontinent are lower than the average values reported by Baker et al. (1969), the hydrocarbon yields and the saturate-to-aromatic ratios of the Excello present in the Illinois basin are much lower, and are only slightly higher than the average values of the coals. This difference suggests that in addition to the high-yield, saturate-rich "sapropelic" type of organic material derived principally from phytoplankton and zooplankton and the fatty tissues of animals, greater amounts of low-yield, aromatic-rich "humic" material derived from the woody parts of plants (cellulose and lignin) were incorporated into the Excello Shale present in the Illinois basin.
This investigation represents a portion of the senior author's Ph.D. dissertation at Rice University. The authors are grateful to the Kansas Geological Survey for supporting summer field investigations.
Funds made available from the Robert A. Welch Grant C-249 to Donald R. Baker and Dieter Heymann purchased much of the laboratory equipment utilized in this investigation. Acknowledgment is made to the donors of The Petroleum Research Fund, administered by the American Chemical Society, for partial support of this research.
The authors also gratefully acknowledge the many people of the Marathon Oil Company, the Indiana Geological Survey, the Illinois Geological Survey, and the Peabody Coal Company who allowed the examination and sampling of many subsurface cores.
Baker, D. R., 1962, Organic geochemistrv of the Cherokee Group in southeastern Kansas and northeastern Oklahoma: Am. Assoc. Petroleum Geologists Bull., v. 46, p. 1621-1642.
Baker, D. R., Ferguson, W. S., and Claypool, G. E., 1969, Organic geochemistry and petroleum distribution of the Cherokee platform, Kansas and Oklahoma (abs.): Am. Assoc. Petroleum Geologists, 54th Annual Mtg., Dallas.
Ferguson, W. S., 1962, Analytical problems in determining hydrocarbons in sediments: Am. Assoc. Petroleum Geologists, Bull., v. 46, p. 1613-1620.
Gamble, J. C., 1967, Paleoenvironmental study of a portion of upper Desmoinesian (Pennsylvanian) strata of the MidContinent region: Unpub. M.S. thesis, University of Illinois, 98 p.
James, G. W., 1970, Stratigraphic geochemistry of a Pennsylvanian black shale (Excello) in the Mid-Continent and Illinois Basin: Unpub. Ph.D. thesis, Rice University, 92 p.
Kvenvolden, K. A., 1966, Evidence for transformations of normal fatty acids in sediments; in, Advances in Organic Geochemistry, G. D. Hobson and G. C. Speers, eds.: Pergamon Press, Oxford, p. 335-366.
Philippi, G. T., 1965, On the depth, time, and mechanisms of petroleum generation: Geochim. Cosmochim. Acta, v. 29, p. 1021-1049.
Wanless, H. R., 1969, Eustatic shifts in sea level during deposition of Late Paleozoic sediments in the central United States; in, Cyclic Sedimentation in the Permian Basin: West Texas Geol. Society, Midland, p. 41-54.
Weiner, J. L., 1961, Environmental study of stages within the Summum cyclothem of Illinois in the east-central United States: Unpub. M.S. thesis, University of Illinois, 31 p.
| Illinois Basin | ||
|---|---|---|
| Warrick (W) core | 35-4S-8W | Warrick Co., Ind. |
| Carrier Mills (CM) core | 4-10S-5E | Saline Co., Ill. |
| Sangamon (S) core | 17-13N-5W | Sangamon Co., Ill. |
| LaSalle (L) core | 15-30N-2E | LaSalle Co., Ill. |
| Midcontinent | ||
| Bevier (B) outcrop | 24-56N-15W | Macon Co., Mo. |
| Concordia (C) outcrop | 35-49N-25W | Lafayette Co., Mo. |
| Jones Quarry (J) outcrop | 7-34N-32W | Vernon Co., Mo. |
| Fort Scott (FS) core | 31-23S-10E | Greenwood Co., Kan. |
| Rexwinkle (R) core | 30-29N-18E | Craig Co., Okla. |
| Drummond (D) core | 4-23N-10E | Osage Co., Okla. |
| Gross (G) core | 34-25N-14E | Osage Co., Okla. |
| Kelly (K) core | 23-20N-14E | Rogers Co., Okla. |
Kansas Geological Survey, Organic Geochemistry of a Pennsylvanian Black Shale (Excello)
Placed on web May 7, 2009; originally published in March 1972.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/204_1A/index.html