
Kansas Geological Survey, Bulletin 187, pt. 1, originally published in 1967

Originally published in 1967 as part of "Short Papers on Research in 1966," Kansas Geological Survey Bulletin 187, part 1, p. 7-8. This is, in general, the original text as published. The information has not been updated.
A dry method for upgrading rock salt that can be adapted to salt mine processing is highly desirable. A photoelectric method based on the differences in light transmission through pure halite and contaminated halite is described. In laboratory tests using this method, 92 percent rock salt was upgraded to 98.5 percent.
Kansas salt, as mined, consists of halite, halite-anhydrite, halite-shale, and halite-anhydrite-shale fragments. The contaminants, shale and anhydrite, are the major contributors to a lower NaCl content. An analysis of a Kansas commercial rock salt is given in Table 1.
Table 1--Composition of rock salt, Reno County, Kansas.
| Acid insolubles (shale) | 0.165% |
| Iron oxide | 0.012% |
| Calcium sulfate | 2.744% |
| Calcium chloride | 0.010% |
| Magnesium chloride | 0.293% |
| Sodium chloride (by difference | 96.776% |
| 100.000% |
From a cost standpoint it is highly advantageous to be able to upgrade this rock salt by some dry method that is adaptable to present processing procedures. With salt of 98 to 99 percent NaCl content, a larger market is available.
Because there are some slight differences in the physical characteristics of halite and anhydrite (Table 2), a number of procedures attempting to separate halite and anhydrite based on these differences have been investigated. These procedures include variations in crushing and screening techniques, centrifugal and drop-bounce methods, and density difference separation. None of these gave sufficiently good, consistent results to really warrant consideration as plant-scale operations.
Table 2--Physical properties of halite and anhydrite.
| Chemical composition |
Hardness | Specific gravity |
Index of refraction |
Transparency | Tenacity | Fracture | Specific heat at 2000°C, BTU/lb |
|
|---|---|---|---|---|---|---|---|---|
| Halite | NaCl | 2.0-2.5 | 2.1-2.6 | 1.544 | Transparent- translucent |
Brittle | Conchoidal | .393 |
| Anhydrite | CaSO4 | 3.0-3.5 | 2.7-3.0 | 1.571-1.614 | Transparent- opaque |
Brittle | Irregular | .239 |
A procedure based on differences in halite and anhydrite diathermancy has been developed to separate these two materials. In essence, this technique calls for exposing the salt-anhydrite mixture to radiant heat of the proper wavelength and allowing the mixture to spread on a high-speed endless belt coated with heat-sensitive resin. Halite, with greater ability to transmit radiant heat, does not become as warm as anhydrite, and thus, does not adhere to the resin. The net result is a separation due to preferential adherence. This method can be incorporated into a production operation and has already performed satisfactorily in some salt mines. However, thermo-adhesive field tests with Kansas salt did not result in the desired improvement.
Industrial use of photoelectric cells for sorting by color, size, or transparency led to the development of this technique as a means of separating clear grains of halite from those clouded with anhydrite or shale. Accordingly, a preliminary series of tests using a very simple photoelectric method gave positive results in upgrading Kansas salt (Fig. 1).
Figure 1--General arrangement of feeder, photoelectric cell, and air nozzle. Feeder has 1/16" slot for light to pass through. Air jet also goes through same slot.
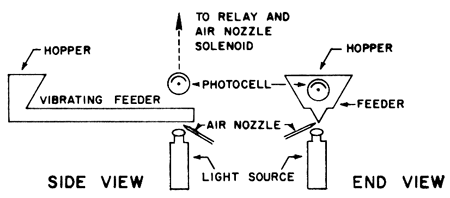
Essentially, the system consists of a means of feeding a single layer of salt particles (<.375" to > .125") between a light source and a photoelectric cell. The cell is connected through a relay to a solenoid-activated air valve to which a fine nozzle is attached. As long as the grains of halite are clear, the cell does not actuate the air valve. A dimming of the light due to anhydrite or shale causes immediate actuation of the air valve, and in turn, the nozzle blows the impure grain out of the stream. The system performs excellently, and by varying the speed of feed and light intensity, a wide range in separation preciseness is available. Results using this method are given in Table 3.
Table 3--Photoelectric grading of a Kansas salt.
| KGS sample |
As received | After treatment | ||
|---|---|---|---|---|
| NaCl content, % |
Acid insolubles, % |
Acid insolubles, % |
NaCl content, % |
|
| 10 | 92.0 | 0.400 | 0.072 | 98.59 |
| 19 | 96.0 | 0.165 | 0.091 | 98.00 |
| 21 | 96.0 | 0.165 | 0.049 | 98.78 |
| 22 | 92.0 | 0.400 | 0.070 | 98.49 |
Speculating on the "after treatment" NaCl content, the data indicate that the top percentage obtainable by this method probably is in the order of 99 percent.
Several areas of study are immediately suggested to determine whether or not this technique is operationally feasible on a plant scale. These include evaluations of photoelectric cells, sources of illumination, and circuitry. Also, experiments should be carried on to determine optimum salt-particle sizes, methods of conveying salt to and away from classification, and handling of rejected salt. This all implies that an optimum method of scanning the salt will also be determined.
Brison, R. J., and Tangel, O. J., 1960, Development of a thermo-adhesive method for dry separation of minerals: Mining Engineering, v. 12, no. 8, August 1960, p. 913-917.
Bleimeister, W. C. and Brison, R. J., 1960, Beneficiation of rock salt at the Detroit mine: Mining Engineering, v. 12, no. 8, August 1960, p. 918-921.
Electronics, 1946, Phototubes control food sorting system: Electronics, July, p. 152.
Pierson, C. V., Jr., 1964, Electronic sorting of crushed rock by color: Mining Congress Jour., October, p. 111-114.
Kansas Geological Survey, Short Papers on Research in 1966
Placed on web July 25, 2011; originally published in Feb. 1967.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/187_1B/index.html