
Kansas Geological Survey, Bulletin 165, pt. 7, originally published in 1963

Originally published in 1963 as part of Kansas Geological Survey Bulletin 165, pt. 7. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (5 MB) is also available.
Versene (ethylenediaminetetraacetic acid) solutions with five different alkali metals or radicals substituted on the basic reagent were compounded for a study in which a range of concentration, temperature, and pH value were variables used in a series of solution rate tests on limestone blocks. These solution rates compared favorably with those of weak acids. Qualitative spectrographic analyses were used to ascertain the metals chelated by the versenate solutions.
The standard technique for the recovery of acid-insoluble or noncarbonate minerals from carbonate rocks is by solution in acetic and mineral acids. Mineral residues are useful for cataloguing carbonate rocks and are especially valuable in sedimentological and geochemical studies. Investigations of particle size, clay minerals present, and kinds and amounts of trace and secondary minerals present provide keys to the understanding of the environment of deposition and to the geochemical aspects of carbonate precipitation. Development of new tools which are useful in deciphering past geological events is paramount to a better understanding of those events.
In spite of the use of very dilute acids, standard insoluble residue techniques, when applied to carbonate rocks, are known to take many accessory minerals into solution. Remedial tactics on this problem have trended primarily toward the use of weaker acids and greater dilution, yielding improved recovery of some minerals. The great advantage in the use of Versene* (EDTA or ethylenediaminetetraacetic acid) and its various alkali metal and other substituted salts is that they are chelating or sequestering agents, which take calcium and magnesium carbonate into solution at a neutral or basic pH. [*Trade name, Dow Chemical Company. Detailed comprehensive discussions of the chemistry of EDTA (Versene) beyond the scope of this paper can be found in Martell and Calvin (1952) and Welcher (1958).] The ions are chelated in solutions of bi-, tri-, and quadri-basic-substituted Versene acid. Versenes leach other elements in addition to calcium and magnesium, but this loss can be controlled partially by adjustment of the pH.
Present techniques used in the solution of calcareous materials by acid are laborious and require considerable laboratory space. The fumes created by this reaction must be removed. Because of the high rate of reaction and evolution of gas the procedure requires either unduly large beakers in proportion to the volume of solution used or repeated acid applications.
The use of EDTA and its various substituted salts does not require special handling, ventilation, unduly large equipment or space, or an unreasonable length of time. It is a convenient and versatile way of removing calcareous materials from sediments.
The experimentation that led to this report was undertaken in order to improve the recovery of acid-soluble minerals from carbonate rocks. This paper reports the observed reaction rates of various Versene solutions (lithium, potassium, sodium, ammonium, and 2-aminoethanol) on blocks of limestone of approximately equal surface area and essentially uniform chemical composition. Loss in weight or changes in solution rate under controlled conditions of concentration, pH, and temperature were appraised for the various substituted Versenes.
Wiessman and Diehl (1954) developed a method using Versene for the determination of calcite-dolomite ratios. Their work was based on the fact that the solvent effect of the quadridentate or sexidentate Versene anion preferentially complexes calcium over magnesium. On the basis of preliminary laboratory studies, Hill and Runnels (1960) suggested the use of Versenes to replace or supplement acids in many facets of carbonate rock study. Glover (1961) gave data on the use of commercially available EDTA compounds for insoluble residue studies, specifically reporting on the effect of concentration, pH, temperature, and particle size of calcium carbonate on solution rate.
The Dow Chemical Company provided the Geochemical Laboratory of the State Geological Survey of Kansas with samples of their commercially available sodium and hydrogen Versenes, which were utilized in preliminary evaluation studies. The x-ray analyses were made by Donald I. Good and the wet chemical analyses by O. Karmie Galle, both of the Kansas State Geological Survey.
Hydrogen- and sodium-substituted Versenes are commercially available. Lithium-, potassium-, ammonium-, and 2-aminoethanol-substituted Versenes were synthesized in the Kansas Geological Survey's Geochemistry Laboratory for use in this experiment. Commercially available technical grade tetra-sodium ethylenediaminetetraacetic acid was used as the basic reagent in these syntheses. The low cost of this form of Versene (approximately 50 cents per pound) adapts it to bulk usage for the solution of large quantities of calcareous materials much more economically than analytical reagent-grade Versenes, which range from $6.00 to $8.00 per pound. [Note: Prices in 1963.]
To make up the different Versenes used in the tests it was necessary first to con vert the tetra-sodium Versene to the Versene acid salt and resubstitute the alkali metals or other compounds desired. This conversion and resubstitution is also the basic method of reclaiming spent Versene for reuse. The tetra-sodium Versene was dissolved in distilled water and precipitated in the tetra-hydrogen Versene acid form by the addition of reagent-grade hydrochloric acid in small increments until a pH of 1 was attained. The solution and precipitation were carried out in 20-quart polyethylene wastebaskets placed on a magnetic stirrer. In an inert-reaction vessel of this size, 3-5 kg of Versene was converted at once. The supernatant liquid was decanted and vacuum-filtered through Whatman 11-cm OK filter paper in a buchner funnel. The filtrate (acidic sodium chloride solution) was discarded. The precipitate was washed with 10 percent hydrochloric acid and vacuum-filtered. These filter cakes of Versene acid were air dried and all placed in a single covered storage container. In this research, in order to minimize the introduction of metallic ions that could interfere with control spectrographic analyses, analytical reagent-grade chemicals were used. A spectrographic analysis run on the precipitated Versene acid showed only trace amounts of sodium remaining, indicating an almost complete conversion to the tetra-hydrogen form. Technical grade chemicals could be used in place of reagent-grade chemicals for precipitation and resubstitution of the Versene in mass application not requiring spectrographic control analyses.
The Versene acid from spent Versene solutions is easily recycled by addition of sufficient hydrochloric acid as described above and washing the precipitate. In this manner, the Versene acid may be reclaimed and reused an indeterminate number of times, thus greatly reducing the cost of reagents. Because sulfuric acid co-precipitates calcium sulfate and complicates the separation of Versene acid, its use is not recommended.
Solutions were made in one-liter polyethylene bottles in the following concentrations: 0.1, 0.5, 1.0, 1.0, 3.0, 4.0, and 5.0 N, based upon the reaction
4 MOH + H4 (EDTA) → M4 (EDTA) + 4H20
Where M is any univalent metal or positive radical.
Weighed amounts of analytical reagent-grade alkali metal hydroxide (lithium*, sodium, and potassium) were dissolved in distilled water; to these solutions weighed amounts of Versene acid (powdered form) were added to give the normalities desired. [*LiOH only available chemically pure.] Table 1 lists the calculated amounts necessary to produce one liter of bialkali-substituted Versene solution. Some adjustment of the amount of alkali metal hydroxide was necessary to attain the desired pH. Aliquots (200 ml) were withdrawn from this stock solution and placed in 250-ml beakers. The pH values of the solutions were adjusted to those desired for the tests by addition of small increments of solid alkali hydroxide, with continuous agitation of the solution by a magnetic stirrer. Frequent checks of pH were made with a Leeds & Northrup model No. 7662 pH meter. When the desired pH was attained, each beaker was covered with a watchglass and reserved for testing. Test solutions of ammonium and 2-aminoethanol Versenes were prepared by titrating the appropriate amount of Versene acid (Table 1) with the liquid base to given pH values, and adjusting to volume. The solutions used in the mixed-base tests (bilithium, bisodium, and bipotassium 2-aminoethanol) were prepared by adding the amounts of alkali base to the amount of Versene acid recommended in Table 1 and titrating to the desired pH with 2-aminoethanol.
Table 1--Amounts of selected reagent required for conversion of Versene acid to desired concentration of bialkali-substituted Versene. [In order to convert to bialkali Versene, use the indicated weight of Versene acid with the indicated weight of one of the alkali hydroxides for the concentration desired and make up to one liter. For quantities greater than one liter, use multiples of both weights. Trialkali Versene is manufactured by using weight of Versene acid and 1.5 times the indicated amount of alkali hydroxide. For quadri-alkali Versene, double the indicated amount of alkali hydroxide.]
| Conc. desired |
g/l Versene acid |
g/l of any one alkali hydroxide below |
Rated efficiency (will dissolve CaCO3 g/l)† |
||
|---|---|---|---|---|---|
| LiOH | NaOH | KOH | |||
| 0.1 N | 7.3 | 1.2 | 2.0 | 2.8 | 2.5 |
| 0.5 N | 36.5 | 5.9 | 10.0 | 14.0 | 12.5 |
| 1.0 N | 73.1 | 11.9 | 20.0 | 28.0 | 25.0 |
| 2.0 N | 146.1 | 23.9 | 40.0 | 56.0 | 50.0 |
| 3.0 N | 219.2 | 35.8 | 60.0 | 84.0 | 75.0 |
| 4.0 N | 292.3 | 47.8 | 80.0 | 112.0 | 100.0 |
| 5.0 N | 365.3 | 59.7 | 100.0 | 140.2 | 125.0 |
| † Based upon the reaction M4 (EDTA) + CaCO3 → CaM2 (EDTA) + M2CO3 where M is hydrogen, alkali metal, or other substituted group. |
|||||
One large block (approximately 24" x 18" x 8") from an outcrop of Warsaw Limestone (Mississippian) in sec. 2, T. 35 S., R. 25 E., in Cherokee County, Kansas, was selected for preparation of a total of 111 smaller blocks (approximately 1" x 1" x 2 1/2"). The large block of Warsaw Limestone is comprised of light-gray, fine-grained limestone with some fine stylolytic markings outlined by very small amounts of "limonite." Coarse crystals of calcite made up about 10 percent of the surface area and generally averaged only 1 or 2 mm in diameter. Small crinoid columnals, brachiopod valves, and fragments of both were abundant in the rock.
The weathered surface of the large limestone block was removed and care was taken to cut it into 111 blocks of uniform size. The small size was chosen because such a block fits nicely into a 250-ml beaker and may be covered with reagent by the addition of 200 ml of Versene solution. The blocks were shaped with a diamond saw, washed free of powdered limestone, dried, serially numbered, and weighed. Variations in the original weight of the blocks (Tables 2, 3, 4, 5) indicated initially a slight variation in size. The effective surface area of approximately 12 square inches on each block allowed comparison of the effects of different alkali metal Versenes at different concentrations and pH values.
Table 2--Weights of limestone blocks after immersion in selected concentrations of versenates under controlled pH at room temperature, at indicated time intervals.
| Sample block no. |
Original block wt., g |
Concentration N |
pH | Weight in grams | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 hours | 8 hours | 16 hours | 24 hours | 48 hours | 72 hours | 168 hours | ||||
| Lithium Versenate | ||||||||||
| 1 | 105.4 | 0.1 | 8 | 104.9 | 104.8 | 104.6 | 104.6 | 104.6 | 104.6 | 104.5 |
| 2 | 91.3 | 0.1 | 10 | 90.9 | 90.7 | 90.5 | 90.5 | 90.5 | 90.5 | 90.5 |
| 3 | 94.4 | 0.1 | 12 | 93.8 | 93.7 | 93.6 | 93.5 | 93.5 | 93.5 | 93.5 |
| 4 | 97.8 | 0.1 | 14 | 97.3 | 97.2 | 97.0 | 97.0 | 97.0 | 97.0 | 97.0 |
| 5 | 95.2 | 0.5 | 8 | 93.9 | 93.4 | 92.8 | 92.7 | 92.5 | 92.4 | 92.4 |
| 6 | 93.6 | 0.5 | 10 | 92.6 | 92.1 | 91.5 | 91.1 | 90.8 | 90.6 | 90.6 |
| 7 | 89.5 | 0.5 | 12 | 88.8 | 88.4 | 87.8 | 87.4 | 86.9 | 86.6 | 86.6 |
| 8 | 93.3 | 0.5 | 14 | 92.5 | 92.1 | 91.6 | 91.3 | 90.9 | 90.6 | 90.4 |
| 9 | 92.3 | 1.0 | 8 | 90.7 | 90.1 | 89.1 | 88.6 | 87.6 | 87.2 | 86.9 |
| 10 | 94.2 | 1.0 | 10 | 93.3 | 92.9 | 92.2 | 91.6 | 90.4 | 89.6 | 88.9 |
| 11 | 87.1 | 1.0 | 12 | 86.4 | 86.0 | 85.4 | 84.9 | 83.9 | 83.2 | 82.5 |
| 12 | 90.9 | 1.0 | 14 | 90.2 | 89.8 | 89.1 | 88.6 | 87.7 | 87.0 | 86.4 |
| 13 | 93.8 | 2.0 | 8 | 92.6 | 92.0 | 91.8 | 89.9 | 88.0 | 87.0 | 85.6 |
| 14 | 98.6 | 2.0 | 10 | 97.8 | 97.5 | 96.8 | 96.2 | 94.5 | 93.8 | 92.4 |
| 15 | 99.5 | 2.0 | 12 | 98.8 | 98.6 | 98.0 | 97.6 | 96.7 | 95.8 | 94.5 |
| 16 | 97.8 | 2.0 | 14 | 97.1 | 96.8 | 96.2 | 95.8 | 95.1 | 94.4 | 93.1 |
| 17 | 103.9 | 3.0 | 10 | 103.3 | 102.9 | 102.3 | 101.7 | 100.3 | 99.4 | 97.3 |
| 18 | 99.7 | 3.0 | 14 | 99.2 | 99.1 | 98.6 | 98.3 | 97.8 | 97.3 | 96.1 |
| 19 | 95.6 | 4.0 | 10 | 95.1 | 94.9 | 94.5 | 94.2 | 93.3 | 92.5 | 90.9 |
| 20 | 90.6 | 4.0 | 14 | 90.1 | 89.9 | 89.6 | 89.3 | 88.7 | 88.1 | 87.3 |
| 21 | 90.4 | 5.0 | 10 | 89.9 | 89.7 | 89.4 | 89.1 | 88.4 | 87.8 | 86.7 |
| 22 | 93.3 | 5.0 | 14 | 92.9 | 92.7 | 92.4 | 92.3 | 91.9 | 91.4 | 90.8 |
| Bilithium 2-aminoethanol Versenate (Mixed Base) | ||||||||||
| 23 | 91.3 | 1.0 | 10 | 91.2 | 90.8 | 90.4 | 89.5 | 89.0 | 87.5 | 85.7 |
| 24 | 116.4 | 2.0 | 10 | 116.3 | 116.3 | 115.9 | 115.3 | 114.8 | 113.6 | 112.3 |
| 25 | 89.1 (for chem. analysis) | |||||||||
| Sodium Versenate | ||||||||||
| 26 | 89.6 | 0.1 | 8 | 89.3 | 89.2 | 89.1 | 89.1 | 89.0 | 89.0 | 89.1 |
| 27 | 97.2 | 0.1 | 10 | 96.8 | 96.7 | 96.7 | 96.6 | 96.6 | 96.6 | 96.6 |
| 28 | 86.1 | 0.1 | 12 | 85.8 | 85.7 | 85.6 | 85.5 | 85.5 | 85.5 | 85.5 |
| 29 | 90.5 | 0.1 | 14 | 90.2 | 90.1 | 90.0 | 90.0 | 89.9 | 89.9 | 90.0 |
| 30 | 103.8 | 0.5 | 8 | 102.5 | 102.0 | 101.5 | 101.4 | 100.7 | 100.7 | 100.7 |
| 31 | 103.6 | 0.5 | 10 | 102.4 | 101.8 | 101.3 | 101.2 | 100.5 | 100.5 | 100.5 |
| 32 | 103.8 | 0.5 | 12 | 102.5 | 101.9 | 101.6 | 101.5 | 100.6 | 100.6 | 100.6 |
| 33 | 115.5 | 0.5 | 14 | 114.3 | 113.8 | 113.4 | 113.2 | 112.8 | 112.8 | 112.8 |
| 34 | 116.1 | 1.0 | 8 | 113.9 | 112.9 | 111.8 | 111.3 | 111.2 | 111.3 | 111.3 |
| 35 | 85.1 | 1.0 | 10 | 83.6 | 82.9 | 81.6 | 80.8 | 80.7 | 80.7 | 80.7 |
| 36 | 94.6 | 1.0 | 12 | 92.9 | 92.1 | 90.9 | 90.1 | 90.1 | 90.1 | 90.1 |
| 37 | 107.6 | 1.0 | 14 | 106.0 | 105.2 | 104.3 | 103.6 | 103.5 | 103.5 | 103.5 |
| 38 | 83.2 | 2.0 | 8 | 81.7 | 80.7 | 78.9 | 77.3 | 74.6 | 73.3 | 72.7 |
| 39 | 84.6 | 2.0 | 10 | 83.2 | 82.3 | 80.6 | 79.1 | 76.5 | 74.5 | 73.8 |
| 40 | 83.8 | 2.0 | 12 | 82.5 | 81.5 | 79.6 | 78.1 | 75.0 | 73.6 | 72.6 |
| 41 | 80.5 | 2.0 | 14 | 79.0 | 78.0 | 76.1 | 74.8 | 72.1 | 70.6 | 69.4 |
| 42 | 83.7 | 3.0 | 10 | 82.5 | 81.7 | 79.9 | 78.3 | 74.4 | 71.7 | 68.2 |
| 43 | 82.0 | 3.0 | 14 | 80.7 | 79.7 | 77.9 | 76.3 | 72.9 | 70.5 | 67.6 |
| 44 | 82.9 | 4.0 | 10 | 82.1 | 81.6 | 80.5 | 79.4 | 76.4 | 73.7 | 68.8 |
| 45 | 89.2 | 4.0 | 14 | 88.2 | 87.6 | 86.2 | 85.1 | 82.2 | 79.9 | 76.0 |
| 46 | 88.6 | 5.0 | 10 | 87.9 | 87.6 | 86.9 | 86.3 | 84.4 | 83.2 | 79.9 |
| 47 | 88.0 | 5.0 | 14 | 87.3 | 86.9 | 86.0 | 85.4 | 83.5 | 81.7 | 78.4 |
| Bisodum 2-aminoethanol Versenate (Mixed Base) | ||||||||||
| 48 | 86.2 | 1.0 | 10 | 85.9 | 85.0 | 83.7 | 82.4 | 81.7 | 81.3 | 81.0 |
| 49 | 83.4 | 2.0 | 10 | 82.8 | 81.6 | 79.8 | 77.6 | 76.5 | 73.9 | 72.3 |
| 50 | 103.0 (for chem. analysis) | |||||||||
| Potassium Versenate | ||||||||||
| 51 | 131.2 | 0.1 | 8 | 131.0 | 130.7 | 130.6 | 130.6 | 130.6 | 130.6 | 130.6 |
| 52 | 129.2 | 0.1 | 10 | 128.8 | 128.7 | 128.6 | 128.6 | 128.6 | 128.6 | 128.6 |
| 53 | 123.4 | 0.1 | 12 | 123.1 | 122.9 | 122.8 | 122.8 | 122.7 | 122.7 | 122.7 |
| 54 | 116.9 | 0.1 | 14 | 116.5 | 116.3 | 116.3 | 116.2 | 116.2 | 116.2 | 116.2 |
| 55 | 108.2 | 0.5 | 8 | 106.7 | 106.2 | 105.8 | 105.8 | 105.8 | 105.8 | 105.8 |
| 56 | 104.4 | 0.5 | 10 | 102.9 | 102.2 | 101.9 | 101.9 | 101.9 | 101.9 | 101.9 |
| 57 | 101.6 | 0.5 | 12 | 100.2 | 99.5 | .99.1 | 99.1 | 99.0 | 99.0 | 99.0 |
| 58 | 98.1 | 0.5 | 14 | 96.7 | 96.2 | 95.7 | 95.7 | 95.6 | 95.6 | 95.6 |
| 59 | 113.5 | 1.0 | 8 | 111.0 | 109.8 | 108.9 | 108.8 | 108.7 | 108.7 | 108.7 |
| 60 | 108.0 | 1.0 | 10 | 105.5 | 104.3 | 103.2 | 1032 | 103.0 | 103.0 | 103.0 |
| 61 | 120.8 | 1.0 | 12 | 118.3 | 116.8 | 115.7 | 115.7 | 115.7 | 115.7 | 115.7 |
| 62 | 111.8 | 1.0 | 14 | 109.4 | 108.2 | 106.9 | 106,9 | 106.7 | 106.8 | 106.8 |
| 63 | 119.5 | 2.0 | 8 | 115.9 | 113.7 | 110.3 | 110.0 | 109.8 | 109.8 | 109.8 |
| 64 | 112.4 | 2.0 | 10 | 108.9 | 106.6 | 103.2 | 102.8 | 102.6 | 102.5 | 102.5 |
| 65 | 101.0 | 2.0 | 12 | 97.6 | 95.3 | 92.1 | 91.6 | 91.4 | 91.3 | 91.3 |
| 66 | 115.9 | 2.0 | 14 | 112.5 | 110.2 | 106.7 | 106.2 | 105.8 | 105.7 | 105.7 |
| 67 | 103.9 | 3.0 | 10 | 100:7 | 98.1 | 92.2 | 90.7 | 89.1 | 88.9 | 88.8 |
| 68 | 103.5 | 3.0 | 14 | 100.2 | 97.6 | 92.0 | 90.7 | 89.1 | 88.8 | 88.6 |
| 69 | 109.4 | 4.0 | 10 | 107.1 | 104.3 | 101.5 | 98.7 | 93.1 | 90.6 | 89.2 |
| 70 | 106.2 | 4.0 | 14 | 103.7 | 101.6 | 98.5 | 96;2 | 91.1 | 88.7 | 86.2 |
| 71 | 104.8 | 5.0 | 10 | 102.7 | 100.8 | 97.7 | 95.1 | 88.6 | 85.1 | 81.4 |
| 72 | 100.7 | 5.0 | 14 | 98.7 | 96.8 | 93.8 | 91.3 | 85.4 | 82.1 | 78.7 |
| Bipotassium 2-aminoethanol Versenate (Mixed Base) | ||||||||||
| 73 | 102.5 | 1.0 | 10 | 100.9 | 99.4 | 98.2 | 97.7 | 97.7 | 97.5 | 97.5 |
| 74 | 101.0 | 2.0 | 10 | 98.5 | 96.0 | 93.6 | 92,1 | 91.5 | 91.3 | 91.1 |
| 75 | 102.9 (for chem. analysis) | |||||||||
Table 3--Weights and average solution rates of limestone blocks in selected concentrations ot acetic and hydrochloric acids at room temperature, at the end of 4- and 4-8-hour periods,
| Sample block no. |
Original wt., g |
Concentration percent |
Acid | Block weight | Average rate of solution g/hr. | ||
|---|---|---|---|---|---|---|---|
| 4 hours | 8 hours | 0-4 hours | 4-8 hours | ||||
| 76 | 97.9 | 1.0 | Acetic | 96.9 | 96.2 | 0.25 | 0.18 |
| 77 | 106.3 | 3.0 | Acetic | 102.7 | 101.8 | 0.90 | 0.23 |
| 78 | 112.9 | 5.0 | Acetic | 107.7 | 106.3 | 1.30 | 0.35 |
| 79 | 111.3 | 10.0 | Acetic | 102.3 | 99.6 | 2.25 | 0.68 |
| 80 | 118.9 | 25.0 | Acetic | 107.5 | 102.0 | 2.85 | 1.38 |
| 81 | 107.4 | 1.0 | Hydrochloric | 106.2 | 105.8 | 0.30 | 0.10 |
| 82 | 99.2 | 3.0 | Hydrochloric | 95.8 | 95.5 | 0.85 | 0.08 |
| 83 | 88.3 | 5.0 | Hydrochloric | 82.8 | 82.4 | 1.38 | 0.10 |
| 84 | 99.5 | 10.0 | Hydrochloric | 87.8 | 87.4 | 2.93 | 0.10 |
| 85 | 98.8 | 25.0 | Hydrochloric | 69.9 | 69.5 | 7.23 | 0.10 |
Table 4--Effects of heat (boiling) on block weight and on average rate of solution at pH 8 using limestone blocks in selected optimum Versene solutions.
| Sample block no. |
Original wt., g |
Concentration N |
Base | Block weight after immersion at boiling | Average rate of solution g/hr. | ||
|---|---|---|---|---|---|---|---|
| 4 hours | 8 hours | 0-4 hours | 4-8 hours | ||||
| 86 | 110.9 | 0.5 | LiOH | 108.8 | 108.7 | 0.53 | 0.03 |
| 87 | 117.2 | 1.0 | LiOH | 113.8 | 113.2 | 0.85 | 0.15 |
| 88 | 113.2 | 2.0 | LiOH | 108.7 | 107.7 | 1.13 | 0.25 |
| 89 | 115.2 | 0.5 | NaOH | 112.9 | 112.9 | 0.58 | 0.00 |
| 90 | 121.0 | 1.0 | NaOH | 117.0 | 116.5 | 1.00 | 0.13 |
| 91 | 113.7 | 2.0 | NaOH | 106.8 | 105.8 | 1.73 | 0.25 |
| 92 | 118.2 | 0.5 | KOH | 116.0 | 115.9 | 0.55 | 0.03 |
| 93 | 116.5 | 1.0 | KOH | 111.9 | 111.7 | 1.15 | 0.05 |
| 94 | 105.1 | 2.0 | KOH | 96.2 | 94.0 | 2.23 | 0.55 |
| 95 | 101.9 | 0.5 | NH4OH | 101.6 | 101.4 | 0.08 | 0.05 |
| 96 | 95.0 | 1.0 | NH4OH | 92.8 | 92.5 | 0.55 | 0.08 |
| 97 | 120.5 | 2.0 | NH4OH | 116.0 | 115.6 | 1.13 | 0.10 |
| 98 | 112.6 | 0.5 | 2-aminoethanol | 112.2 | 112.1 | 0.10 | 0.03 |
| 99 | 127.0 | 1.0 | 2-aminoethanol | 124.8 | 124.4 | 0.55 | 0.10 |
| 100 | 118.2 (for chem. analysis) | ||||||
| 101 | 126.6 | 2.0 | 2-aminoethanol | 122.4 | 121.7 | 1.05 | 0.18 |
Table 5--Weights of limestone blocks immersed in selected concentrations of 2-aminoethanol Versene and ammonium Versene at room temperature and pH 8, at indicated time intervals.
| Sample block no. |
Original wt., g |
Concentration N |
Block weight after immersion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 hours | 8 hours | 16 hours | 24 hours | 48 hours | 72 hours | 168 hours | |||
| 2-aminoethanol Versene at Room Temperature | |||||||||
| 102 | 112.4 | 0.1 | 112.2 | 112.1 | 111.9 | 111.9 | 111.9 | 111.9 | 111.9 |
| 103 | 122.3 | 0.5 | 120.9 | 120.4 | 120.0 | 120.0 | 120.0 | 120.0 | 120.0 |
| 104 | 115.9 | 1.0 | 113.4 | 112.3 | 111.4 | 111.3 | 111.1 | 111.1 | 111.1 |
| 105 | 107.7 | 2.0 | 104.9 | 102.9 | 100.4 | 99.4 | 98.0 | 98.0 | 98.0 |
| 106 | 114.6 | 3.0 | 112.5 | 110.8 | 107.8 | 106.2 | 102.7 | 101.0 | 99.3 |
| Ammonium Versene | |||||||||
| 107 | 108.9 | 0.1 | 108.7 | 108.4 | 108.2 | 108.2 | 108.2 | 108.2 | 108.2 |
| 108 | 105.9 | 0.5 | 104.5 | 103.9 | 103.6 | 103.6 | 103.6 | 103.6 | 103.6 |
| 109 | 116.6 | 1.0 | 114.1 | 112.8 | 112.3 | 112.0 | 111.8 | 111.8 | 111.8 |
| 110 | 102.0 | 2.0 | 98.8 | 96.3 | 93.9 | 93.0 | 92.3 | 92.3 | 92.3 |
| 111 | 103.2 | 3.0 | 100.1 | 97.2 | 93.8 | 91.5 | 89.0 | 88.5 | 88.4 |
Sample blocks numbered 25, 50, 75 (Table 1), and 100 (Table 4) were designated as representative blocks. These blocks were crushed and quantitative analyses were run on them (Table 6), using the methods outlined by Hill, et al. (1961). Because of slight dissimilar chemical composition of the blocks, x-ray diffractometer patterns were run on each of the four samples. The samples were found to be predominantly calcite. Any accessory minerals present were below the detection limit of the instrument. Although minor variations did occur, the blocks were very similar chemically and no significant difference in solution rates of the blocks was anticipated.
Table 6--Results of quantitative chemical analyses of 4 arbitrarily selected limestone blocks.
| KSGS sample no. | Sample block no. | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | P2O5 | SO3 | K2O | Na2O | S | D.L.O.I. | Total | Calculated | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 105°-550°C | 550°-1000°C | CaCO3 | MgO3 | ||||||||||||||
| 62125 | 25 | 0.35 | 0.07 | 0.22 | 0.04 | 55.18 | 0.44 | 0.05 | Trace | 0.05 | Trace | Nil | 0.27 | 43.47 | 100.14 | 98.36 | 0.56 |
| 62150 | 50 | 0.47 | 0.21 | 0.41 | 0.03 | 55.08 | 0.53 | 0.02 | Trace | 0.05 | Trace | Nil | 0.40 | 43.22 | 100.42 | 98.22 | 0.10 |
| 62175 | 75 | 0.34 | 0.21 | 0.66 | 0.01 | 55.14 | 0.44 | Trace | Trace | 0.05 | Trace | Nil | 0.54 | 43.21 | 100.60 | 98.27 | Nil |
| 62200 | 100 | 0.27 | 0.03 | 0.36 | 0.03 | 55.13 | 0.48 | Trace | Trace | 0.06 | Trace | Nil | 0.56 | 43.32 | 100.24 | 98.39 | 0.11 |
After the Versene test solutions were prepared, a 200-ml aliquot from each solution was placed in a numbered 250-ml beaker. The blocks were immersed in the solutions and covered with a watchglass. These solutions were maintained at room temperature, 23°C ± 20, without provision for agitation. Agitation occurred only when the blocks were removed for drying and weighing and then reimmersed in their respective solutions. The samples were removed after immersion in the Versene solution for periods of 4, 8, 16, 24, 48, 72, and 168 hours in the same solution (Tables 2 and 5). At the end of each of these total immersion times the blocks were carefully removed and rinsed with distilled water. Possibly a small but negligible amount of material was washed from the blocks by this procedure. The blocks then were placed in a container of circulating distilled water for one hour and dried 1 1/2 hours in an oven at 110°C. After the blocks were removed from the oven, they were cooled to room temperature, weighed, and returned to their respective solutions for the next period of immersion (Tables 2, 5). At the conclusion of this test series, samples of each Versene solution were reserved in glass vials for qualitative spectrographic examination.
The calculated efficiency of 1.0 N Versene used for the solution of CaCO3 is 25.0 grams per liter or 5.0 grams per 200-ml aliquot. The calculated efficiencies of other concentrations are given in the right-hand column of Table 1. These values must be divided by 5 to arrive at the rated efficiency of the solutions for each beaker. The effects of heat (boiling) on the optimum Versene solutions are shown in Table 4.
A comparative series of solution-rate tests were run using hydrochloric and acetic acids at various dilutions (Table 3). Samples of these solutions were reserved for qualitative analysis, also.
The qualitative spectrographic analyses were run on an Applied Research Laboratory (ARL) 1.5 m emission spectrograph with a grating of 24,600 lines per inch. One-quarter-inch crater electrodes were treated with a water-proofing solution of 1 percent paraffin in benzene. The benzene was allowed to evaporate at room temperature. The crater was then loaded with 10 mg of spectrographically pure graphite powder and placed in an aluminum block holder. Each sample solution (0.2 ml) was carefully introduced into the electrode crater with a graduated micro-syringe. Subsequently, the aluminum holder containing the loaded electrodes was placed in an oven at 100°C and the samples evaporated to dryness.
The dry samples were ignited for 60 seconds in a direct-current arc at 12 amperes with a hemispheric-tipped-cone counter-electrode. The resulting exposed films (Eastman SA1) were developed 3 minutes in D-19, fixed, washed, and dried. The developed films were read on an ARL densitometer to determine the elements present.
Because the chelation of bi- and trivalent metals by Versene is a reaction based upon the ionization of a weak base, it is to be expected that the more concentrated solutions, being less highly ionized than some of the weaker solutions, would be less efficient within a given reaction period. The observed solution rates (CaCO3 in g/hr) of the various versenate solutions (Fig. 1, 2, 3) averaged at the end of 24- and 48-hour periods tend to verify this supposition. With the exception of the ammonium and 2-aminoethanol versenates, which were carried only to 3.0 N, the solution rates showed a general reversal with increasing concentrations and higher pH value.
Figure 1--Bar graphs showing solution rates of CaCO3 averaged at the end of 24- and 48-hour periods in sodium and potassium versenate solutions at room temperature, using various concentrations and pH values (Stippled 0-24 hr., Black 0-48 hr.).
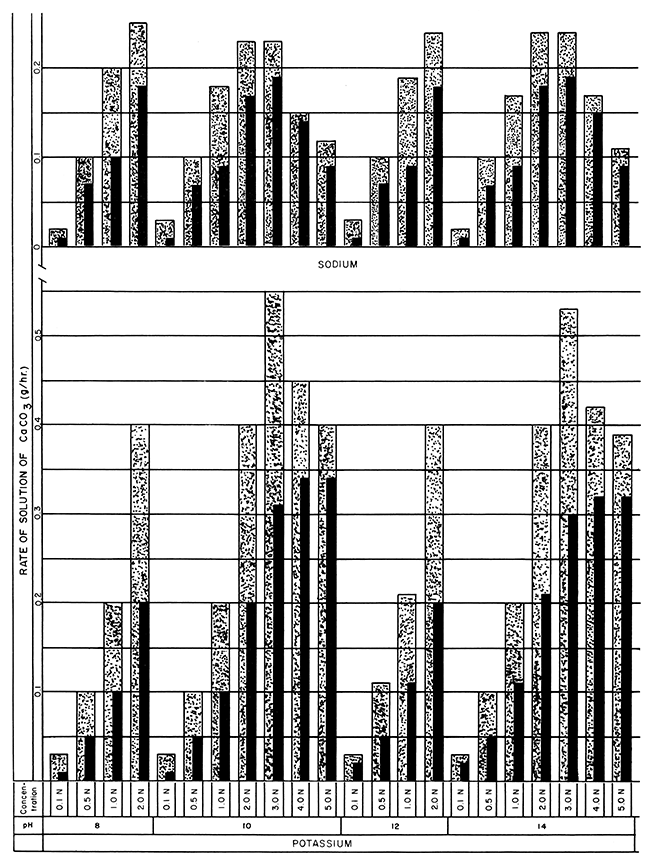
Figure 2--Bar graphs showing solution rates of CaCO3 averaged at the end of 24- and 48-hour periods in lithium versenate solutions at room temperature, using various concentrations and pH values (Stippled 0-24 hr., Black 0-48 hr.).
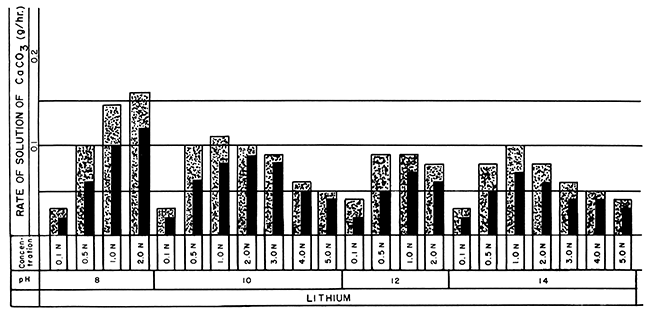
Figure 3--Bar graphs showing solution rates of CaCO3 averaged at the end of 24- and 48-hour periods of 2-aminoethanol; ammonium; and bilithium, bisodium, and bipotassium 2-aminoethanol versenate solutions at room temperature, using various concentrations and pH values (Stippled 0-24 hr., Black 0-48 hr.).
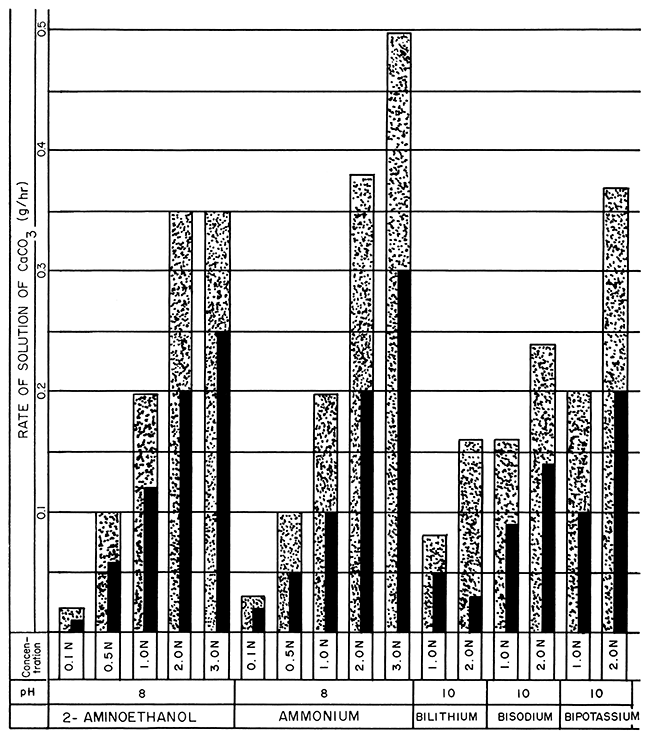
The optimum concentration values of the different Versene solutions appear on the bar graphs in Figures 1, 2, and 3. The optimum concentrations are not those showing the highest rate of solution but are the first and second concentrations preceding the maxima. These concentrations more closely approximate their rated efficiencies to dissolve calcite (Table 1) and an; suggested for gross economic applications. For a 24-hour solution period 1.0 N or 2.0 N Versene solutions (except for lithium) at room temperature are optimum. The optimum concentration of lithium versenate is 0.5 N. Although many of the 3.0 N solutions showed a higher rate of solution, that rate was not proportionately higher than that of the weaker solutions. Immersion periods of 24 hours in the weaker solutions, with the spent solution drawn off and fresh reagent added, resulted in solution of more calcite in any given period of time than could be achieved by using a single treatment with a more concentrated reagent.
For a 48-hour period of solution, the optimum concentration for lithium versenate (Fig. 2) increased only slightly from that of the 24-hour period. The optimum concentration of sodium versenate increased to 2.0 N; potassium to 3.0 N; ammonium to 3.0 N; and 2-aminoethanol to 2.0 N. A faster rate of solution may be achieved by reapplication of weaker solutions of Versenes at 24-hour intervals than by allowing continuous submergence in the more highly concentrated solutions for 48 hours.
Two Versenes not commercially available gave the highest rates of solution. The potassium- and ammonium-substituted Versenes, reacting with the limestone blocks at room temperature, removed 0.40 grams and 0.38 grams respectively per hour for the first 24 hours in 2.0 N solutions at pH 8 (Fig. 1, 3). For these two versenates, the 3.0 N concentration exhibited the highest rate of solution, potassium at 0.53 g/hr at pH 10, and ammonium at 0.49 g/hr at pH 8, but the solutions were not completely spent in 24 hours. Tests with the 2.0 N, 2-aminoethanol-substituted Versenes under the same conditions dissolved 0.35 g/hr (Fig. 3). The sodium versenate (2.0 N at pH 8) dissolved 0.25 g/hr, and the lithium versenate (2.0 N at pH 8), with the slowest reaction rate in the series, dissolved 0.16 g/hr.
The effect of pH on the rate of solution was minimal; lithium versenate solutions exhibited a slightly higher solution rate at pH 8 than those at higher pH values, but the other alkali metal Versenes exhibited almost identical solution rates for any given concentration at any of the pH values used. Ammonium and 2-aminoethanol Versenes were tested only at pH 8.
Solution of calcite with Versene at pH 8 is recommended for economy and safety. Use of solutions of pH 8 is much safer than those of higher pH values, lessening the likelihood of caustic burns and the ruin of equipment in the event of spillage. The cost of additional reagent to attain a higher pH is unwarranted in view of the extremely small increase in solution rate.
The weight loss of the block samples from the 200-ml aliquots of the selected optimum concentrations of the different Versenes with the addition of heat are shown in Table 4. The three alkali-substituted Versenes showed a 5-fold increase in solution rate during a 4-hour period compared with the 24-hour rate at room temperature. The ammonium and 2-aminoethanol Versenes showed only a 2- to 3-fold increase during the same period of time, because of evolution of the base from the solution during boiling.
Apparatus such as large hot plates or steam baths could be easily and inexpensively constructed to handle large quantities of samples, and the increased rate of solution induced by heat would more than justify the cost of this equipment and any additional handling required by the operator.
The Versene solutions incorporating mixed bases (Table 2 and Fig. 3) exhibited approximately the same properties and rates as the corresponding concentrations of alkali Versenes. At present no significant advantages are derived from using Versenes prepared from mixed bases.
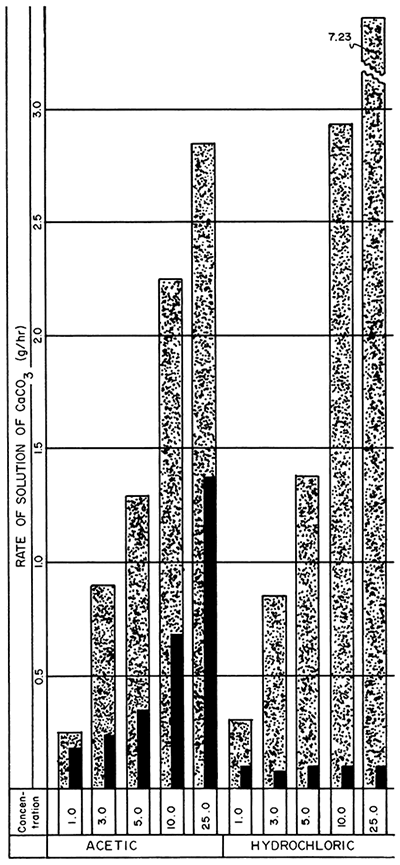
The loss in weight of the limestone blocks in various concentrations of hydrochloric and acetic acids at room temperature are given in Table 3, and the rates of solution for a 4-hour period and from 4-8 hours are shown in Figure 5. At room temperature for a 24-hour period, the selected optimum concentrations of sodium, potassium, ammonium, and 2-aminoethanol versenate solutions exhibited about the same solution rates that normally would be expected from a 1.0 to 2.0 percent hydrochloric or acetic acid solution in 8 hours.
The accelerated rates of solution exhibited by the heated Versene solutions (Table 4 and Fig. 4) compare favorably with the rate for acid at room temperature. Lithium versenate (2.0 N), at boiling, shows a slightly lower rate of solution than does 5.0 percent acetic or hydrochloric acid at room temperature. The 2.0 N sodium versenate rate at boiling is greater than the 5 percent acids but not as great as the 10 percent acids. The 2.0 N potassium versenate at boiling shows the same solution rate as 10 percent acetic at room temperature and approximately the same rate as 7.5 percent hydrochloric acid at room temperature. The ammonium and 2-aminoethanol Versenes did not maintain highly efficient solution rates at boiling. They would compare with 3.0 to 5.0 percent acids at room temperature.
Figure 4--Bar graphs showing average rates of solution at the end of 4- and 4-8-hour periods using optimum concentrations of selected versenate solutions, at pH 8, and boiling (Stippled 0-4 hr., Black 4-8 hr.).
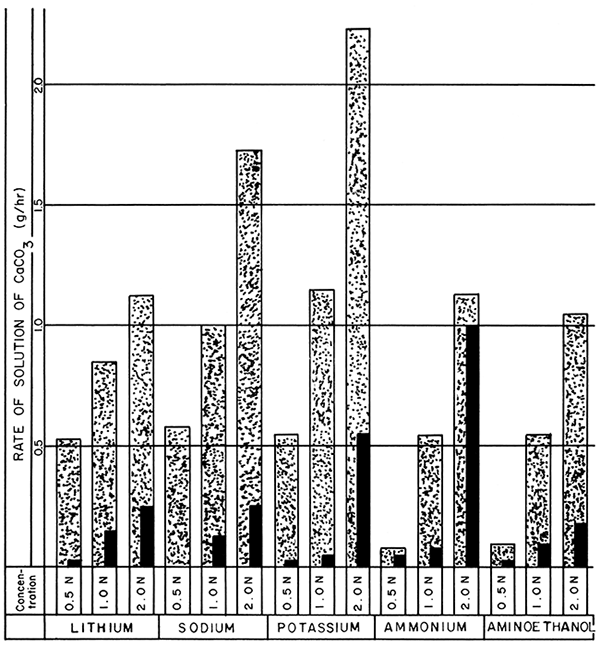
Figure 5--Bar graphs showing average rates of solution at the end of 4- and 4-8-hour periods, at room temperature, using acetic and hydrochloric acids (concentration is given in percent) (Stippled 0-4 hr., Black 4-8 hr.).
The removal of iron-containing minerals, fossils, or replicas from carbonate rocks pose special problems. Glover (1961) reported the extraction and recovery of fragile, glauconite-replaced or coated sponge spicules from the Wapanuka Limestone (Pennsylvanian) at an alkaline pH in sodium Versene solution. We have found that with increasing strength of solutions or with higher values of pH, gelatinous precipitates of ferric hydroxide and aluminum hydroxide often are formed. This reaction indicates that pH values lower than those used in this experiment probably would be better suited to the recovery of iron and aluminum minerals.
Heated or highly concentrated solutions of lithium versenate throw down a precipitate of lithium carbonate upon reaction with calcium carbonate or when allowed to absorb carbon dioxide from the air. The precipitation of lithium carbonate precludes the recovery of the insoluble fraction. The other Versene solutions used in the tests did not precipitate detectable primary or secondary by-products.
Qualitative spectrographic analyses were run at the conclusion of the test series on aliquots of the test solutions, to ascertain the effects of concentration, pH, and type of Versene upon metal ions present in the limestone as secondary and accessory minerals. These analyses show that all the Versene solutions contained major amounts of calcium and the alkali metals from the Versene, and minor amounts of the following elements: magnesium, strontium, manganese, sodium, silicon, aluminum, vanadium, titanium, and iron. The 0.1 N solutions did not dissolve manganese as readily as the higher concentrations. Copper and silver were taken into solution by hydrochloric and acetic acids, but these elements were not chelated by the Versene solutions. This indicates that although the solution effects of Versenes and acids seem comparable on bi- and trivalent elements, the Versenes do not affect univalent elements.
The main advantages of Versene solutions over acid solutions in geologic applications are: (1) Versenes do not cause formation of foam. (2) Solution of calcareous materials may be carried out in safety, without fume hoods, in large multiples, requiring little space. (3) When heated solutions are utilized, rates of solution compare favorably with rates of solution of weak acids. (4) Although use of Versene as a reagent is more costly than acid, most of the effective Versene solution can be reclaimed and reused, lowering the prorated cost per unit.
Ammonium Versene solution (a nonmetallic Versene), one of the least expensive to make, is also one of the most reactive of the five Versenes tested at room temperature. The simple titration method used in making up the ammonium Versene solution facilitates use by nonchemists.
The 2-aminoethanol-substituted Versene is another completely nonmetallic Versene which compared well in some of the solution rate tests, even surpassing the rates of sodium versenates at room temperature.
Potassium versenate solution rates greatly exceeded the rates for all other alkali metal Versenes. Because of the ease of dissolving and mixing a single powder in water to get a working solution, the commercially available sodium Versenes, although slower in reaction rate than potassium Versene, can be used in geological laboratories lacking chemical facilities.
The solution rate of lithium versenate was the slowest of the entire group. The precipitation of lithium carbonate will limit the use of lithium Versene to dilute solutions for special purposes or where the introduction of the lithium ion is desirable.
The effects of Versene solutions on clay minerals, on accessory minerals which normally occur in carbonate rocks, and on the relative rates of solution of carbonate rocks are under investigation. Possible economic application to the beneficiation of industrial minerals is being investigated, also. Use of substituted Versenes in the making of peels of various sedimentary rocks is underway. Current work on creating or improving porosity and permeability in carbonate rocks by preferentially chelating calcium is a project of high priority. Studies of fluid flow through porous media utilizing the sequestering characteristics of Versenes are being considered.
Glover (1961) recovered glauconite-replaced or coated sponge spicules from carbonate materials using a sodium Versene solution. Phosphatic fossils, including conodonts and orbiculoid brachiopods, have been successfully extracted from the Clarita member of the Chimney Hill Limestone (Silurian) of Oklahoma in a mass recovery technique utilizing substituted Versenes which has been developed at the Kansas Geological Survey.
Glover, E. D., 1961, Method of solution of calcareous materials using the complexing agent, EDTA: Jour. Sedimentary Petrology, v. 31, no. 4, p. 622-626.
Hill, W. E., Jr., and Runnels, R. T., 1960, Versene, a new tool for the study of carbonate rocks: Am. Assoc. Petroleum Geologists Bull., v. 44, no. 5, p. 632-633.
Hill, W. E., Jr., Waugh, W. N., Galle, O. K., and Runnels, R. T., 1961, Methods of chemical analysis for carbonate and silicate rocks: Kansas Geol. Survey Bull. 152, pt. 1, p. 1-30.
Martell, A. E., and Calvin, Melvin, 1952, Chemistry of the metal chelate compounds: Prentice-Hall, New York, 613 p.
Weissmann, R. C., and Diehl, H. C., 1954, A new method utilizing Versene for determination of the calcite-dolomite ratio in carbonate rocks: Iowa Acad. Science, Proc. 1953, v. 60, p. 433-437.
Welcher, F. T., 1958, The analytical uses of ethylencdiaminetetraacetic acid: D. Van Nostrand Company, Inc., New York, 366 p.
Kansas Geological Survey
Placed on web March 28, 2014; originally published in December 1963.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/165_7/index.html