Kansas Geological Survey, Open-file Report 2003-75
by
Margaret Townsend
Kansas Geological Survey
KGS Open File Report 2003-75
December 2003
Groundwater Associates, Inc. of Wichita, KS requested that personnel at the Kansas Geological Survey (KGS) assist to determine sources of high nitrate found in the municipal water at Horton, KS. The study initially involved running chemical and isotopic analyses on water collected in May 2002 and submitted by the company. The samples arrived without ice and the resulting isotopic results were considered suspicious. The KGS requested permission to pump the wells and collect samples for additional analysis. Personnel from Groundwater Associates Inc. and KGS went to the field site in Atchison County, KS, in January 2003 to collect samples.
The nitrate-N values were above 10 mg/L, which is the U.S. EPA drinking water limit. Chloride and sulfate values are generally low. Soil at the site is a Grundy silt clay with measurable organic carbon and nitrogen in the soil profile. The cores taken throughout the study area are generally gray clays and silts, with brown fine-grained sand interspersed. Limestone and shale occur at depth.
Results from the January sampling showed enriched δ15N values and depleted δ13C values. The nitrogen-15 values and nitrate-N concentrations are in the range typical for animal-waste sources. However, the study area is a dryland farm that rotates milo and soybeans. Manure was not used as a fertilizer. Cattle graze on the site in the winter but the numbers are not large and the cattle are rotated through fields near the site. It is possible that that animal waste from the cattle is the source, but the chloride concentration is much lower than has been observed at other study sites in Kansas. The δ13C values are in the range of native grasses and not row crops. This suggests that nitrate is moving faster than carbon through the system and that carbon from the "modern" crops has not yet reached ground water.
Possible sources for the high nitrate-N include nitrification and/or volatilization enrichment, limited denitrification enrichment, or a possible source away from the site that is transported via ground water in buried valley sediments.
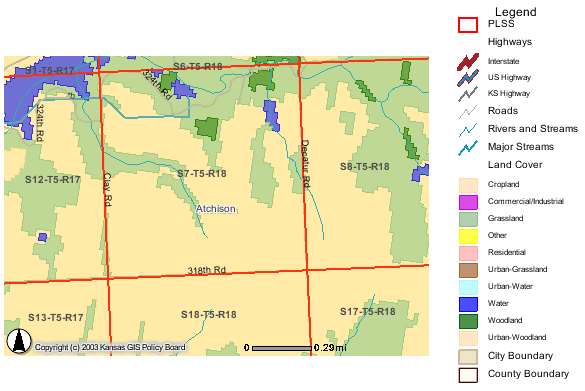
The study area is located in Atchison County in sec. 7, T. 5 S., R. 18 E. Many test holes and test wells were installed. For this study, only 10 wells were sampled at different times. Complete analyses were done on 5 of the wells. One water analysis is from a 1981 study by Denne et al. (1990). The present study was undertaken because of observed increasing nitrate-N concentrations in the city of Horton's water supply.
Land use in the study area is primarily cropland and grassland. The primary area of testing by Groundwater Associates was SW sec. 7 (figure 1). Average rainfall is 36 inches/year (NASS, 2003).
Figure 1. Land-use cover for the study area in sec. 7, T. 5 S., R. 18 E. Primary land use is cropland and grassland. Source of data is from KanView ARCIMS map (DASC, 2003A).

Soils at the study site are classified as Grundy--silt and clay soil with measurable carbon throughout the profile (USDA, NRCS, 2003). The area is dryland farmed with only fertilizer application; no manure is used. The main crops are milo (60-100 lb N/acre) or soybeans (7-10 lb/acre). Total nitrogen concentration of 0.139% to 0.092% was measured in a soil pit in Grundy soil in Brown County. Organic carbon in the soil ranged from 1.62 to 0.29% with depth. A small herd of cattle graze on the stalks remaining after harvest. However, the animals are rotated to other fields and are not confined to the study area for long periods of time.
The geology in the area consists of unconsolidated deposits of a basal limestone and chert gravel, a fine-grained silty sand of the Atchison formation, at least two different tills (with a locally occurring layer of outwash between them), the Nortonville clay, loess, terrace deposits, and alluvium (Ward, 1973, Denne et al., 1998). The total thickness of sand and gravel in the study area is greater than 25 ft thick. Work by Denne et al. (1990) and Ward (1973) indicate that a major buried valley occurs in the center of the county. Work by Dreeszen and Burchett (1971) indicates that preglacial drainageways existed in this portion of the county.
Drillers' logs from the site show layers of gray and brown silt, clay and fine sands, which suggest reducing conditions at some time in the past. At depth, several of the wells showed limestone and gray or green shales. The presence of gray or green shales suggests reducing conditions.
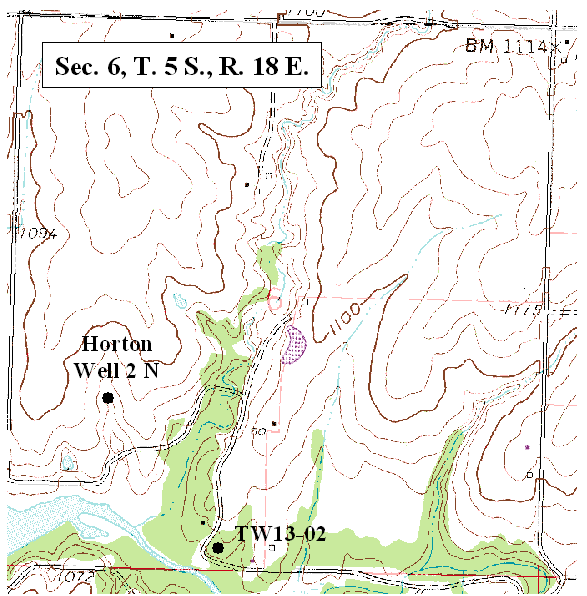
Ground water occurs at depths of 20 to <100 ft in the study area. Depth to water decreases as a neighboring stream is approached. Location of sampled wells and test holes in sec. 7 are shown in figure 2. Location of wells sampled in section 6 are shown in figure 3.
Figure 2. Location of test holes and wells sampled in this study (DASC, 2003B).
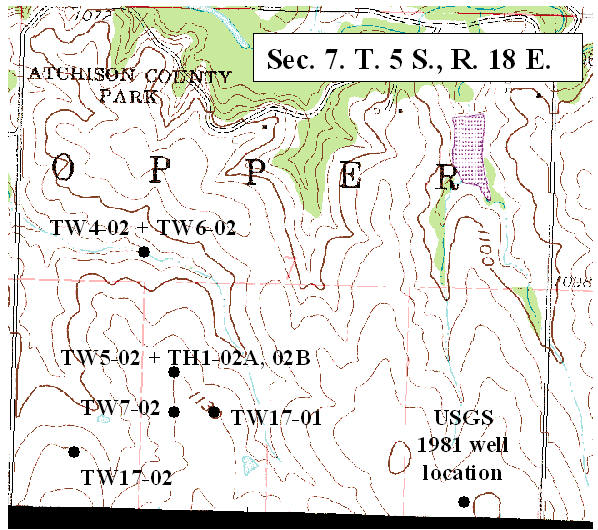
Figure 3. Location of test well and city well sampled in this study (DASC, 2003B).
Development of wells by Groundwater Associates consisted of air purging of the wells. Sampling of wells was done using a submersible pump. Field tests of nitrate indicated very low values for several of the wells. Analyses of water from the same wells by Servi Tech labs in Dodge City, KS, and by Kansas Department of Health and Environment in Topeka, KS as well as by the Kansas Geological Survey in Lawrence, KS, showed much higher concentrations for the same wells (Appendix A). It is possible that the field-kit measurements were in error.
The Kansas Geological Survey (KGS) collected samples in January 2003 using a submersible pump. Samples were collected after the specific conductance and temperature of the samples stabilized, usually about 15 to 20 minutes after pumping began. Samples for nitrate analyses were collected in 250-ml polyethylene bottles marked to a 200-ml level with 2-ml HCL as a preservative. Samples for other constituents were collected in 500-ml bottles. Samples for isotope analyses were collected in 80-ml polyethylene bottles. All samples were stored on ice until return to the KGS where the bottles were refrigerated until analyzed. Standard methods of analysis were used for all major cations and anions (Hathaway et al., 1981).
The isotope samples were placed in a freezer until sent for analysis within two weeks of collection. Samples for carbon-13 and nitrogen-15 were sent to Dr. Stephen Macko at the University of Virginia Department of Environmental Sciences for analysis.
Natural-abundance nitrogen-isotope analysis is a frequently used method to assist in determining sources of nitrogen to ground water. The isotope analysis involves establishing the ratio of nitrogen-15 (15N) to nitrogen-14 (14N) on the nitrogen in nitrate compared to the ratio observed in the standard, atmospheric nitrogen (air). Comparisons of these values indicate if there is more (positive) or less (negative) 15N in the sample. The values thus indicate whether the sample is enriched (+) or depleted (-) in relation to the standard.
Isotopic values are reported as δ15N in per mil (‰) (Hoefs, 2001):
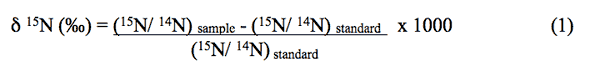
Figure 4 illustrates the range of δ15N values for various sources of nitrogen and associated processes affecting the 15N abundance (Heaton, 1986). Generally, biological activities use 14N preferentially, resulting in an increased δ15N value in the remaining nitrogen. Previous work has shown that nitrate from commercial fertilizer sources has δ15N values of -2 to +8‰, from soil nitrogen a range of +5 to +7‰, and from animal waste generally greater than +10‰ (Heaton, 1986, Herbel and Spalding 1993). Other information such as dissolved oxygen, salinity, iron, and manganese concentrations, and proximity to potential sources, aid in source determination.
Figure 4. Range of δ15N signatures for different sources of nitrogen (Heaton, 1986).
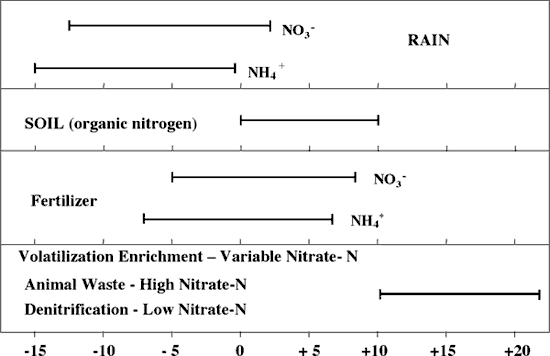
Sources such as fertilizer and legumes have very light δ15N signatures (-2 to +2 ‰) (figure 4). Ground water impacted by fertilizer frequently has measurable nitrate as well (generally greater than 3 mg/L in Kansas). Because of possible volatilization of anhydrous ammonia by bacteria, the δ15N values frequently are in the +2 to +8‰. Legume sources generally have low nitrate concentrations because the nitrogen is often tied up in an organic form, and the plant nodules need to degrade before the nitrogen is released.
Other sources such as human-septic waste or animal waste have starting δ15N values around +5‰. Because animal waste has a high ammonia component, the release of the ammonia when the waste is produced causes an immediate enrichment of the δ15N because the δ14N in the waste is released by volatilization. As a result, the δ15N of the remaining nitrogen is much higher, in the range of +10‰ or more. Generally with animal-waste sources, nitrate-N values are high (usually more than 10 mg/L) as well.
Volatilization also can occur in soils and rocks with carbonate zones. Carbonates can increase the pH of water towards 8.5, which means the water is more basic. In this range nitrate can be converted to ammonia gas. The lighter 14N isotope is released with the gas. The remaining nitrogen becomes enriched with δ15N of a higher value.
Another process that can result in an enriched δ15N value is denitrification. In this process, bacteria degrade nitrate to nitrogen gases that are released to the atmosphere. The 14N of the nitrate is utilized first resulting in an enriched δ15N in the remaining nitrate. Signs of possible denitrification are low nitrate values and enriched δ15N values. Table 1 lists the range of (15N values and the types of sources usually identifiable with the method.
Table 1. Range of Nitrogen-15 and Nitrogen Sources
| δ15N Values | Nitrogen Sources |
|---|---|
| δ15N < 8‰ | Fertilizer (Nitrate-N usually > 2 mg/L) |
| δ15N 8 to 10‰ | Mixed Sources (Variable range of nitrate-N) |
| δ15N > 10‰ | Animal waste (Nitrate-N > 10 mg/L) or Denitrification (Nitrate-N < 1 mg/L) |
The ranges of nitrate-N observed in other areas in Kansas are similar to what is observed at this site (Appendix A). However, most of the δ15N values from this study are much more enriched than the author has observed at other site studies. The δ15N values suggest the possibility of animal waste except that there is no known observable animal waste source in the area. The soils and subsurface geology may result in denitrification enrichment during time of travel from the surface downward. Volatilization enrichment is possible because of interaction with limestone fragments or calcareous zones throughout the unsaturated zone. However, the level of enrichment is much higher than observed at other sites (Oberlin, KS, for instance; Townsend, 2001).
The samples with measurable nitrate-N of greater than 3 mg/L should have recorded large to very large peaks in the nitrogen-15 analyses. Previous work with Dr. Macko (isotope chemist at University of Virginia) indicates that generally there is an excellent correlation between peak height (as a representation of concentration) and the reported nitrogen concentrations. The fact that this correlation did not hold this time (all peak heights were very small even for samples with measurable nitrogen) suggests that there was deterioration in the sample or else an error in the analyses for the nitrogen. The samples received from Groundwater Associates personnel in May 2002, arrived with the ice melted and took several days to arrive at the Kansas Geological Survey. There may have been a transit problem and thus the possibility of temperature changes and sample degradation by bacteria. The samples were run for complete analyses with very little difference in the calculated chemical balance. Other than the isotopic values for the samples, the inorganic parameters seemed to be little affected by the temperature and possible bacterial impacts on the sample.
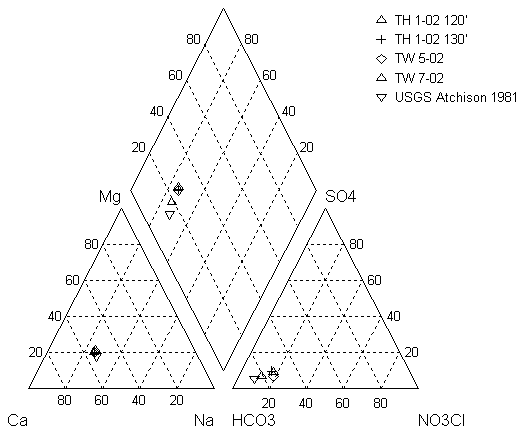
In January 2003, personnel from KGS and Ground Water Associates, Inc. went to the area near Horton, KS, that was being tested for a possible water source for the city of Horton. The samples were collected for complete analyses for wells that had not been tested previously and for nitrogen-15 and carbon-13 analyses. There are complete analyses for May 2002 and partial analyses and one complete analysis for January 2003 (Appendix A). Figure 5 shows a plot of water samples from this study and from the 1981 work done by Denne et al. (1990) on a trilinear diagram. The trilinear diagram permits plotting of complete water-chemistry analyses to determine the water type and to observe possible variation between water samples. The samples fall in the calcium-bicarbonate water type. Although there is a slight increase in nitrate from 1981 to the 2002-03 samples the samples, all have similar values (Appendix A).
Figure 5. Trilinear diagram of water analyses from study area near Horton, KS. Sample collected from 1981 study by Denne et al. (1990) is shown for reference. The NO3CL is a calculated value of nitrate + chloride so that nitrate values, which are a significant portion of the anion concentration of the samples, could be included on the plot.
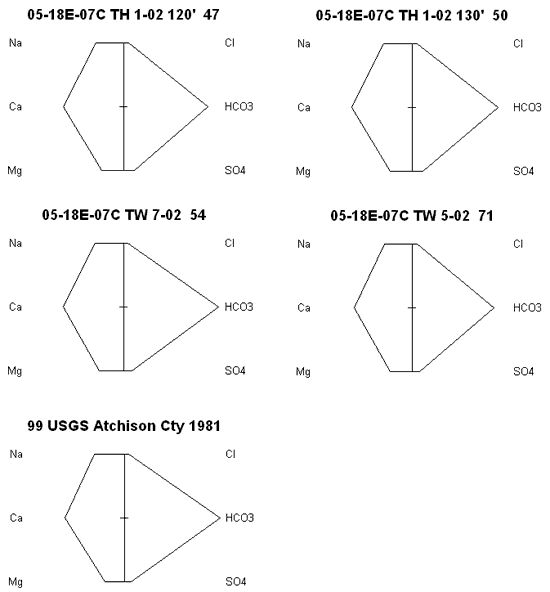
Stiff diagrams were used to look at the water chemistry from each of four wells plus a water sample collected by Denne et al. (1990) and analyzed by the USGS (figure 6). The similarity of the analyses as represented by the graphs indicates that variation in water chemistry from the section is low. In addition, the similarity of the samples to the 1981 USGS sample indicates that there has been little change in water chemistry over time. Chloride concentrations are low which suggests that the cattle that graze on the site have not impacted the ground water quality. Other studies on feed lots indicate a large chloride concentration because of the limited space available for the animals to move around and the use of salt licks for the animals (Maule and Fonstad, 2000).
Figure 6. Stiff diagrams of five wells sampled in sec 7., T. 5 S., R. 18 E. Chemistry of the samples is very similar suggesting that variation within the section is low. The water is predominately a calcium-bicarbonate type.
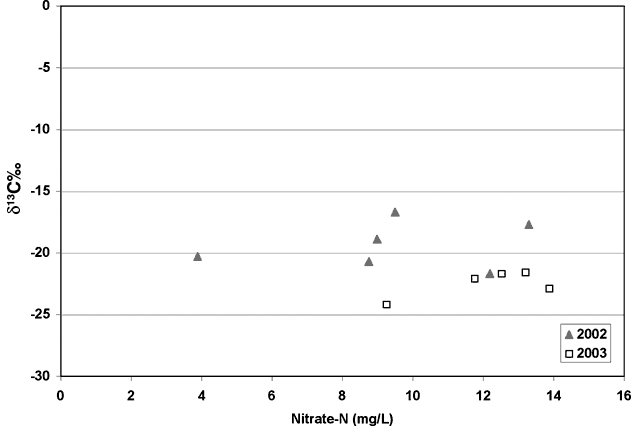
Carbon-13 isotope analyses were done to try to determine if the carbon present in the system was from native grasses (C3 type plants) or modern row crops such as milo or sorghum. The results from the analyses showed quite a range of values. The samples collected in 2002 were the samples that arrived without ice. These samples show enriched δ13C values (-16.7 to -21‰), which suggest a row crop source (figure 7; diamonds). However, when samples were collected in January 2003, the resulting values were more depleted suggesting a native-grass source of carbon (figure 7; squares). The fact that the isotope values were more enriched in the May collection suggests that some bacterial enrichment had occurred.
Figure 7. Nitrate-N values versus δ13C graph show possible effects of bacterial processes causing enrichment of carbon-13 values in the 2002 samples. The 2002 samples are more enriched (-16.7 to -21‰) than the 2003 samples, which are more depleted (-21 to -24‰).
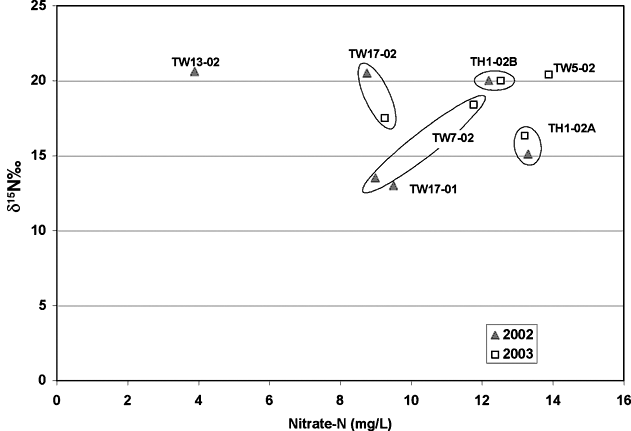
The nitrogen-15 values for the two sampling periods also indicate some possible enrichment although the amount of enrichment is not as noticeable as with the carbon-13 results. Figure 8 shows the nitrate-N versus nitrogen-15 values for the samples. For pairs of samples, the nitrogen-15 signature varies. Although the signature values are somewhat different for the two sampling periods, the δ15N values are above 10 for all samples. The presence of iron and manganese for some of the samples (Appendix A) suggests that reducing water chemistry may have been present in the system at one time.
Figure 8. Nitrate-N versus nitrogen-15 values for samples collected during this study. Values fall in the animal waste zone for nitrogen-15 values (table 1). However, there is no record of manure use as fertilizer in the immediate study area.
The soils and sediments of the vadose zone are of glacial origin with a predominance of gray and black clays in the profiles. Work by Denne et al. (1986) in Nemaha County, KS, showed that reducing water chemistry frequently occurred in sediments that had gray, tan, or black colors that are indicative of reducing chemistry. In addition, presence of measurable carbon in the vadose zone provides an energy source for bacteria.
The drilling logs from the test holes and wells at the site indicate the presence of gray clay and tan silts and clays as well as black and gray shales, indicating that reducing zones have occurred in the sediments.
The site is dryland farmed. The main source of recharge is from rainfall. The soil chemistry for the Grundy soil indicates measurable carbon and nitrogen in the soil profile (USDA, NRCS, 2003). In addition the shallow soil profile has a more acidic pH (5.5 to 6.8) than the deeper profile (7.0 to 7.4). The change in pH may have resulted in possible volatilization enrichment of fertilizer moving downward through the profile. In addition, nitrification enrichment of fertilizer would result in fractionation enrichment of the formed nitrate.
Considering that the site has been farmed for many years, it is likely that some of the fertilizer nitrogen has moved into the vadose zone and to the water table. Because dryland areas are subject to storage of nitrogen in dry periods, pulses of higher nitrate concentration may be moving through the soil profile. The travel time through relatively dry soil will be great. The presence of carbon-13 in the range of native grasses (-20 to -24‰) suggests that movement of the nitrate is faster than the carbon in the system. Slow movement through the profile with bacteria and carbon present in the soil and aquifer (Denne et al., 1998) could permit enrichment of the nitrogen-15 by denitrification.
Another possibility is that water is moving through a buried valley from outside the area. Work by Denne et al. (1998) indicates that tributaries to the major buried system exist in the study area. Mapping of these tributaries through a drilling program would help to verify possible outside sources of nitrogen entering the area.
The water quality of the area shows a calcium-bicarbonate water type with some iron and manganese present. Chloride and sulfate concentrations are low. Nitrate-N values are variable in the study area. Values range from 3 to greater than 10 mg/L. All of the nitrate-N values are above the USGS pristine background level of 2 mg/L suggesting that an anthropogenic source is affecting the water quality. The area is dryland farmed. The average rainfall in the area is 36 inches/year (NASS, 2003). The area also is subject to drought conditions and excess rainfall. Work by USGS in southwest Kansas indicated storage of up to 2,000 mg/L of nitrate in the upper foot of soil at the Cimarron National Grasslands site (McMahon et al., 2003). It is likely that evapoconcentration of nitrate as well as other salts occurs in the Atchison County study area. It is possible that pulse movement of enriched nitrate moves through the profile to the ground water.
Nitrification or volatilization may be a factor in causing the enriched δ15N values observed at this site. In addition, the soil and vadose zone contain enough organic carbon to provide an energy source for bacterial denitrification of nitrate resulting in enriched δ15N values. An animal waste source upgradient that has impacted the glacial buried valley system in the area also is a possibility.
The use of nitrogen-15 natural abundance method did not resolve the defining of the source of nitrate in the study area. Recommendations for future research include collection of cores to do sequential nitrogen-15 analyses with depth to the ground water. Sampling of available domestic and municipal wells in the area might provide additional information as to possible sources for the enriched nitrogen-15 in the area. Sampling of streams and lakes in the area also might provide additional information of what might be occurring in the area. Most importantly, coring and installation of additional wells throughout the area with a long-term sampling period might provide insight into possible seasonal sources that occur in the area.
Data Access and Support Center (DASC), 2003A, KanView map program, http://www.kansasgis.org/ (verified December 2003).
Data Access and Support Center (DASC), 2003B, Kansas Geodatabases, Digital Raster Graphics (DRG) UTM NAD 83 Clipped: http://www.kansasgis.org/ (verified December 2003).
Denne, J. E., Randtke, S. J., and Hathaway, L. R., 1986, Geological and geochemical factors influencing water quality in a buried valley in northeastern Kansas: Kansas Water Resources Research Institute Contribution 253, 53 p.
Denne, J. E., Miller, R. E., Hathaway, L. R., and O'Connor, H.G., 1990, Hydrogeology and geochemistry of glacial deposits in northeastern Kansas: water-quality data: Kansas Geological Survey Open-file Report 90-23b.
Denne, J. E., Miller, R. E., Hathaway, L. R., O'Connor, H. G., and Johnson, W. C., 1998, Hydrogeology and geochemistry of glacial deposits in northeastern Kansas: Kansas Geological Survey Bulletin 229, 127 p. [available online]
Dreeszen, V. H., and Burchett, R. R., 1971, Buried valleys in the lower part of the Missouri River basin; in, Pleistocene Stratigraphy of Missouri River Valley along the Kansas-Missouri Border, Guidebook, 20th Annual Meeting of the Midwest Friends of the Pleistocene: Kansas Geological Survey, Special Distribution Publication 53, p. 21-25.
Hathaway, L. R., Waugh, T. C., Galle, O. K., and Dickey, H. P., Chemical quality of irrigation waters in the Equus Beds area, south-central Kansas: Kansas Geological Survey Chemical Quality Series 10, 45 p.
Heaton, T. H. E., 1986, Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review: Chemical Geology, v. 59, p.87-102.
Herbel, M. J., and Spalding, R. F., 1993, Vadose zone fertilizer-derived nitrate and δ15N extracts: Ground Water, v. 31, no. 3, p. 376-382.
Hoefs, J., 2001, Stable Isotope Geochemistry, 4th ed. Springer, 201 p.
Maule, C. P., and Fonstad, T. A., 2000, Impacts of cattle penning on groundwater quality beneath feedlots: Canadian Agricultural Engineering, v. 42, no. 2, p. 87-93.
McMahon, P. B., Dennehy, K. F., Michel, R. L., Sophocleous, M. A., Ellett, K. M., and Hurlbut, D. B., 2003, Water movement through thick unsaturated zones overlying the central High Plains aquifer, southwestern Kansas, 2000-2001: U. S. Geological Survey, Water-Resources Investigations Report 03-4171, 39 p., http://webserver.cr.usgs.gov/nawqa/hpgw/reports/wrir03_4171.pdf (Verified December 2003).
National Agricultural Statistics Service, 2003, 1998 Kansas County Profiles, http://www.nass.usda.gov/ks/coflyer/1998/005.htm (Verified December 2003).
Townsend, M. A., 2001, Use of natural abundance nitrogen-15 isotope method to identify sources of nitrate in ground water near Oberlin, Kansas: Kansas Geological Survey, Open-file Report 2001-50, 22 p. [available online]
U.S. Department of Agriculture, Natural Resources Conservation Services, 2003, NSSC Soil Survey Laboratory Research Database, http://ssldata.nrcs.usda.gov/ (verified December 2003).
Ward, J. R., 1973, Geohydrology of Atchison County, northeastern Kansas: U.S. Geological Survey, Hydrologic Investigations, Atlas HA-467, scale 1:62,500.
Appendix A. Water-chemistry analyses for wells and test holes near Horton, KS.
| Sample ID |
Date | Location | Specific Conductance (μmhos/cm) |
pH | SiO2 ppm |
Ca ppm |
Mg ppm |
Na ppm |
K ppm |
Sr ppm |
Total Hardness ppm |
HCO3 ppm |
SO4 ppm |
Cl ppm |
F ppm |
NO3 ppm |
NO3-N ppm | B ppm |
Fe ppb |
MN ppb |
δ13C (per mil) |
δ15N (per mil) |
Depth (feet) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TH1-02A | 1/15/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 640 | 75 | 19 | 265.7 | 12.63 | 11 | 0.4 | <0.1 | 0.09 | 0.15 | 120 | ||||||||||
| TH1-02A | 5/31/2002 | Sec. 7 C., T. 5 S.,R.,18 E. | 682 | 7.55 | 35.5 | 76.2 | 17.1 | 40.0 | 1.1 | 0.54 | 323 | 32.0 | 10.8 | 0.40 | 58.9 | 13.3 | 45 | -17.7 | 15.1 | 120 | |||
| TH1-02A | 1/15/2003 | Sec. 7 C., T. 5 S.,R.,18 E. | 677 | 35.2 | 10.4 | 58.5 | 13.2 | -21.6 | 16.33 | 120 | |||||||||||||
| TH1-02B | 1/15/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 790 | 80 | 21 | 286.4 | 26.95 | 52 | 1.8 | 0.4 | 0.35 | 0.18 | 130 | ||||||||||
| TH1-02B | 5/31/2002 | Sec. 7 C., T. 5 S.,R.,18 E. | 687 | 7.65 | 33.9 | 75.8 | 17.0 | 41.1 | 1.2 | 0.60 | 329 | 33.0 | 11.4 | 0.41 | 54.0 | 12.2 | 59 | -17.8 | 12.1 | 130 | |||
| TH1-02B | 1/15/2003 | Sec. 7 C., T. 5 S.,R.,18 E. | 679 | 37.2 | 10.9 | 55.5 | 12.5 | -21.7 | 20 | 130 | |||||||||||||
| TW7-02 | 1/31/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 680 | 257.5 | 36.00 | 19 | 57.1 | 12.9 | 0.02 | 0.02 | 124 | ||||||||||||
| TW7-02 | 4/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 257 | 14 | 57.0 | 12.86 | 0.051 | 0.019 | 124 | ||||||||||||||
| TW7-02 | 5/31/2002 | Sec. 7 C., T. 5 S.,R.,18 E. | 680 | 7.45 | 34.8 | 75.5 | 18.5 | 41.6 | 1.4 | 0.60 | 364 | 24.8 | 8.8 | 0.39 | 39.8 | 9.0 | <27 | -18.9 | 13.5 | 124 | |||
| TW7-02 | 1/15/2003 | Sec. 7 C., T. 5 S.,R.,18 E. | 680 | 27.3 | 10.3 | 52.1 | 11.8 | -22.1 | 18.4 | 124 | |||||||||||||
| TW17-01 | 1/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 620 | 254.1 | 19.91 | 7 | 42.1 | 9.5 | 0.03 | 0.02 | -16.7 | 13.0 | 140 | ||||||||||
| TW17-01 | 4/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 242 | 7 | 47.7 | 10.76 | 0.058 | 0.014 | 140 | ||||||||||||||
| TW17-01 | 5/1/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 0.03 | 0.02 | 140 | ||||||||||||||||||
| TH4-02 | 1/16/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 620 | 72 | 18 | 254.1 | 13.84 | 9 | 13.7 | 3.1 | 0.04 | 0.004 | 90 | ||||||||||
| Horton Well 2 N | 1/15/02 | Sec. 6 C., T. 5 S.,R.,18 E. | 560 | 73 | 14 | 240.1 | 13.84 | 6 | 25.7 | 5.8 | <0.005 | <0.001 | |||||||||||
| Horton Well 2 N | 4/17/02 | Sec. 6 C., T. 5 S.,R.,18 E. | 240 | 6 | 25.7 | 5.8 | <0.005 | <0.001 | |||||||||||||||
| TW13-02 | 4/17/02 | Sec. 6 C., T. 5 S.,R.,18 E. | 231 | 3.00 | 3.88 | 0.01 | 0.001 | 65 | |||||||||||||||
| TW13-02 | 5/22/02 | Sec. 6 C., T. 5 S.,R.,18 E. | 3.89 | -20.3 | 20.6 | 65 | |||||||||||||||||
| TW17-02 | 4/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 239 | 12.00 | 32.3 | 7.3 | 0.01 | 0.002 | 110 | ||||||||||||||
| TW 17-02 | 1/15/2003 | Sec. 7 C., T. 5 S.,R.,18 E. | 642 | 39.6 | 7.1 | 41.0 | 9.3 | -24.2 | 17.5 | 110 | |||||||||||||
| TW 17-02 | 5/22/2002 | Sec. 7 C., T. 5 S.,R.,18 E. | 38.8 | 8.8 | -20.7 | 20.5 | 110 | ||||||||||||||||
| TW6-02 | 1/30/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 630 | 71 | 18 | 251.6 | 30.00 | 11 | 24.4 | 5.5 | 0.01 | 0.004 | 85 | ||||||||||
| TW6-02 | 4/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 249 | 5 | 14.8 | 3.35 | 0.029 | 0.007 | 85 | ||||||||||||||
| TW5-02 | 1/30/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 680 | 74 | 18 | 259.1 | 39 | 17 | 48.3 | 10.9 | 0.066 | 0.116 | 140 | ||||||||||
| TW5-02 | 4/17/02 | Sec. 7 C., T. 5 S.,R.,18 E. | 253 | 11 | 43.9 | 9.91 | 0.066 | 0.116 | 140 | ||||||||||||||
| TW 5-02 | 1/15/2003 | Sec. 7 C., T. 5 S.,R.,18 E. | 654 | 7.60 | 36.5 | 73.3 | 16.6 | 39.8 | 0.7 | 0.49 | 314 | 22.3 | 10.7 | 0.42 | 61.5 | 13.9 | 30 | -22.9 | 20.4 | 140 | |||
| USGS 1981 | 3/18/1981 | Sec 7 DCD 01 T. 5 S.,R.,18 E. | 592 | 7.3 | 44 | 1.1 | 15 | 75 | 366 | 19 | 9 | 0.3 | 23 | 1.1 | 0.03 | 0.01 | 120 |
Kansas Geological Survey, Geohydrology
Placed online Jan. 12, 2006
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/2003/OFR03_75/index.html