Kansas Geological Survey, Open-file Report 1994-29
by
M. A. Townsend, S. A. Macko, D. P. Young, and R. O. Sleezer
KGS Open File Report 1994-29
Nitrogen-15 isotopes have been used as a tool for identifying sources of nitrate contamination in groundwater for almost 20 years. The method is used successfully in areas with thin, permeable vadose zones with a shallow ground water table and in rock units such as fractured limestone.
Kreitler (1975, 1979) showed that 15N signatures of animal wastes generally occurred in the range of +10 to +22 ‰. Work by Herbell and Spalding (1993) and Heaton (1986) indicates that highly enriched nitrogen-15 signatures (+8 to +42 ‰) can occur because of fractionation of the nitrogen by denitrifying bacteria. In addition, enriched nitrogen-15 can occur because of volatilization of ammonia to the atmosphere leaving enriched ammonium behind. As a result of multiple causes for enrichment, either a clearer understanding of processes involved in the fractionation needs to be resolved, or the application of 15N is at best qualitative at high enrichments.
Our work on Kansas water quality problems presented in this paper suggests that caution is needed when applying the technique for identification
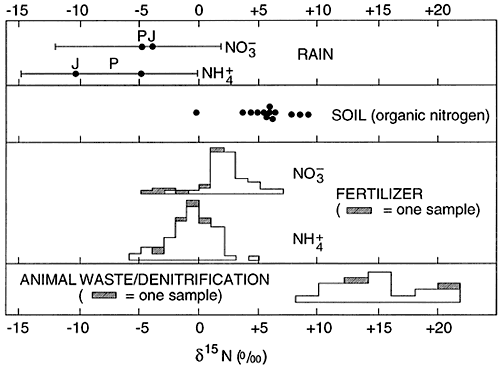
The major use of 15N isotopes in ground water studies is to determine a source for the nitrate concentration in the water. Figure 1 shows the ranges of 15N isotopes for a variety of source materials.
Figure 1--Range of 15N values. After Heaton, 1986.

The typical isotope range for fertilizer nitrogen is +2 to +6 ‰. The range for animal waste is +10 and above. Work by Heaton (1986) and Herbell and Spalding (1993) show that denitrification can also result in enriched 15N isotope values in the same range as animal waste. This variability of cause of isotope enrichment makes the use of 15N isotopes less reliable for source detection than previously thought.
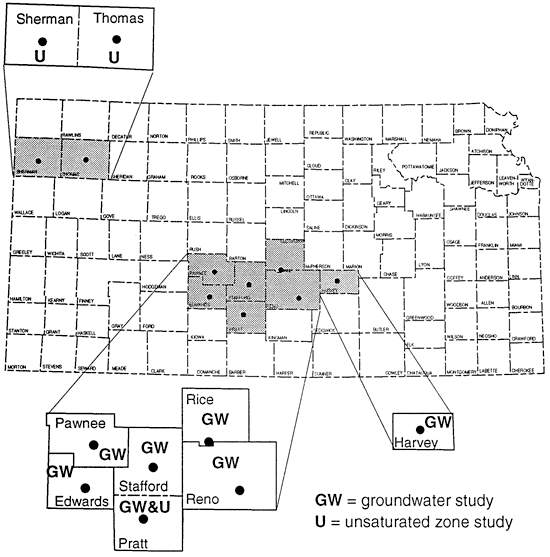
This paper presents three ground water studies and three unsaturated zone studies in Kansas (fig. 2) that attempted to use 15N for source detection with mixed results.
Figure 2--Location of study areas.
In south-central Kansas the stratigraphy consists of Quaternary alluvium overlying Cretaceous or Permian bedrock. Work throughout the area indicates that the major aquifer consists primarily of framework of sands and gravels with discontinuous clay zones throughout (fig. 3). Approximately 31 % of the area is covered by sandy loam soils.
Figure 3--Schematic stratigraphic cross section.
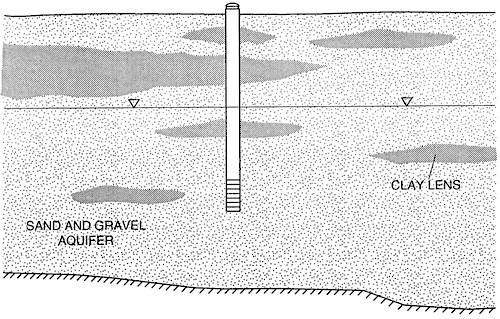
Figure 4 shows the location, depth, and nitrate-N concentrations of domestic and irrigation wells sampled in south-central Kansas. Much of the area is cultivated in irrigated corn. Anhydrous ammonia is the dominant fertilizer with an 15N signature of -2 to -1 ‰ (Gormly and Spalding, 1979).
Figure 4--Location of sampling points, south-central Kansas.
Because the sandy soils in the area have low organic matter content and moderate pH the loss of NH3 by volatilization, resulting in enrichment of the residual NH4-N, or the mineralization of the fertilizer in the soil zone is not expected to occur. Therefore the 15N of the nitrate-N in the ground water should reflect the source. Figure 5 shows that most of the shallow wells are affected by fertilizer nitrogen (table 1). There are a few that have indications of enriched isotope values because of denitrification above fine-textured zones or proximity to upgradient barnyards (table 1, Sites 8 and 9).
Figure 5--Nitrogen-N vs. Nitrogen-15 concentration for groundwater studies.
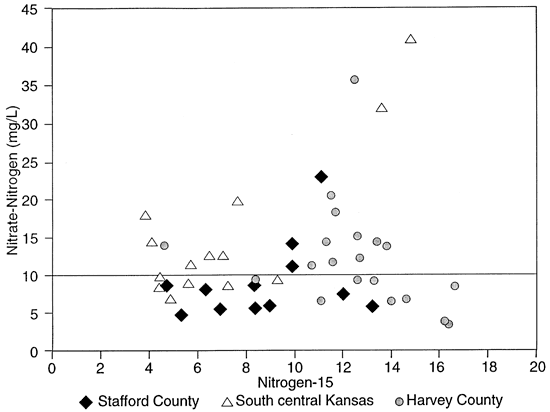
Table 1--Nitrate-nitrogen and Nitrogen-15 Isotope Data, South-Central Kansas.
| Location | County | Well type |
Depth (m) |
NO3-N mg/L |
15N ‰ |
Landuse |
|---|---|---|---|---|---|---|
| 1 | Pawnee | D | 13.7 | 9.3,8.5 | 9.3,7.15 | Irrigated field. Manure as fertilizer? |
| 2 | Pawnee | D | 17.8 | 12.6 | 7.0 | Irrigated fields. |
| 3 | Pawnee | D | 15 | 9 | 5.6 | Irrigated fields. |
| 4 | Edwards | D | 16.5 | 14,10 | 4.6,4.45 | Irrigated fields. |
| 5 | Rice | D | 12.3 | 8.3 | 4.4 | Irrigated fields. |
| 6 | Rice | 1 | 22.8 | 7 | 4.9 | Irrigated corn. |
| 7 | Pratt | D | 20 | 14.4, 18 | 4.1,3.81 | Irrigated cropland. |
| 8 | Pratt | m | 9 | 7.6 | 9.3 | Irrigated corn. Denitrification above clays. |
| 9 | Reno | m | 9.6 | 40.8,32 | 14.8,13.6 | Irrigated fields. Manure as fertilizer. Barnyard nearby. |
| 10 | Reno | m | 6.1 | 19.8 | 7.6 | Irrigated field nearby. |
| 11 | Stafford | D | 16.4 | 12.4,11.5 | 6.5,5.76 | Irrigated fields nearby. |
| D = domestic; I = irrigation; M = monitoring well; S = stock well | ||||||
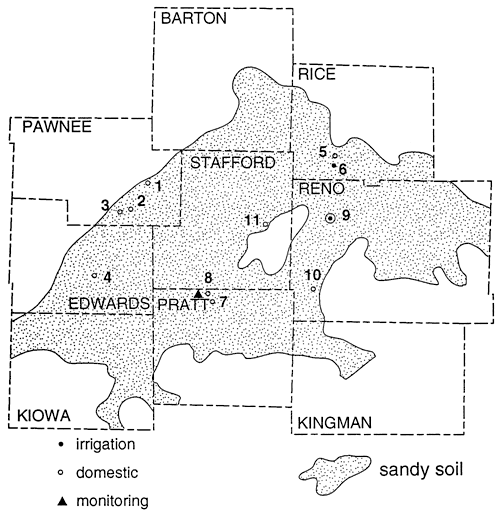
Figure 6 shows the sampling sites in northern Stafford County. The area has predominantly sandy soil with irrigated cropland. The stratigraphy of the county is similar to figure 3. Figure 5 and table 2 show that wells are affected by fertilizer, animal waste and possible enrichment by denitrification above fine-textured zones in and around the wells.
Figure 6--Location of sampling points, northern Stafford County, Kansas.
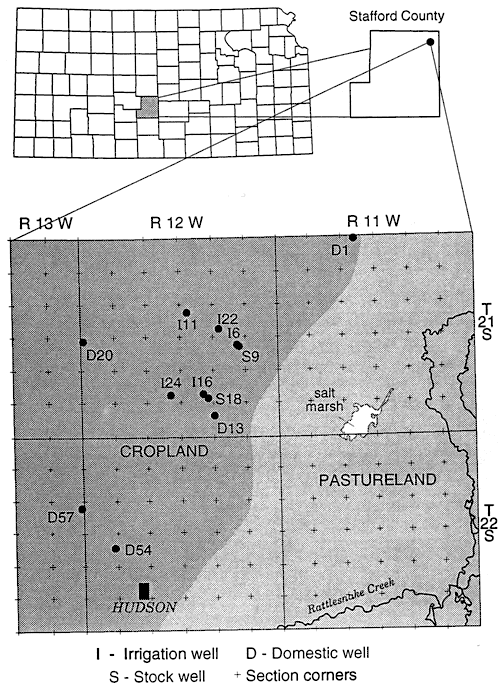
Table 2--Nitrate-nitrogen and Nitrogen-15 Isotope Data, Stafford County, Kansas
| Location | Well type |
Depth (m) |
NO3-N mg/L |
15N ‰ |
Landuse |
|---|---|---|---|---|---|
| D1 | D | 12.8 | 14.2 | 9.9 | Intermittently used livestock pens |
| I6 | D | 42.7 | 8.1 | 6.3 | Irrigated com |
| S9 | S | 24.7 | 23 | 11.1 | Stock well used intermittently |
| I11 | D | 35 | 11.1 | 9.9 | Irrigated croplands; source of manure; Denitrification above clay lenses |
| D13 | D | 27.4 | 8.8 | 4.7 | Irrigated cropland |
| I16 | D | 36.6 | 8.7 | 8.3 | Irrigated cropland; source of manure; Denitrification enrichment above clays |
| S18 | S | 27.4 | 5.7 | 8.3 | Irrigated cropland; source of manure; Denitrification enrichment above clays |
| D20 | D | 22.5 | 5.4 | 6.8 | Irrigated cropland |
| I22 | I | 30.5 | 5.9 | 8.9 | Irrigated cropland; source of manure; Denitrification enrichment above clays |
| I24 | I | 31 | 6.0 | 13.2 | Irrigated cropland; source of manure; Denitrification enrichment above clays |
| D54 | D | 29 | 4.9 | 5.3 | Irrigated cropland |
| D57 | D | 24.4 | 7.6 | 12 | Abandonned barnyard to west |
| D = domestic; I = irrigation; S = stock well | |||||
In Harvey County a number of wells are associated with dairy farms and feedlots (table 3; fig. 7). These wells were sampled with the expectation of enriched 15N to explain the high nitrate-N concentrations that were measured. As expected most of these wells were enriched (wells east of Halstead, figs. 5 and 7).
Figure 7--Location of sampling points, Harvey County, Kansas.
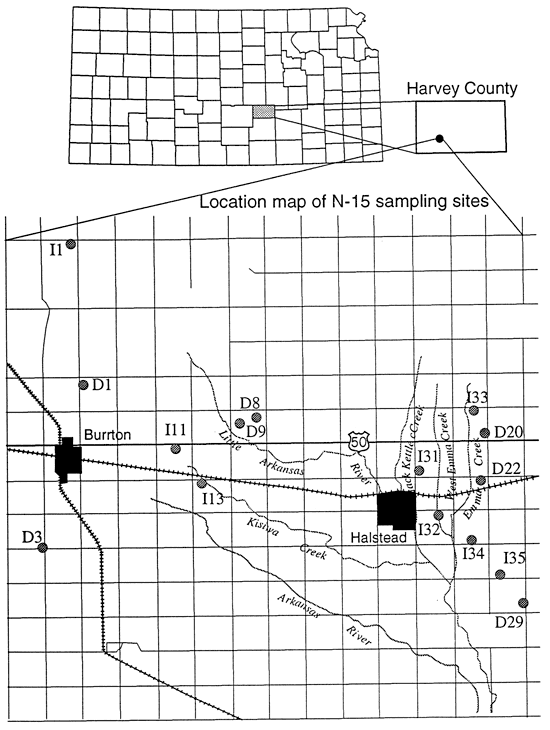
Table 3--Nitrate-nitrogen and Nitrogen-15 Isotope Data, Harvey County, Kansas
| Location | Well type |
Depth (m) |
NO3-N mg/L |
15N ‰ |
Landuse |
|---|---|---|---|---|---|
| D1 | S | 7.6 | 9.2 | 13.3 | Well in feedlot |
| I1 | D | 18.3 | 3.8 | 16.2 | Irrigated fields; denitrification above clays |
| D3 | D | 15.3 | 12.2 | 12.7 | Irrigated fields; denitrification above clays |
| D8 | D | 32.9 | 6.9 | 14.6 | Dairy feedlot near well |
| D8 | S | 22.9 | 15.1 | 12.6 | Dairy feedlot near well |
| D9 | D | 22.3 | 14.4 | 13.4 | Feedlot near well |
| I11 | D | 13.7 | 6.5 | 14 | Irrigated fields; denitrification above clays |
| I13 | D | 20.7 | 6.5 | 11.1 | Irrigated fields; intermittent feedlot |
| D20-1 | D | 24.4 | 35.6 | 12.5 | Dairy farm in past |
| D20-2 | D | 15.3 | 20.5 | 11.5 | Dairy farm in past |
| D22 | D | 27.5 | 9.3 | 12.6 | Irrigated fields; denitrification above clays; Possible manure source |
| D29 | D | 21.4 | 11.2 | 10.7 | Feedlot nearby |
| 131 | D | 19.8 | 14.4 | 11.3 | Well in dairy barn |
| 132 | D | 22.6 | 11.6 | 11.6 | Dairy and irrigated fields |
| 132 | s | 22.6 | 9.4 | 8.4 | Dairy and irrigated fields |
| 133 | D | 21.4 | 18.2 | 11.7 | Longterm dairy; manure as fertilizer |
| 133 | S | 32 | 13.9 | 13.8 | Longterm dairy; manure as fertilizer |
| 134 | D | 24.4 | 3.6 | 16.3 | Irrigated fields; denitrification above clays |
| 135 | D | 24.4 | 8.5 | 16.6 | Feedlot nearby; denitrification above clays |
| D = domestic well; S = stock well | |||||
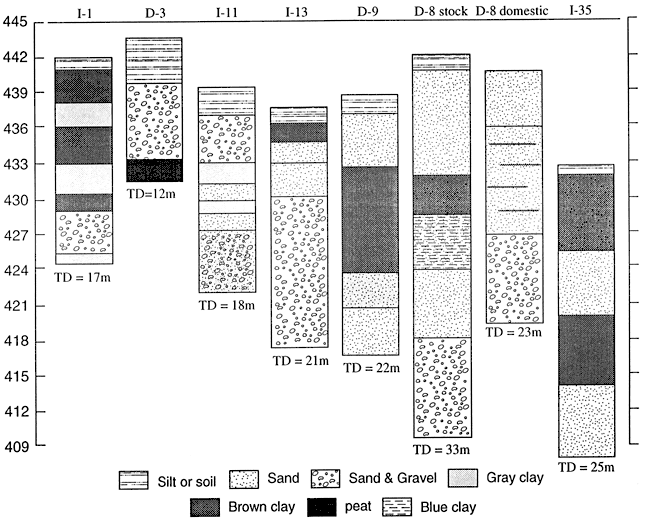
The finding that wells with moderate nitrate also had highly enriched 15N was unexpected (table 3). Much of this enrichment may be attributed to denitrification above fine-textured zones. As can be seen from figure 8, wells I-1, D-3 and I-11 all have gray clays that may indicate reducing conditions conducive for denitrification (table 3).
Wells I-13, D-8 and D-9 all lack fine-textured zones in the upper stratigraphy of the wells. The enriched 15N signature is probably due to a point source from the dairy farm feedlots. Well 1-35 is the only available well-log from the area east of Halstead (fig. 8). Because of the stratigraphic variability observed in well logs from the area, we did not try to use substitutes.
Figure 8--Selected well logs from Harvey County.
The movement of nitrate-nitrogen through the unsaturated zone to ground water was studied in Pratt, Sherman, and Thomas counties (fig. 2). Table 4 summarizes the depth of sample collection, nitrate-nitrogen concentration, 15N value, and sample type. The water samples from Pratt and Sherman Counties were collected using lysimeters. The samples from Thomas County were extracts from soil samples.
The 15N values for UAN fertilizer (-1.3 ‰) and anhydrous ammonia (-2 to -1 ‰) are listed on figure 11 for comparison to the collected samples.
Table 4--Summary of Unsaturated Zone Studies
| County | Sample Site |
Depth (m) |
NO3-N (mg/kg) |
15N ‰ |
Sample Source |
|---|---|---|---|---|---|
| Pratt | 0.3-A | 0.3 | 9.9 | 10.6 | Lysimeter |
| Pratt | 3.0-A | 3.0 | 10 | 8.7 | Lysimeter |
| Pratt | 0.9-B | 0.9 | 2.3 | 13.5 | Lysimeter |
| Pratt | 1.2-B | 1.2 | 4.1 | 10.6 | Lysimeter |
| Pratt | 0.6-C | 0.6 | 53 | 5.6 | Lysimeter |
| Pratt | 1.2-C | 1.2 | 115 | 8.6 | Lysimeter |
| Pratt | 3.0-C | 3.0 | 30 | 5.5 | Lysimeter |
| Pratt | M | 9.0 | 7.6* | 9.3 | Groundwater |
| Pratt | D | 20 | 18* | 3.8 | Groundwater |
| Sherman | 5 | 7.6 | 53 | 6.0 | Lysimeter |
| Sherman | 5 | 12.2 | 115 | 3.5 | Lysimeter |
| Sherman | 5 | 18.9 | 142 | 3.3 | Lysimeter |
| Sherman | 5 | 7.6 | 52 | 5.1 | Lysimeter |
| Sherman | 5 | 18.9 | 135 | 3.9 | Lysimeter |
| Sherman | 5 | 7.6 | 47 | 5.2 | Lysimeter |
| Sherman | 5 | 12.2 | 84 | 4.4 | Lysimeter |
| Sherman | 5 | 18.9 | 124 | 3.3 | Lysimeter |
| Sherman | 10 | 8.2 | 16 | 4.7 | Lysimeter |
| Sherman | 10 | 21.9 | 9 | 6.5 | Lysimeter |
| Thomas | S9 | 2.4 | 7.2 | 15.9 | Soil extract |
| Thomas | S13 | 2.4 | 5.9 | 10.7 | Soil extract |
| Thomas | S14 | 2.4 | 10.1 | 17.1 | Soil extract |
| Thomas | S15 | 2.4 | 10 | 11.7 | Soil extract |
| Thomas | S31 | 2.4 | 4.1 | 18.5 | Soil extract |
| Thomas | S32 | 2.4 | 4 | 10.7 | Soil extract |
| Thomas | S66 | 2.4 | 7 | 9.0 | Soil extract |
| Thomas | S71 | 2.4 | 10.3 | 16.4 | Soil extract |
| Thomas | S72 | 2.4 | 13.2 | 14.2 | Soil extract |
| M = monitoring well; D = domestic well; * = units in mg/L | |||||
Figures 9 and 10 show the location and sampler distribution at the site study in Pratt County. The samplers are adjacent to an irrigated field which is fertilized with anhydrous ammonia in the spring and 28% N liquid fertilizer during the growing season. The samples were collected in the spring of an irrigated corn rotation.
Figure 9--Location of study site on Pratt County, Kansas.
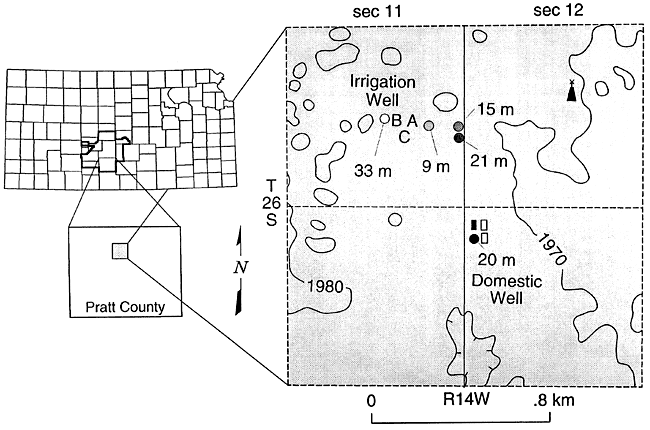
Figure 10--Location of lysimeters in field, Pratt County, Kansas.
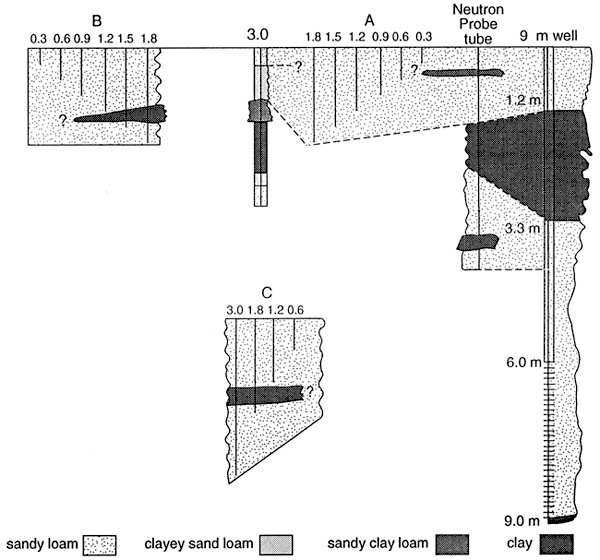
The samples that have enriched isotope signatures are located above fine-textured zones (fig. 11; table 4). The samples that have isotope values in the range of fertilizer are located in sands (0.6 and 3.0 C samplers, table 4, figs. 10 and 11). This suggests that denitrification is occurring above the fine-textured zones and causing enrichment. The fact that all of the samplers do not have enriched isotope values suggests that volatilization of the anhydrous ammonia is not a reasonable source for the enriched isotopes.
Figure 11--Nitrate-N and 15N isotopes in the unsaturated zone.
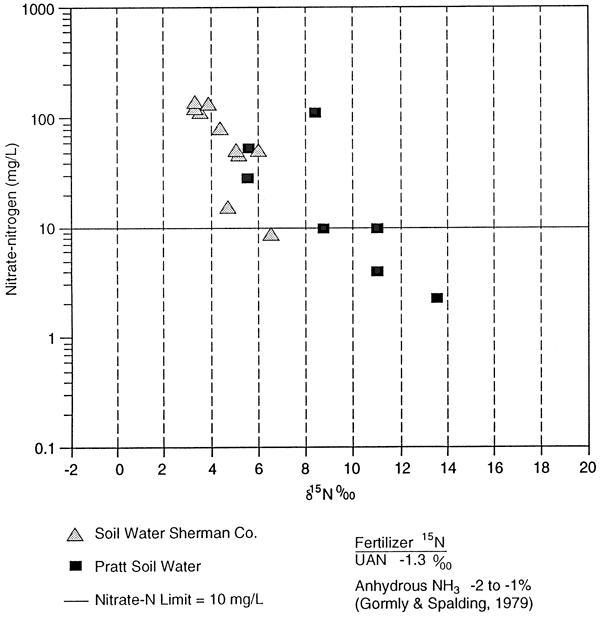
The 9 m monitoring well and the 20 m domestic well are listed in table 4 for comparison. The 9 m well is sited above a clay zone and has an enriched 15N value. The domestic well is sited in sands and gravels with no clay zones and has an 15N in the fertilizer range (table 4).
Figure 12 shows the location of sites 5 and 10 along the South Fork of Beaver Creek in Sherman County. Samples from the lysimeters at these sites all fall within the fertilizer range (fig. 11). Both of these sites are in pastureland and which has never been cultivated. Loess and the Ogallala Fm. are the dominant unsaturated zone materials. The thicknesses of these units vary up to 30 m or more.
Figure 12--Core descriptions of lysimeter sites 5 and 10, SOuth Fork of Beaver Creek, Sherman County.
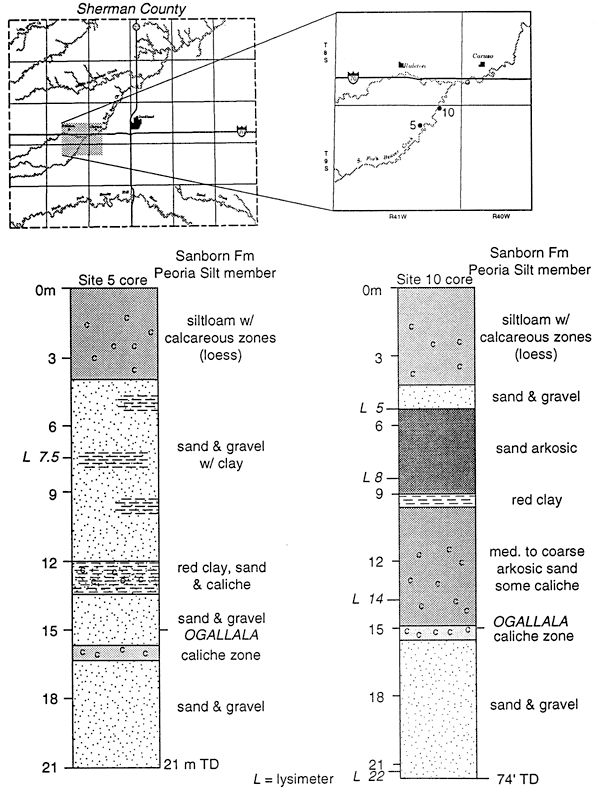
Beaver Creek was used as an irrigation tailwater runoff stream from the mid 1950s to 1980s. Both of the study sites are located in the floodplain of the creek. Based on the nitrate concentration and the isotope data we feel that these values represent the last flood events by irrigation water prior to the changeover to center pivot irrigation. The presence of the nitrate at depths up to 21 m is not unwarranted given the documented presence of deep fractures in the loess of the area (Prescott, 1953). Fracture or macropore flow may be responsible for the presence of nitrate-N at these depths.
Figure 13 shows the soil series found at the Northwest Research Extension Center for Kansas State University. Nine samples were analyzed for nitrate-nitrogen and 15N isotopes to determine if a fertilizer signature could be used for comparison with 15N values found in other parts of northwest Kansas. The samples were collected from a field fertilized with approximately 60 kg N/ha from UAN fertilizer. The site had minimal irrigation.
Figure 13--Soils description for site in Thomas County.
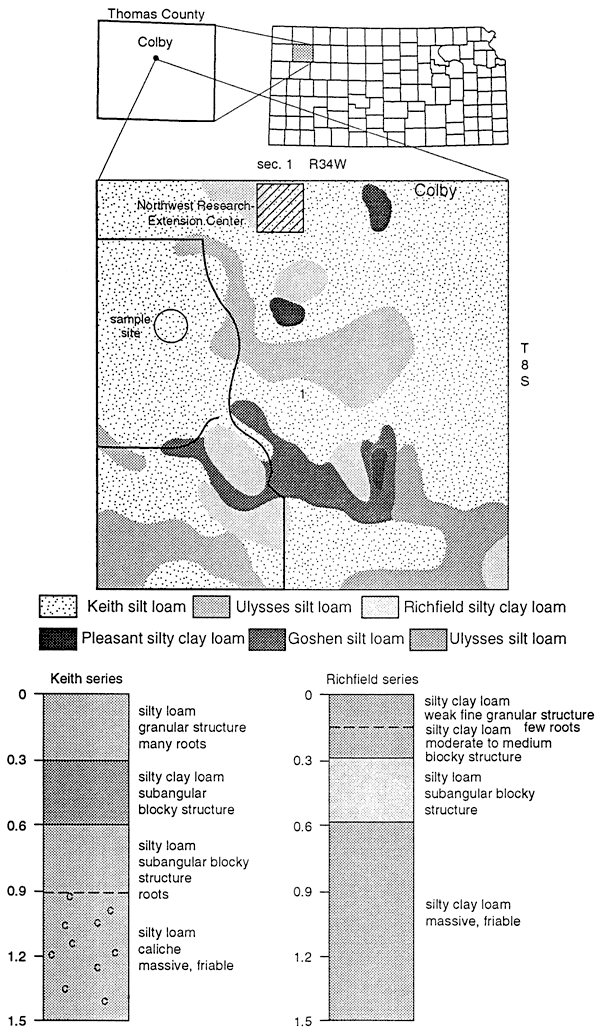
The soil extract samples showed an enriched 15N range from +9.0 to 18.0 ‰ with a small range of nitrate-N concentration (table 4). These 15N values suggest that the nitrogen is residual and is a result of denitrification processes prior to mineralization in the soil. The 15N values show no effect of recent application of UAN fertilizer (5 15N = -1.3 ‰). More work is needed to determine organic carbon content, temperature variations, soil moisture variations, and biomass effects on the isotope values.
Studies using 15N isotopes to determine sources of nitrate in ground water and soils have been done for approximately 20 years. The method is used successfully in areas with thin, permeable vadose zones with a shallow ground water table and in rock units such as fractured limestones. The results of the work in Kansas using this method suggests that the geology of the study area, the depth to ground water, and land use need to be closely evaluated before depending solely on 15N to identify nitrogen sources.
The presence of fine-textured layers such as buried loess, clay, or paleosols can result in enriched isotope signatures because of denitrification processes. The presence of an enriched isotope value in the range of animal waste (+10 to +22‰) does not necessarily mean there is an animal waste source. Denitrification enrichment of the 15N may have occurred or perhaps enrichment by volatilization of an ammonia based fertilizer if the soil conditions are correct. Studies in Kansas indicate that areas with extensive fine-textured layers make it difficult to interpret an isotope signature.
As a result of our work in Kansas we feel that the 15N isotope method is useful in certain circumstances but that the method should be used with caution. More work is needed to clarify the denitrification enrichment process such as additional marker compounds that could be tested for in order to verify the denitrification process. Also, testing of the method in areas with thick unsaturated zones and deep water tables would help to clarify the range of usefulness of the method. Another area of study is to measure the fractionation enrichment processes because of volatilization of UAN and anhydrous ammonia fertilizers.
Gormly, J.R., and Spalding, R.F., 1979, Sources and concentrations of nitrate-nitrogen in ground water of the central Platte region, Nebraska. Ground Water, v. 17, p. 291-301.
Heaton, T.H.E., 1986, Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: a review: Chemical Geology, v. 59, p. 87-102.
Herbell, M.J., and Spalding, R.F., 1993, Vadose zone fertilizer-derived nitrate and 15N extracts: Ground Water, v. 31, p. 376-382.
Kreitler, C.W., 1975, Determining the source of nitrate in ground water by nitrogen isotope studies: University of Texas (Austin), Bureau of Economic Geology, Report of Investigations 83, 57 p.
Kreitler, C.W., 1979, Nitrogen-isotope ratio studies of soils and groundwater nitrate from alluvial fan aquifers in Texas: Jour. Hydrology, v. 42, p. 147-170.
Prescott, G. C., Jr., 1953, Geology and ground-water resources of Sherman County, Kansas: Kansas Geological Survey, Bulletin 105, 130 p. [available online]
Kansas Geological Survey, Geohydrology
Placed online Nov. 6, 2008
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/1994/OFR94_29/index.html