


Kansas Geological Survey, Open-file Report 88-39
Great Plains and Cedar Hills Aquifers--Page 13 of 25
The origin of the water is probably surface recharge in the local or subregional vicinity that flows from the surface through Cretaceous rocks to the location of the well. Some of the fresh ground waters are derived from shallow wells, while others are from wells a few to several hundred feet in depth. Many of the fresh waters also have nitrate concentrations exceeding 10 mg/l (as nitrate) in comparison with the low nitrate contents of most of the ground waters in the Great Plains aquifer. The nitrate is an indicator of surface contamination, supporting the mechanism of a local surface source of water.
Several of the slightly brackish (1000-3000 mg/l total-dissolved solids) ground waters also contain relatively high concentrations of nitrate. These waters could be a mixture of shallow ground waters from surface recharge mixed with deeper, saline water. Many of the wells are open over large intervals that include several different water-bearing zones, thus, the water pumped from such wells can be derived from different sources.
The relative concentrations of the major cationic constituents--i.e., calcium, magnesium, and sodium--in ground waters in the Great Plains aquifer are derived not only from mixing of varying amounts of local or regional recharge with saline ground water, but also are affected by ion exchange. Many of the ground waters collected in this study have a Na/(Ca + Mg) equivalent ratio greater than 10 in comparison with the oil brines which have a Na/(Ca + Mg) of 2-4. The high ratios can be explained by the movement of fresher water into the aquifer and exchange of calcium and magnesium for sodium on clays in the Great Plains aquifer. Most of the ground water containing high nitrate concentrations have Na/(Ca + Mg) equivalent ratios less than 0.8, further indicating that these waters are derived from surface recharge through carbonate rocks and have not been appreciably affected by ion exchange in the Great Plains aquifer. Other waters with both high nitrate contents and high Na/(Ca + Mg) equivalent ratios are probably a mixture of waters from different formations due to the construction of the wells.
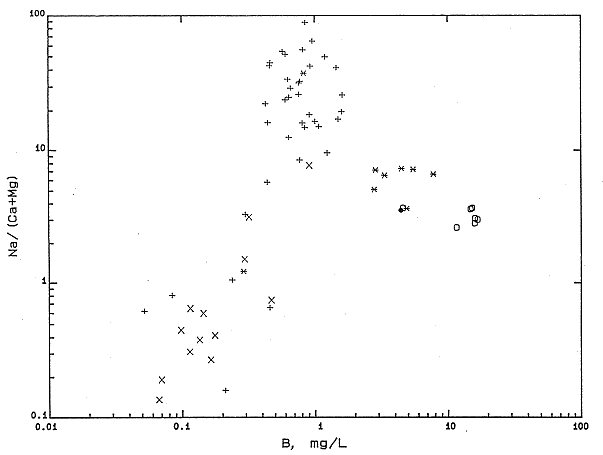
In order for the exchange process that increases the Na/(Ca + Mg) to occur, the clays must have previously been in contact with waters of higher Na/(Ca + Mg), i.e., more saline waters with a Na/(Ca + Mg) equivalent ratio appreciably greater than unity. The origin of the clays or the saline water cannot be determined on the basis of the major constituents alone. The distribution of boron concentrations in the fresh to moderately saline waters collected in this study suggest that some of the exchanging clays causing chemical alteration of the water chemistry had a marine origin. Boron concentrations in the fresh to moderately saline (less than 10,000 mg/L total-dissolved solids) waters are positively correlated with the Na/(Ca + Mg) ratios (Figure 27). Marine clays contain substantially more boron than continental clays, both in structural and adsorbed positions (Fairbridge, 1972). As fresher waters flowed through the marine clays, both major cation and boron exchange could occur.
Figure 27. Equivalent ratio of Na/(Ca+Mg) versus boron concentration for KGS data.

Concomitant processes with the cation exchange have further affected the water chemistry within the Great Plains aquifer. As calcium and magnesium decreased during the exchange, the waters became under-saturated with respect to calcite. Calcite could then dissolve resulting in increases in calcium and bicarbonate concentrations. The dissolved calcium would then be readsorbed on the clays, while most of the bicarbonate would stay in solution. Many of the ground waters in the Great Plains aquifer have relatively high bicarbonate concentrations (greater than 500 mg/l). However, calcite contents in the sediments of the Great Plains aquifer are small. Therefore, many of the waters affected by exchange during flow through clays are still undersaturated with respect to calcite, thereby limiting the increase in the bicarbonate contents.
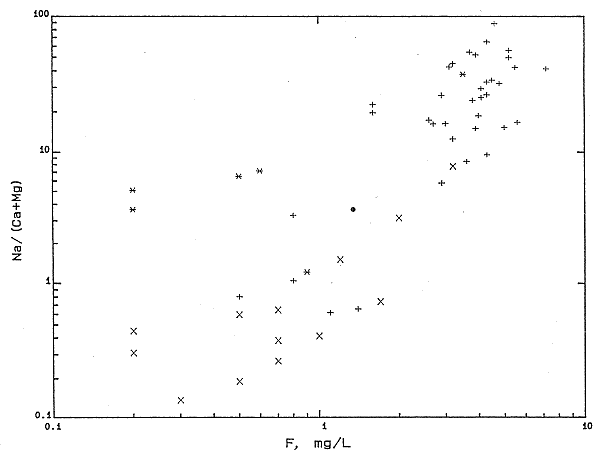
The decrease in the calcium concentrations caused by ion exchange have also caused increases in the fluoride contents of ground waters in the Great Plains aquifer as shown by a positive correlation between Na/(Ca + Mg) ratios and fluoride concentrations (Figure 28). Chemical equilibrium calculations indicate that the mineral fluorite is the probable control on the fluoride content of high fluoride (greater than 1 mg/l) waters. As the calcium content of waters flowing through clays in the aquifer decreased during exchange, fluorite dissolved to bring the water back into equilibrium with the mineral. Many of the fluoride concentrations in the usable waters are greater than the drinking water standard.
Figure 28. Equivalent ratio of Na/(Ca+Mg) versus fluoride concentration for KGS data.
Previous page--Chemical Characteristics ||
Next page--Salinity Sources
Start of Report ||
Report Contents