


Kansas Geological Survey, Open-file Report 88-39
Great Plains and Cedar Hills Aquifers--Page 12 of 25
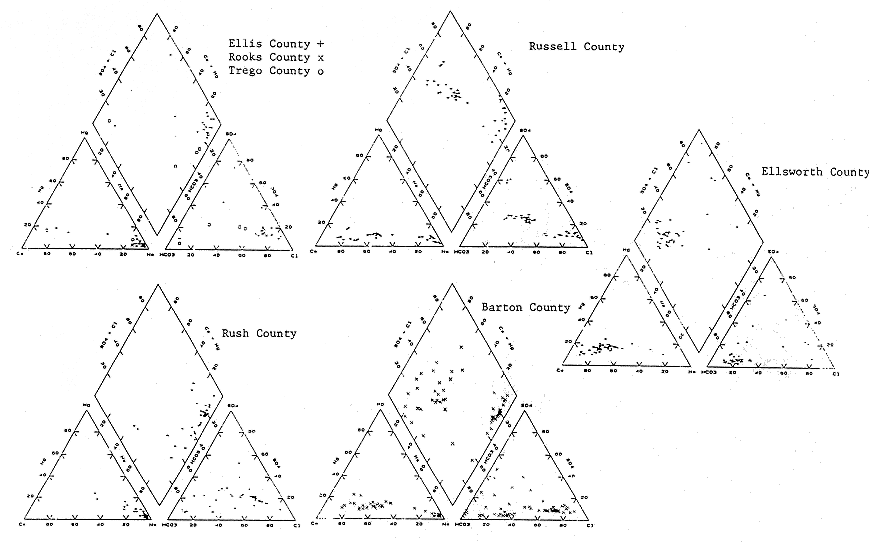
Trilinear diagrams (Piper, 1944) were drawn for the USGS and KGS data sets to evaluate the occurrence of different chemical types of waters from water-supply wells in the study area (Figure 22). These diagrams were plotted on a county by county basis or groups of counties depending upon the quantity of data available in the area. The depths reported for the wells were compared to formation tops picked from geophysical logs of nearby boreholes where available.
Figure 22. Distribution of water chemistry by county (USGS WATSTORE data set).

The trilinear diagrams indicate a trend from calcium-bicarbonate type water in Ellsworth County and parts of Barton County, the eastern part of the study area, toward a calcium-bicarbonate-sulfate water in Russell County, to sodium-bicarbonate and sodium-chloride waters in parts of Russell, Barton, Ellis, Rooks, Rush, and Trego counties. A trilinear diagram for the samples collected by the KGS indicates similar trends (Figure 23 and Table 2). At this writing it is uncertain if these transitions are related to well depth.
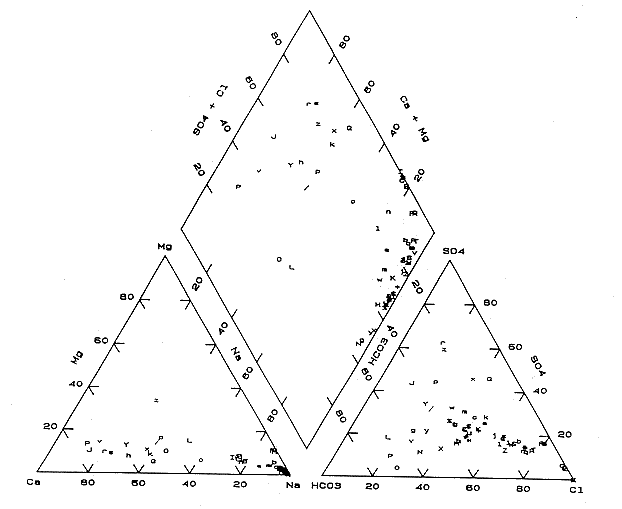
Figure 23. Trilinear diagram of water samples collected by KGS in June, 1987 (see Table 1 for symbols).
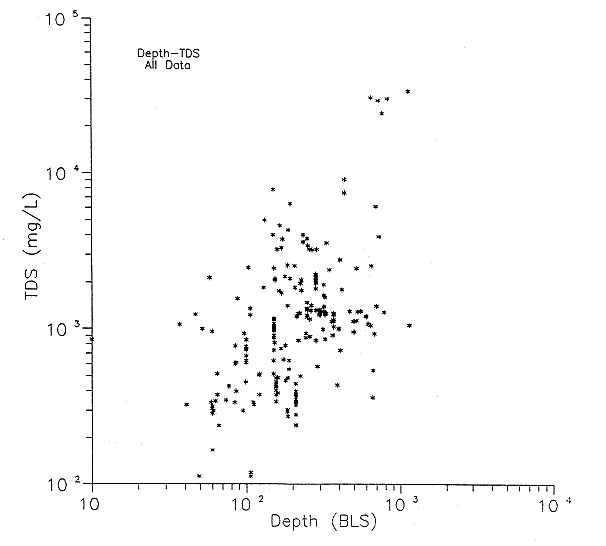
A generalized graph of all of the well depths versus total-dissolved-solids concentrations does not indicate a prominant trend (Figure 24). It is possible that depth versus total-dissolved-solids contents by county may correlate better with the variation of water types found throughout these counties. This aspect will be evaluated at a later time.
Figure 24. Total dissolved solids (TDS) versus depth for all data (see Appendices A and B).
The chemical data on the samples collected by KGS shows that there are two major divisions of water types: sodium-chloride and mixed-type waters (Figure 25; Table 2). The mixed types include sodium-bicarbonate to calcium-sodium-sulfate-chloride waters and all combinations in between. The mixed type waters are represented by one symbol in Figure 25 as there is not enough information at present to fully explain the origin of these types of waters. Their presence suggests that one or more of the following processes may have been important in the origin of water types:
Figure 25. Distribution of water types from KGS sampling June, 1987 (see Table 1).
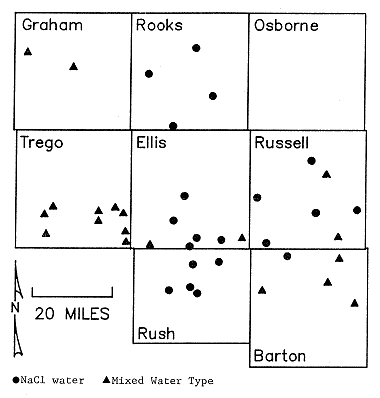
Figure 25 indicates that a corridor of sodium-chloride water exists through Rush, Ellis, Rooks, and parts of Russell counties. The occurrence of sodium-chloride waters appears related to the Stockton and Fairport Natoma Anticlines in central Ellis and Rooks counties and in western Russell county. The oil-field brine samples (Table 3) plot near the sodium and chloride ends of the trilinear diagram (Figure 23).
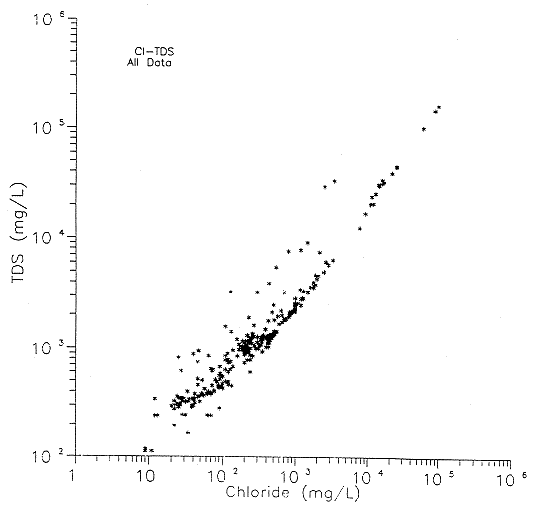
A strong linear relationship exists between TDS and chloride concentrations (Figure 26). The scatter of points to the left of the line is probably related to waters with appreciable sulfate and/or bicarbonate concentrations relative to the chloride concentration. The waters represented as points to the left of the trend may be related to the mixed cation/anion type waters shown in Figures 23 and 25. Further information on flow dynamics in the system is needed in order to determine the origins of these waters.
Figure 26. Total dissolved solids (TDS) versus chloride concentration for all wells.
Swineford and Williams (1945) characterized the waters in south-central to southwest Russell county. The percentage distribution of the major cation and anion equivalent concentrations in their water samples are given in Table 4 for comparison with the waters collected by the KGS in 1987. In general, the Dakota and lower formations contain sodium-chloride waters with relatively low magnesium, calcium, bicarbonate, and sulfate concentrations. A few of the lower Dakota waters have low calcium, magnesium, and proportionately higher bicarbonate contents that are due to base-exchange and concomitant adjustment of carbonate equilibria. Swineford and Williams stressed the extreme difference between waters in the Upper Dakota and some of the waters in the Lower Dakota and those in the Kiowa, Cheyenne and Cedar Hills. The major chemical characteristics of the waters they examined are not much different from the water quality of the samples recently collected. They also stated that oil-field brines have probably affected the overall water chemistry of parts of the Dakota. The following results and discussion more conclusively address the effects of natural mechanisms and oil-brine disposal on water chemistry.
Previous page--Chemical Data Types ||
Next page--Dissolved Constituents
Start of Report ||
Report Contents