Prev Page--Introduction || Next Page--Systematic Paleontology
Shell Morphology
Glossary of Shell Terms
In some particulars, pectinoid shells are so unlike the shells of other pelecypods that a special terminology has been used for them by some authors; hence, it is desirable to define briefly some of the terms used in the text. A list of these, arranged in alphabetical order, follows.
Adductor impression—The single large muscle "scar" just back of the middle of each valve will be called the adductor impression. The insertion of the retractor muscle of the foot is continuous with the anterodorsal part of the adductor impression, and it is generally indistinguishable from that of the adductor proper.
Auricle—The anterior and posterior extensions of the pectinoid shell along the dorsal margin are the auricles. These are sometimes called "ears" or "wings."
Auricular crura—In shells like Amussium, Pernopecten, and Entolium there is a pair of internal ridges diverging from the beak of each valve along the juncture of the auricles with the shell body. These internal ridges or crura (singular, crus) do not function primitively as teeth, since they are not in contact and are not alternate, but are in direct apposition. In Spondylidae, however, they assume the role of interlocking teeth.
Auricular sulcus—This is a more or less distinct external furrow at the juncture of an auricle with the body of the shell.
Byssus—The group of conchiolin strands by which many pelecypods make a temporary attachment is known as the byssus. It is secreted by special glands of the foot, and while still fluid is attached by the foot to extraneous objects.
Byssal notch—When at rest the pectinoid shell lies on the right valve. A deep notch under the anterior auricle of this valve permits the small foot to be extruded without opening the valves widely. In some forms, like Pernopecten and Amussium, the notch is lost in the adult, but its existence in the juvenile can be seen in early growth lines.
Byssal sinus—The front edge of the anterior auricle in the left valve generally has a broad, slight indentation or sinus. Most commonly the margin of the auricle is recurved inward toward the beaks at the hinge line forming a sigmoidal outline, somewhat unlike the simple sinus below the posterior auricle. The relatively deep anterior notch in the right valve and the shallow anterior byssal sinus in the left afford a ready means for orienting Pecten-like shells.
Cardinal area—In many shells, like members of the Arcidae and Pteriidae, two flat areas diverge upward from the hinge axis of the two valves. The flattened area of each valve is generally called cardinal area. In many of the Paleozoic pectinoids, as in modern Arcidae and Pteriidae, the cardinal area corresponds to the ligament area, or surface of ligament attachment. A few of the Paleozoic forms, such as Pernopecten, like most modern Pectinidae, have no cardinal area.
Cardinal axis—This is the axis of rotation for the valves. The axis in the Aviculopectinidae and Pterinopectinidae, as in Arca or Pteria, lies well below the dorsal margin of the shell.
Cardinal costa—In some genera, a pronounced ridge occurs on both sides of the beak, lying at the angle between the cardinal area and the outer shell surface. These ridges, the cardinal costae, are not comparable to ordinary ornamentation, because they may occur in otherwise unornamented forms.
Cardinal margin—The cardinal margin is the dorsal margin of the shell. In those forms possessing cardinal areas the cardinal margin is not quite straight, but forms the sides of a low triangle, the base of which is the cardinal axis. The triangular shape of the cardinal area is more distinct in some forms than in others.
Chevron grooves— The V-shaped ligament grooves typically developed on the cardinal areas of Arcidae and Pterinopectinidae may be termed chevron grooves. In some pelecypods, like Myalina, the anterior part of the chevron is suppressed or obsolete.
Convexity—This term is used for the maximum convexity of a single valve, measured from the plane of commissure to the outer surface of the shell.
Costae—Costae (singular, costa) are external radial ridges. When costae increase in number by bifurcation or intercalation, a shell is said to be multicostate; when they are fascicled, the shell is fascicostate. When the costae do not increase in number the shell is simplicicostate. Those costae which appear first at the umbones are said to belong to the first order; the next ones to appear belong to the second order, etc.
Fila—Fila (singular, filum) are regular, concentric lirae, They may be distinguished arbitrarily from ordinary growth lines which are irregular and relatively fine; and from imbricating lamellae. If there are two ranks of fila, the coarse ones are said to be of the first order, and the finer ones are of the second order. Fila, like the edges of imbricating lamellae, are only a special kind of growth lines.
Gape—In some pelecypods the valves, when closed, are only in contact along the dorsal and ventral margins. Such shells gape anteriorly and posteriorly.
Growth lines—These are irregular and generally obscure lines marking the successive advances of the shell margin.
Height—It is convenient to measure the height in pectinoids, as in dimyarian pelecypods, from the beaks to the ventral margin, at right angles to the hinge.
Hypostracum—The term hypostracum is applied to a filmlike calcareous secretion of the muscles, deposited between the muscle and shell proper, coextensive with the muscle impressions.
Interspaces—Interspaces are striae, or external furrows separating adjoining fila or costae.
Lamellar layer—The calcareous shell, or ostracum, is commonly divisible into two or more layers of unlike structure. Ordinarily one or more of the layers is constructed of microscopic lamellae of calcite or aragonite separated by equally thin layers of conchiolin. The two most common types of lamellar structure are the nacreous or pearly structure and the crossed lamellar structure. Shell structure is more fully discussed in "Shell Structure."
Length—In pectinoids the length is the greatest dimension of the body of the shell measured parallel to, and below the hinge. The hinge is in some instances longer than the shell body. Its length is designated separately as the "hinge length."
Levator impressions—Small pits on the inner surface under the beaks mark the insertions of the superior retractor muscles, or levator muscles of the foot. Such muscles characterize the Pteriidae and older Pectinacea but are absent in the Pectinidae sensu stricto.
Ligament (see resilium)—The ligament commonly consists of two unlike kinds of elastic conchiolin. One kind is impregnated with calcareous spicules, the fibrous ligament, and is elastic chiefly to compressional stresses. This part of the ligament is located mainly below (ventral to) the hinge axis. The other type of ligament, the lamellar ligament, contains no calcareous material. It is elastic to both compressional and tensional stresses.
Midumbonal line—An imaginary, usually curved, line bisecting the umbonal angle may be termed the midumbonal line.
Musculature—The muscles of the modern Pectinidae or Pteriidae that produce an impression on the shell are: the levators of the foot, generally two to each valve (absent in Pectinidae), attached at the interior of the umbones; retractors of the foot, one to each valve (right retractor missing in Pectinidae), inserted at the anterodorsal part of the large adductor impression; adductor system, inserted slightly behind the center of both valves; orbicular or pallial system, inserted at a pallial line; and the gill suspensories (in the Pectinidae), just below the adductors.
Obliquity—The obliquity of a shell may be stated conveniently in terms of the inclination of the midumbonal line. Shells with a forward obliquity, like most Pteriidae, are prosocline; upright shells, like most Pectinidae, are acline; and shells with a backward obliquity, like Streblochondria, n. gen., are opisthocline. As determined by ontogeny, acline shells are derived in every case from prosocline shells, and in turn acline shells give rise to opisthocline shells. In the latter group, the midumbonal line tends to describe a semicircle or C, open toward the anterior end of the shell.
Ostracum—In many kinds of pelecypods, such as Aviculopectinidae, Unio, Pteria, there are two separate layers constituting the ostracum or shell proper. The outer layer is commonly prismatic or homogeneous and will be termed the outer ostracum. The inner layer is commonly nacreous or crossed-lamellar and will be termed the inner ostracum.
Pallial line—The insertion of the shell of the orbicular or pallial muscle system is called the pallial line. The line may be continuous or consist of a series of pits. The mantle is not attached to the shell outside of the pallial line.
Periostracum—An outer film of conchiolin, called the periostracum, is present in many groups of pelecypods. It is generally present in the modern Pteriacea and absent in modern Pectinacea.
Plane of valves—The plane of commissure or plane approximately coinciding with the valve margins may be called the plane of the valves. In valves with front and rear gape the plane of the valve.s includes only the hinge line and ventral margin.
Plicae—Plicae (singular, plica) are ribs or folds involving the entire thickness of the shell and interlocking at the margin. Many of the post-Paleozoic pectinoids are plicate, whereas almost all of the Paleozoic forms are either smooth or costate. The costae in the early forms are generally confined to a thin outer layer of the shell, but in some of these individuals the shell margin is obscurely plicate.
Prismatic layer—The outer layer of the ostracum is in some cases prismatic. It occurs in nearly all modern Pteriacea and many Paleozoic pectinoids. The layer consists of closely spaced polygonal prisms of calcite in a matrix of conchiolin. Such a layer appears to be invariably absent in the adults of modern Pectinidae, but it is present in the juvenile right valves of some species. The prismatic layer in general occurs in only the right valves of the Paleozoic forms.
Resilifer—The triangular or rectangular fossette for the reception of the resilium, below the dorsal margin of both valves, is called the resilifer.
Resilium—As generally employed, the term applies to a triangular ligament structure residing in a central pit along the inner margin of each valve. The resilium, as properly defined, is invariably an organ of compression, the functional part being ventral to the hinge axis. The fibrous ligament of Arca is morphologically and mechanically a resilium, but the term is never applied to the areas because the fibrous ligament is not contained in triangular pits. Structurally the "resilia" of Pteria, Yoldia, and Pecten are different and I doubt the propriety of employing the term to diverse structures. I have avoided employing the term resilium wherever possible.
Retractor—In Pteriidae a muscle extends from the anterodorsal margin of the adductor impression in both valves to the foot. These muscles are the pedal retractors. In modern Pectinidae there is no retractor in the right valve, the single retractor present occurring in the left valve.
Shell body—The shell body is the pectinoid or pterioid shell minus the auricles.
Shell structure—In its complete development the pelecypod shell consists of three parts, ostracum, hypostracum and periostracum. The ostracum makes up most of the calcareous shell and may have a complicated microstructure. The hypostracum is a thin filmlike calcareous layer secreted by cells of the muscles at their insertions in the shell. The hypostracum may or may not have a fibrous structure. The periostracum is a thin layer of conchiolin covering the outer surface of the ostracum.
Shell thickness—The shell material of a pelecypod is thickest near the hinge and thinnest near the ends and ventral margin, with more or less gradation in thickness in the intermediate areas. The thickness is, however, for practical purposes nearly uniform over the central area of both valves, and measurements of shell thickness in this report are given for the central parts of shells having a stated height and length.
Sinus—This is an indentation of the shell margin which may be shown in growth lines.
Subinternal mold—Very commonly the outer calcite layer of the ostracum is preserved in the Paleozoic pectinoids after there is no trace of the inner, aragonite layer, or the hinge structures. In all such examples the inner as well as the outer surface of the preserved layer exhibits distinct growth lines and general features of ornamentation. A natural mold of this surface presents a striking, but generally misleading, resemblance to the external surface of the shell. Such molds are not replicas of either the inner or outer surface of the original shell and the term subinternal mold is applied to them.
Transition zone—This term is used for an area along the outer slopes of the umbonal folds of some pectinoid shells where there is a transition between the ornamentation of the shell body and a slightly different ornamentation of the auricles.
Umbonal angle—The umbonal angle is the angle of divergence of the umbonal folds. When either of the umbonal folds is particularly obscure, a precise, or even approximate, value of the umbonal angle cannot be obtained. Commonly the angle flares outward so abruptly that a different measurement can be obtained at different growth stages. Ordinarily it is convenient to record only the maximum umbonal angle at a given shell size.
Umbonal folds—The umbonal folds are the low folds or shoulders that bound the umbo and set it off from the auricles.
Modern Pelecypods Compared with Ancient Pectinoids
It has often been said that the aviculopectens are structurally intermediate between the Pteriidae and Pectinidae. A proper approach to an understanding of the ancient shells lies in an examination of similar modern ones. In the following pages a review is made of some shell characters of modern Pectinidae and Pteriidae and the ligament structures in Arca, with emphasis on those features that are also displayed by the Paleozoic shells. The modern examples that are most frequently referred to are Pinctada vulgaris (Schum.) and Pecten tenuicostatus Mighels, because the literature on these species is particularly satisfactory. Personal observations were made on the shells and anatomy of some other modern species.
Orientation
The animal of Pecten or Pteria has undergone a remarkable torsion as compared to more primitive pelecypods. In most pelecypods the hinge is dorsal and the opposite margin of the valves ventral, but in the Pectinidae, Pteriidae and similar monomyarian forms there has been a torsion of the body within the shell concurrent with the progressive reduction and loss of the anterior adductor muscle. The relation of the soft parts to the shell of Pteria and Pecten are such that the hinge line properly can be said to be anterior, the auricles, therefore, are ventral and dorsal. The correct orientation of these shells is further complicated by the circumstance that the animal in every case rests on what is anatomically the right valve. There has been a slight further torsion of part of the body to permit the foot to be extruded through a slit in the lower valve. As regards the actual living position of the valves, they might be considered dorsal and ventral. The torsion in these forms is comparable to that of the flounder among fishes. It is much less confusing for purposes of description to consider the hinge margin dorsal and the valves right and left, than to employ a more accurate terminology.
Difficulty has been experienced by several authors in orienting Paleozoic pectinoid shells properly. There are three criteria, anyone of which is infallible for distinguishing right and left valves of pectinoids and pterioids. (1) The most obvious distinction is the deep byssal notch or slit below the anterior auricle of the right valve. A corresponding sinus occurs below the anterior auricle of the left valve, but it is quite shallow as compared with the deep notch of the complementary valve. Concerning the orientation of modern Pecten, Davenport (1900, p. 864) says:
I was interested to see whether the Pecten ever lies on its left side—a condition which would be comparable in a way with left-handedness in dextral gastropods. If the abnormal condition ever occurs, it will show itself by the circumstance that the notch will appear on the left side of the (left) valve, viewed exteriorly. Now although over a thousand notched shells of Aequipecten irradians (Lamarck) were examined by me, I found no exception to the condition of being notched on the right (anterior) ear.
Jackson (1890, p. 334) found no exception to this rule:
Professor Hyatt informs me in a letter that he has examined over three hundred specimens of Pecten irradians, all of which lie habitually on the right valve . . . This habit of lying on the right valve is characteristic of many related genera, as Perna, Spondylus, Plicatula, Hinnites, and Anomia. Patten, in his studies of Pecten Jacobeus, says that this species, which has a deeply convex right valve and a flat left valve, lies habitually on the right side, and if turned on the left side in an aquarium soon rights itself. The studies of young Pecten irradians were interesting on this point. I turned them several times on the left valve intentionally and in every case they almost immediately extended the foot with which they laid hold of the glass, and with a sudden jerk righted themselves, showing that they were uneasy in the reversed position.
(2) Pterioids and pectinoids pass through the same form stages during their early development. At an early stage the shells are subrhombic in form with a forward, or prosocline, obliquity, and there is a distinct anterior lobe. The proper orientation of any pectinoid or pterioid shell can be determined by an examination of the growth lines at the beaks. (3) Finally the adductor impression without exception occurs just behind the middle of each valve.
In adults of almost all of the Paleozoic pectinoids, and the early growth stages of later forms, the left or upper valve is more convex than the right one. But the adult shells of many post-Paleozoic Pectinidae have it right valve much more convex than the left.
There is no known Paleozoic pectinoid in which the right valve is markedly more convex than the left, although a very few forms have valves of subequal convexity.
Relation of Form to Habit
The particular characters of form embraced by the term "pectinoid" have a very ancient origin. Many shells from the Middle and Upper Paleozoic rocks are superficially so like modern Pectinidae that they were described by early workers under the name Pecten. The details of shape which have been so persistent through the vast ages of geologic time suggest that the pectinoid form is truly a fundamental character which endured while other characters underwent progressive change.
It has been suggested often that the high degree of symmetry in some of the modern pectinoids has been acquired by their swimming habit. There seems to be a definite correlation between form and habits in the modern Pectinidae. Drew (1906, p. 4) says:
Pecten is one of the ablest swimmers among lamellibranchs. The whole structure of the animal is modified for this purpose. The valves have become rounded in outline, flattened, and comparatively light. The posterior adductor muscle, which is very powerful, is well developed so the shell may be opened quickly when the muscle relaxes, and the hinge line is straight so there may be no unnecessary strains in opening and in closing the shell. Each gill is attached by one lamella only, so water in the temporary cloacal chamber may be thrown out without injuring the gills, and the gills and margins of the mantle are provided with muscles to withdraw them from the margins of the shell when the shell is closed.
According to Verrill (1897, p. 48):
Those species (of modern Pectinidae) that are best specialized for swimming have a broadly rounded, symmetrical, and compressed shell, frequently with thin, nearly smooth valves, but generally strengthened by corrugations, undulations, external radial ribs, or internal lirae or flutings. Species that swim but little when adult often have a high and narrow form, with auricles oblique and usually unequal, and the byssal notch is often highly developed, while the shell itself may become oblique and unsymmetrical, or heavy and thick, with strong ribs and grooves.
Perhaps the development of the pectinoid symmetry out of some pterioid radical was determined by unusually active habits which ultimately led to a locomotion by swimming. Some of the modern Pteriidae exhibit a degree of activity such as the ancestral Pectinacea might have had. Regarding Pinctada vulgaris (Schum.), one of the typical Pteriidae, Herdman (1904, p. 50) says:
Although the pearl oyster has not the power of moving rapidly through the water or over the sea bottom after the fashion of Lima and of some species of Pecten, by the violent expulsion of water caused by a sudden closure of the valves, still it can eject a jet of water with some force to a distance of 9 to 12 inches.
It is a curious fact that the right or lower valve in the primitive Pectinacea is the less convex of the two valves. In a few genera of Paleozoic forms, and many of the later ones, the valves are subequal. There are several post-Paleozoic forms in which the lower valve is very convex, whereas the upper one is flattened or concave. However, in the latter group as well as the equivalve forms the early ontogenetic stages preserved in the umbones show that the juveniles possessed a flattened lower valve, and a more convex upper one.
The convexity of the valves has a mechanical effect on the swimming habit in modern Pectinidae. According to Verrill (1897, p. 44) the group having the upper valve relatively flat is not well suited for a swimming habit because—
If currents of water should be expelled in the usual way, from the subauricular margins, the currents would naturally be forced upward and out of the convex lower valve, and thus the reaction. would be strongly downward, so that the shell would not be raised from the bottom. In those species that are able to rise to the surface and swim actively about, the left or upper valve is always the more convex, and therefore the expelled currents of water must be directed more or less down as well as backward.
This statement must be qualified by recalling that the equivalve Amussium, and Aequipecten irradians, in which the lower valve is the more convex, are good swimmers. Nevertheless the mechanical principles indicated by Verrill possibly have had some effect on the development of the Pectinacea.
A characteristic feature of all Pectinacea and the more typical Pteriacea is the deep slit or byssal notch at the ventral margin of the anterior auricle of the right or lower valve. (The Limidae, commonly placed in the Pectinacea by paleontologists, are classified by Pelseneer, on gill structures, in the Ostracea; shells do not have a byssal notch, and are essentially bilaterally symmetrical). This notch generally is ascribed to the habit of fixation by means of a byssus. Jackson (1890, p. 344) points out, however, that the notch owes its existence to the location of the foot so close to the line of union of the valves. If the notch were not present, the valves would have to open very wide to allow the extension of the foot. He shows, further, that the very young Pecten has no byssal fixation, and the notch at this period may be considered as a foot aperture. The presence of a byssal notch, apparently, is not conclusive evidence of a byssal habit. Jackson says (p. 344):
This has important bearing on fossil forms which are considered as byssated or attached if a notch exists in this region; whereas they may with equal reason be considered, as far as the notch is concerned, as free forms which crawled while lying on one side with the foot extended through a special notch produced by the existence of such a habit.
In a masterly discussion of the problem Yonge (1936, p. 95) has presented appealing evidence that the "power of swimming possessed by certain Lamellibranchia is secondary, all the modifications which have made it possible having been evoluted in the first place in response to the needs of monomyarians originally attached by the byssus." He believes (p. 95), with Drew (1906), that the water current produced by Pecten in clapping the valves together is "primarily of service in cleansing the mantle chamber (of sediment), and is used habitually for locomotion by only a few forms, it seems quite possible that those forms that do use it for locomotion may have simply perfected an already existing mechanism primarily designed for another purpose."
According to this hypothesis pectinoids had acquired something of their characteristic expression and morphology before the adoption of the swimming habit.
Whatever may have been the habits of locomotion of the ancient pectinoids, it scarcely can be doubted that they were active, because pectinoid shells perforated by borings, or covered by encrusting organisms are relatively uncommon, even where they are associated with pelecypod shells that are so modified.
Ornamentation
The shell ornamentation in all modern and ancient Pectinacea can be resolved into concentric and radial elements, or the shells may be smooth. The radial ornamentation may consist of plications in which the entire shell is radially folded so that the shell margins interlock, or the shell may simply be costate. Of these two types, the latter is the more common. In plicate shells the plications are commonly simple, that is, they do not multiply in number during growth. Costate shells present a greater variety in ornamentation. The costae may appear in successive orders during shell growth by implantation either singly or in pairs between older costae; or the increase may take place by longitudinal division of costae. Furthermore, costae may be grouped into fasicles. Imbricate lamellae of growth commonly give rise to more or less regularly spaced spines which may originate either from the furrows between costae, or from the costae themselves. A concentric ornamentation may be produced by fila, that is, regularly spaced concentric ridges. Fila are, in reality, only a special kind of growth varices, recording regular habits of growth, and usually they are consistent in a given species or genus.
The basic types of ornamentation are so few that it is not surprising that some unrelated stocks of pectinoids are similarly ornamented. Even in such instances generally there are characters by which different stocks of Pectinacea can be distinguished.
As a rule, the two valves in the ancient pectinoids are differently ornamented. In such instances, the ornamentation is somewhat subdued in the lower valve, but it may be also quite different in other respects. Much confusion has arisen in the past through failure to realize that the two valves of the same pectinoid individual may be, and generally are, very unlike. The problem of correlating two unlike valves of a species is not so difficult as might be supposed. Although the right valves are, as a rule, much scarcer than the left ones because of their greater fragility, it is nevertheless possible, with a few exceptions, to obtain some specimens of the right valve in any good collection of Paleozoic pectinoids. In my own field experience I have never found right valves without also discovering left valves at the same place, because the left valves are for some reason more abundant than right ones. If there are two sets of complementary valves in a collection from a given horizon and locality, the reasonable assumption is that they belong to a single species unless there is convincing evidence to the contrary.
The fact that the pectinoid invariably lies on one valve when at rest, has tended undoubtedly to modify the ornamentation of the lower valve. Such an idea, however, is not favored by observation of a number of instances in which the ornamentation of the two valves is very nearly alike.
To Davenport (1900, p. 869) belongs the discovery in his brilliant biometric studies, that the ornamentation of the lower valve in modern Pectinidae is more conservative than that of the upper or left valve:
We may conclude that the right valve is the more conservative, or responds less to varying environmental conditions. This small variability of the lower valve is in accord with the fact that the young shell of Pecten is larger and better preserved on the right valve than on the left. Again in P. squamosus, in which the scales are becoming obsolete in the adult, they are found at a later stage on the right valve than on the left. In other cases the grooves of the left valve divide and become ornamented while the right valve remains simple. Here, then, the index of variability is an index of phylogenetic changeableness.
He found also that "the anterior, or notched ear is the more constant; it may be regarded as a generic character. The posterior ear may be regarded as a specific character." (1903a, p. 135). This statement originated from statistical studies in which he found the coefficient of variation to be 17 percent higher in the posterior than in the anterior ear of Aequipecten irradians. Certainly it is true in many types of Paleozoic Pectinacea that the right valves in two species cannot be distinguished. There is also less variability in the ornamentation of the anterior auricles than in the posterior ones. I have found that the number of costae in the anterior auricle of the left valve is much more constant in a suite of specimens than costae on other parts of the same valve. Consequently, this character has proved to be a valuable one for discriminating species.
The importance of surficial ornamentation in the taxonomy of the Paleozoic pectinoids has not been appreciated, in spite of the fact that ornamentation has long been employed in genetic classifications of brachiopods, cephalopods, gastropods, and even other pelecypod groups.
As shown by the ontogeny of an individual, the development of adult ornamentation in the pectinoid shell generally involves a series of changes, correlated with changes in form. If the principle of recapitulation is applicable in pelecypods, and I believe that it is, each fundamental type of ornamentation observed in an adult pectinoid shell represents the culmination of a series of evolutionary modifications. Although I am not prepared to argue that ornamentation in pectinoids is strictly nonadaptive, it appears that these shell features are less readily influenced by external environment than some others. Consequently, I have been impelled to set aside as separate genera, shells having certain fundamental and distinctive types of ornamentation.
Musculature
The muscle system in various genera of modern Pteriidae appears to be fairly uniform, and distinct from that of the Pectinidae. The Paleozoic pectinoids in this respect, as in some others, embody features of both Pteriidae and Pectinidae. The most striking distinction in musculature between the two modern groups lies in modifications of the pedal muscles and pallial muscle system.
In typical Pteriidae, such as Pinctada and Pteria, the shell bears impressions of one adductor, one pair of retractors for the foot, two pairs of small pedal levator muscles (superior retractors), and a series of discontinuous pits marking the insertion centers of fanlike muscles of the orbicular or pallial system. [Note: Pinctada and Meleagrina are synonymous names.]
In the adult shell there is but one adductor muscle, morphologically equivalent to the posterior one of the primitive dimyarian shell. This adductor is located just back of the center of each valve. There are two distinct parts of the muscle—a narrow tendonous strip, forming the posterior border, and a thick, massive part, making up the remainder.
The chief retractor muscles of the foot are two in number, symmetrically disposed in reference to the two valves. They originate in the walls of the byssal gland of the foot, and then diverging, pass backwards in the form of a V to be inserted, one in the right valve, the other in the left at the anterodorsal part of the adductor impression. Generally, the retractor part of the impression cannot be distinguished from the remainder of the real adductor impression, the posterior edge of the retractor blending indistinguishably with the anterior edge of the adductor "scar."
There are four levator muscles of the foot, two anterior and two posterior. Both muscles of the anterior pair have their insertion at the innermost point of the umbonal recess, directly dorsal to the mouth region. From this place the fibers pass downward at right angles to the hinge, on either side of the mouth spreading laterally, fanlike, until they join the mass of the foot. The left anterior levator is the stronger of the two. By contraction of this muscle, the foot is drawn over to the left side, which is its normal position when in a state of rest. An explanation of this asymmetry is seen in the fact that the left valve is more convex, and, therefore, more spacious than the right, and so the foot is more readily accommodated on the left side.
The posterior levators are short, weak muscles. which originate high up in the fibers of the anterior levators at the level of the mouth. From their origin they extend backward and upward to an insertion behind and slightly below the impression of the anterior levators.
The orbicular system of the mantle in the Pteriidae consists of a series of fan-shaped retractor muscles radiating outward to the mantle edge from a number of insertion centers at the pallial line. The purpose of the orbicular system is the retraction of the mantle edge from the shell margin preparatory to the closing of the valves.
Figure 1—Musculature in modern Pecten islandicus, a typical representative of the Pectinidae, and Pinctada vulgaris, a typical species of the Pteriidae. 1a-b, Pecten islandicus, X 5/8, right and left valves, respectively; note that the musculature is not identical in the two valves. 2, Interior of right valve in Pinctada vulgaris, X 1; note the discontinuous pallial line, and the fact that the pallial line, unlike Pecten, is open dorsally, that is, U-shaped; the musculature in the two valves of Pinctada is almost identical. a, b, Impressions of pedal levator muscles; c, pallial line; d, pedal retractor impression; e, f, adductor impression; g, gill suspensory, generally visible only on the right valve of Pecten, although the gills are attached to both valves of Pecten and Pinctada; h, superior suspensory for gills; i, impression of uncertain significance, possibly attachment for auricle of heart; k, impressions for circular pallial muscle.
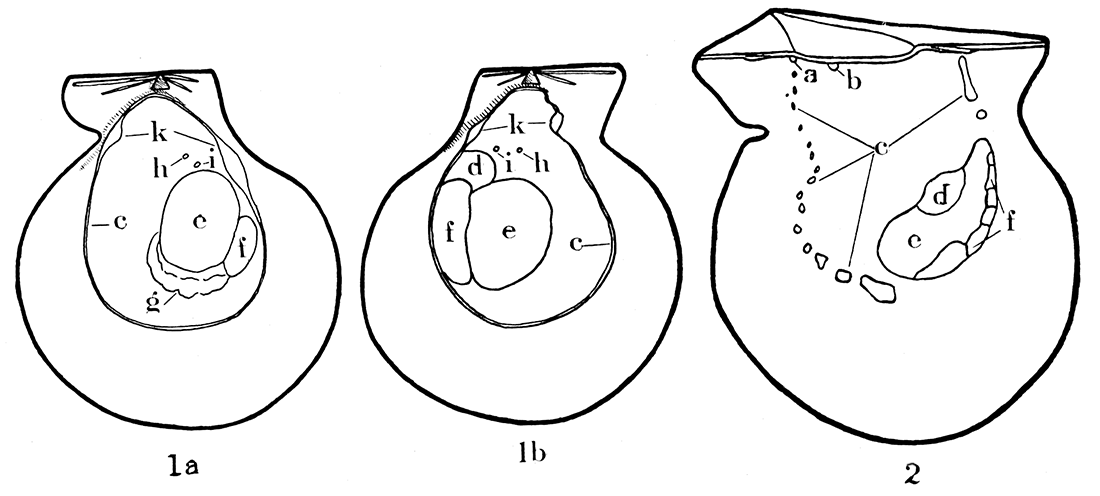
It will be seen that the shell musculature in the Pectinidae is considerably different from that outlined above (see fig. 1a, b). The adductor impression is large and located just behind the center of either valve. This muscle corresponds to the posterior one in dimyarian pelecypods. In very young Pectinidae, as in other monomyarian forms, an anterior adductor muscle is present which for a time is the only functional one, but the posterior muscle soon makes its appearance and the anterior muscle is lost. In adult Pectinidae, the adductor has a greater area of attachment on the left, the upper, valve than on the right. The muscle impression is divided and crescent-shaped. The physiology of the two parts of the adductor is quite different. The large anterior part may be entirely severed in Placopecten tenuicosiatus (Drew 1906, p. 31), and the posterior will close the shell with only slightly less vigor; but if the smaller posterior part is severed and the anterior portion not injured, the animal cannot close its shell.
There are no levator muscles and there is but one pedal retractor muscle. It is attached to the left valve of the shell along the dorsal border of the adductor muscle. The impression of the retractor cannot be distinguished from the scar of the adductor muscle in some forms, but in Chlamys there is a dorsal extension from the adductor impression, corresponding to the pedal retractor. The retractor muscle extends along the dorsal border of the foot and is about equally in evidence on its right and left sides. It extends from the foot over to the left side and is attached to the left valve. The right retractor has been lost, apparently during the phylogenetic development of the group.
The mantle is attached to each valve along a continuous pallial line that is farther removed from the shell margin than in Pteriidae. The muscles that are attached at the pallial line radiate toward the free edge of the mantle, but they are not gathered into insertion centers as is the case in the Pteriidae. The Pectinidae differ in another respect in that the mantle is also provided with circular muscle bands that are collected into large bundles near the hinge line and attached to the shell. The impressions on the shell caused by the attachment of these circular pallial muscles are commonly more distinct posteriorly than anteriorly. The mantle is attached to the shell continuously below the hinge and on both sides of the liver, whereas in the Pteriidae there is no attachment of the mantle between the extremities of the pallial line.
There are perhaps two factors that might have operated in establishing the pallial line in Pectinidae so far from the shell margin, (1) the active habits, and (2) specializations of the mantle margin. Obviously a high development of the orbicular system is essential in this group to insure a quick withdrawal of the mantle from the margin. An increase in the distance from the shell margin to the pallial line not only would permit a development of longer and more effective muscle strands, but also would produce a greater shell buoyancy compatible with rapid locomotion. Modern Pectinidae have extraordinary specializations of the mantle margin which have resulted in an unusually thick edge. The surprisingly complicated eyes and tactile organs of the thickened margin of the mantle require a distant withdrawal before the valves can be closed safely. According to Drew (1906, p. 12):
In specimens (of Placopecten tenuicostatus) that have been disturbed so the shell valves are closed together, the margins of the mantle lobes are drawn far back into the shell so there may be a strip three quarters of an inch or more of the inner border of each shell valve that. is left uncovered. This retraction of the mantle is necessary in order that the thickened and highly modified margins of the mantle may not be injured by the closing of the shell.
In general, the pallial line in Paleozoic pectinoids is relatively closer to the margin than in modern Pectinidae and more nearly comparable to the Pteriidae in this respect. This fact suggests that the sense organs and habits of the Paleozoic pectinoids might have been more like those of modern Pteriidae, than of the Pectinidae. The mantle margin in Pteriidae, although somewhat thickened, bears only short tentacles.
In modern Pectinidae there is generally an indistinct line beneath the adductor impression that marks the attachment of the suspensory muscles of the gills. Similar, and presumably homologous impressions, occur in some Paleozoic forms.
In Chlamys islandica there are two small impressions in each valve just above the adductor system. It appeared to me, upon dissecting preserved specimens of this species, that the inner pair of gill lamellae is attached to the shell at one pair of the small impressions. The significance of the other pair of impressions was not so evident. It seems, however, that a connection extends from each auricle of the heart to the two valves at these impressions.
Figure 2—Musculature in three types of Pennsylvanian pectinoids.
- Aviculopecten exemplarius Newell, n. sp., camera lucida drawing of an internal mold of left valve, X 4.
- Camera lucida drawing of the interior of a right valve of the same species, X 4. Note particularly the pterioid features of the U-shaped pallial line, impressions of levator muscles, and existence of a pedal retractor in both valves; the pallial line is not disconnected, however, being in this respect like modern Pectinidae.
- Pernopecten clypeatus Newell, n. sp., camera lucida drawing, X 2, of the internal mold of a left valve. Note the peculiar pallial line, continuous dorsally, as in modern Pecten or Amussium, but giving way to a number of radial furrows ventrally, as though the pallial system had a marked development of radial muscles. The lack of apparent pedal retractors, like in modern Amussium, is correlative with the loss of a byssal notch and atrophy of the foot at the adult stage.
- Camera lucida drawing of internal mold of a right valve of Pernopecten clypeatus. The muscle systems are like those of the opposite valve, except for the addition of a distinct scar made by gill suspensories.
- Pseudomonotis equistriata Beede, camera lucida drawing of internal mold of left valve, X 4. This form has about the same musculature as Aviculopecten except for the absence of pedal retractor scars, fully in accord with the sessile habit of Pseudomonotis, consequent loss of the byssal notch, and atrophy of the foot at maturity.
- Dorsal view of same specimen shown in 5, X 6. a, b, impressions of pedal levator muscles; c, pallial line; d, impressions of pedal retractor; e, f, adductor impressions; g, gill suspensory; h, superior suspensory for gills.
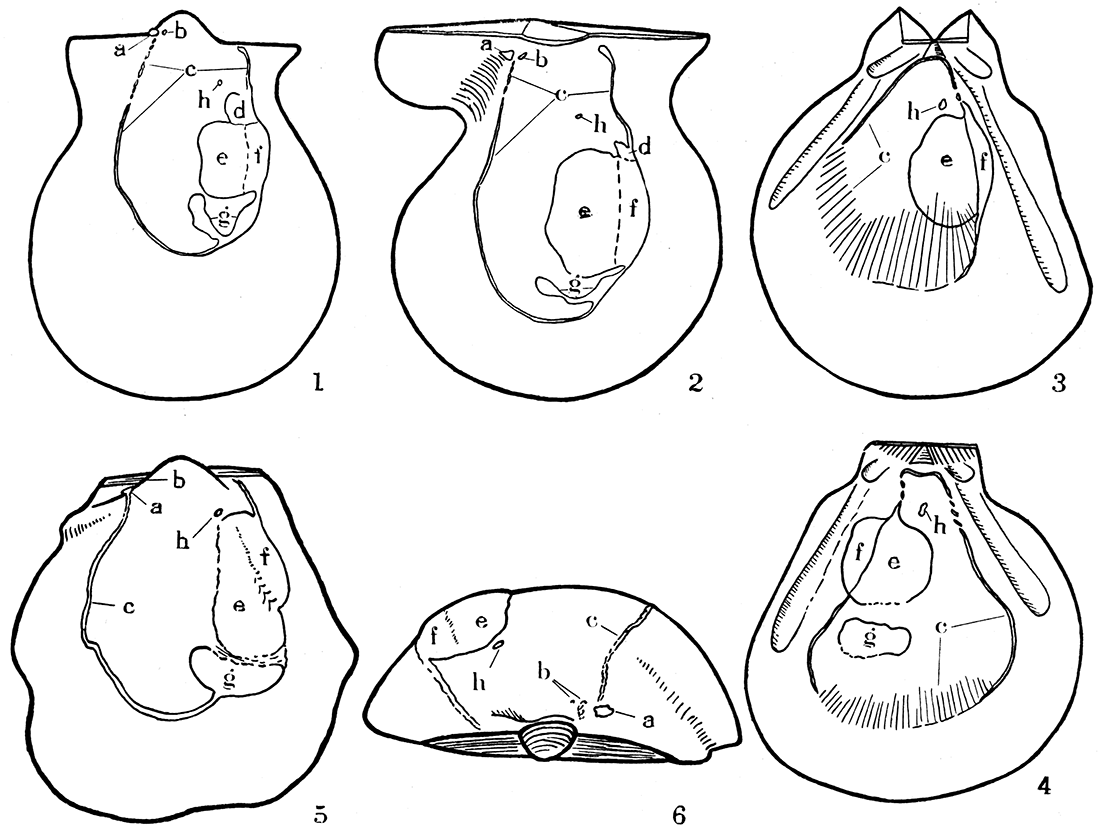
The muscle system in Aviculopectinidae does not correspond exactly to that of either the Pteriidae or Pectinidae (see fig. 2, Nos. 1, 2, and 5, 6). The pallial line is continuous except a short portion near the anterodorsal extremity of the line where, at least in some individuals, the line is broken into obscure pits, indicating a bundling of the fibers in insertion centers. This differentiation of an anterior part of the orbicular system takes on added interest when we recall that anterodorsal shell features appear to be more conservative than other parts of the shell (see "Ornamentation").
In Aviculopectinidae that have been investigated there are three muscle pits along the hinge line. Two of these are within the umbonal cavity and correspond in appearance and position to the levator impressions of the Pteriidae. The other impression is intermediate between the umbonal cavity and the posterior termination of the hinge, located at the end of the pallial line.
In Aviculopecten muscle impressions occur in both valves at the dorsal extremity of the adductor impressions, appearing to occupy the position of the retractor muscles of modern Pectinidae and Pteriidae. The fact that the retractor impression in the left valve is larger than in the right, is in accordance with the obsolete condition of the right retractor in modern Pecten. Pseudomonotis and Pernopecten, on the other hand, appear to have had no retractors, or at least the retractors were degenerate. In both of these genera the byssal notch is obsolete in adults, indicating a loss of the foot at maturity, and therefore a degeneration of the pedal retractors. A similar condition is seen in modern Amussium, which has a byssal notch and an active foot early in the ontogeny, but at maturity the foot, pedal muscles, and byssal notch are lost.
Although the characters of musculature are useful supplementary criteria for differentiation of groups of the ancient pectinoids, there are many puzzling questions that I cannot at present answer. For example, I suspect, but cannot yet demonstrate, that the modern Pteriidae are not closely related to many of the "pterioids" of the Paleozoic. In fact, it appears that the Paleozoic pterioids more closely resemble ancient pectinoids than modern Pteriidae. The fact that some of the aviculopectens possess musculature incorporating features of both modern Pectinidae and Pteriidae is not intelligible until we can determine the nature of the musculature in the Paleozoic pterioids. Obviously the Paleozoic pectinoids were not derived from modern kinds of Pteriidae, and it appears that most, if not all, of the Paleozoic pterioids should be placed in families other than the Pteriidae.
Shell Structure
The microscopic structure of the molluscan shell has been poorly understood, and the recent treatise in English on the subject by Bøggild (1930) should be enthusiastically received by students of mollusks. Although Bøggild's biologic treatment is not entirely satisfactory, his work will prove a valuable tool in the hands of systematists.
There are several distinctive structural elements that may compose a pelecypod shell. The particular distribution of these elements, as pointed out by Bøggild, is constant in some taxonomic groups, in other groups, variable. Consequently, the value of shell structure in classification of pelecypods has to be demonstrated separately for each group.
Contrary to the belief of Bøggild and other workers, the preservation of original shell microstructure in Paleozoic mollusks is not a rare phenomenon—at least it is not rare in collections from the Pennsylvanian rocks. The condition of preservation can be determined readily by examining the shells under some liquid, such as xylol. In some instances the distribution of the shell elements can be studied satisfactorily without the need for thin sections.
The pelecypod shell may possess three parts. These are named, in order from the inner to the outer surface: hypostracum, ostracum, and periostracum. The hypostracum is a relatively thin layer of prismatic or fibrous aragonite, secreted by special glands at the muscle insertions. Commonly the hypostracum is negligible except at the insertions, or "scars," of the adductor muscles. Because the hypostracum is secreted only by the muscles, addition to this layer takes place in isolated areas that are bordered laterally by a different shell substance. As the body of the pelecypod migrates ventrally in the shell, due to increasing size, the muscles migrate, leaving abandoned areas of insertion, or "muscle tracks," imbedded under later deposits of shell material. Cross-sections of a shell show that each hypostracum layer (one for each muscle insertion) is a triangular layer having an acute apex directed toward the beaks of the shell, buried by later shell addition except at the last position of the muscle. In some forms, the hypostracum is exceedingly thin and easily overlooked; in some others, particularly large adults or gerontic individuals, the hypostracum is relatively thick.
The ostracum includes, with the exception of the hypostracum, all of the calcareous shell. This part is commonly composed of more than one layer of either calcite, aragonite, or combination of the two. In the majority of the Paleozoic forms, the ostracum consists of two layers, an outer thin one (outer ostracum ), of either prismatic or homogeneous calcite, and an inner one (inner ostracum), either nacreous or crossed lamellar. A foliated structure characterizes the entire ostracum in modern Pectinidae, but this shell is probably homologous with only the inner ostracum of the Paleozoic shells.
Jackson (1890, pp 343, 348-350) has shown that the juvenile shell has a prismatic outer ostracum in the right valve of modern Pectinidae, whereas the adult shell, except perhaps in a few deep sea amussiums, does not show prismatic structure in either valve. Apparently in these the outer ostracum is lacking, the valve being constructed of foliaceous calcite inner ostracum and an obscure fibrous hypostracum. In several, and probably all of the genera of Aviculopectininae, the outer ostracum of the right valve is prismatic at all observed growth stages, whereas the outer ostracum of the left valve is homogeneous calcite. This restriction of the prismatic structure to the right valve is clearly demonstrated in all of the Pennsylvanian species of Limipecten from America, and also in every well-preserved Aviculopecten that I have examined, except Aviculopecten mazonensis Worthen, which has a prismatic outer ostracum on each valve. In modern Pteriidae the outer ostracum in both valves is prismatic throughout all but the earliest growth stages.
The nacreous structure is characterized by thin, exceedingly uniform lamellae of aragonite, all of the same thickness, separated by equally thin leaves of some organic SUbstance, probably conchiolin. The thickness of each lamella is slightly less than 1 micron. The lamellae are always parallel to the lines of shell growth and never irregular like the calcite lamellae in the oyster or the foliaceous structure in modern Pectinidae. In the older fossils the organic lamellae are, of course, lacking, but the characteristic pearly luster is preserved in some cases. Nacreous structure is not found outside the mollusks and should not be confused with the pearly sheen shown on fresh fractures of some brachiopod shells. Nacreous structure is well exemplified by the pearly part of the shell in Nautilus, Unio, Pteria, and Pinctada.
Crossed-lamellar structure (see Bøggild, 1930, p. 251) is found in all classes of Mollusca except the cephalopods, and is found in no other phylum. This structure generally occurs in aragonite, but is also found in calcite. It is made up of two elements, larger lamellae of the first order, and smaller ones, called lamellae of the second order. Only the first order lamellae, of which three are shown in figure 4 (lower), are readily visible at low magnification. They are minute lath-shaped pieces, arranged either with the long axis parallel to growth lines (concentric crossed-lamellar structure), or radiating more or less regularly from the beak (radial crossed-lamellar structure). The intermediate dimension of the first order lamellae is either normal to the shell surface or slightly inclined to it. The short axis is parallel, or nearly so, to the surface of the shell. These lamellae are built up of smaller elements, lamellae of the second order, which are oriented normally to the broad faces of the first order lamellae, but form with their edge an angle of 41 degrees. In adjoining first order lamellae the second order lamellae are inclined in opposite directions, producing a characteristic crossing.
Figure 3—Diagrammatic cross-section through a Paleozoic pectinoid showing the three fundamental shell constituents. a, Outer ostracum, composed of calcite; b, hypostracum, composed of aragonite; c, inner ostracum, composed of aragonite.

The lamellae of the first order produce a characteristic pattern when viewed in planes parallel to the shell surfaces. The lamellae usually branch and wedge out in the direction of their long axis and are replaced by others. The boundaries may be fairly rectilinear and parallel, or very irregular. In a type characteristic of many pelecypods the lamellae are grown together in a peculiar way to form a network of rhomboidal figures.
The breadth of a single lamella may equal or be less than the entire thickness of the crossed-lamellar layer of the shell, and its length, although commonly small, may reach several millimeters. As viewed in cross-sections the lamellae may be normal to the shell surfaces, oblique, curved, or have nearly a zigzag form.
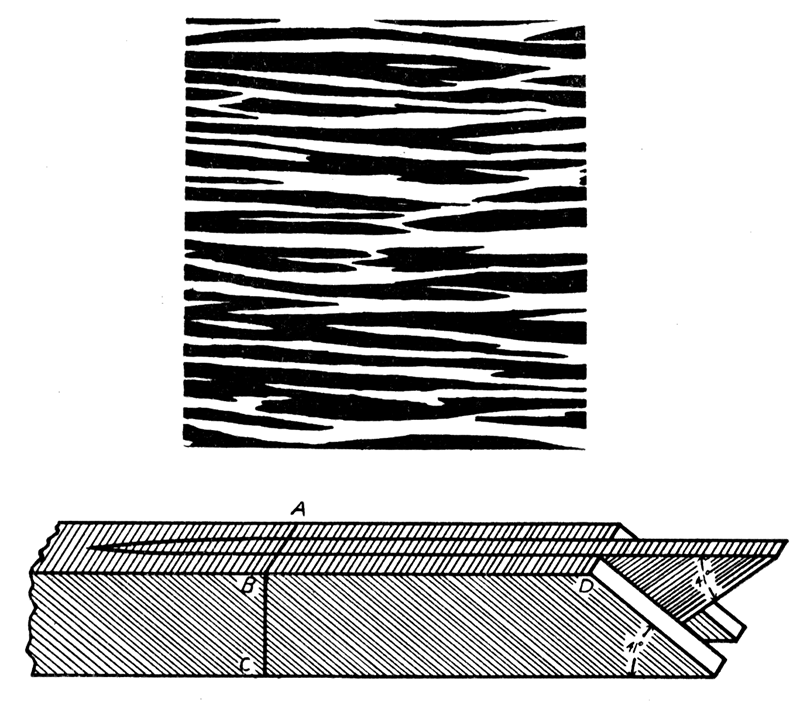
Figure 4—Diagrams illustrating crossed-lamellar structure. Upper, a section of shell, about X 90, cut parallel to shell surface, showing edges (white and black) of branching and wedging first-order lamellae. Lower, three first-order lamellae greatly magnified, composed of second-order lamellae which are inclined differently in alternate lamellae of the first order; the surface, ABD, corresponds to a small part of the surface represented in the diagram above; BC may equal the entire thickness of the crossed lamellar layer as in Aviculopecten, or the layer may be built of successive tiers of first-order lamellae as in Arca. [Image scaled and magnification adjusted for web presentation.]
Nacreous and crossed-lamellar structure occur in modern Pteriidae, but the latter is extremely rare. So far as I know, both are lacking in modern Pectinacea. In the Aviculopectinidae both kinds of structure are found to characterize the inner ostracum. The two kinds of structure are not found together in the same shell, however, and this rule appears to hold for modern shells. In the Pteriidae, as in the Aviculopectinidae, the outer ostracum is composed of calcite, whereas the inner is aragonite. There may be exceptions to this rule, of course, but it is a useful generalization. There are several reasons for believing that the inner ostracum in the Aviculopectinidae was made of aragonite. In many of the aviculopectens only the outer ostracum is preserved, suggesting that the inner ostracum was made of the less stable aragonite. If both layers were composed of the same substance, there would be no differential preservation. In other specimens the outer ostracum of the right valve retains the original prismatic structure, whereas the inner ostracum has been altered to coarsely crystalline calcite. Aragonite alters readily to calcite, and because of the notable differences in density of the two, the original microscopic structures are commonly destroyed completely when aragonite undergoes this change. A direct test by Meigen's method of staining aragonite showed that the inner ostracum of some exceptionally well-preserved specimens of Limipecten has not been altered from the original aragonite.
A very interesting outcome of my examination of shell microstructure in the Paleozoic pectinoids has been the discovery that easily recognizable and consistent differences in shell structure exist between tribes that are also separable on characters of form and ornamentation.
Ligament
The ligament has been badly neglected by students of pelecypods and its usefulness in classification certainly has not generally been appreciated. Few extensive studies on the pelecypod ligament have been published. That of Reis (1902) is one of the most outstanding, but it leaves much to be desired.
Mr. F. Stearns MacNeil, of the United States Geological Survey, who is now engaged in comprehensive studies of pelecypod ligaments, is convinced of the importance of the ligament in working out the phylogeny of various pelecypod groups. I have had the pleasure of an extended correspondence with Mr. MacNeil concerning various problems of the pelecypod ligament, and our mutual cooperation has thus far proved very fruitful.
In many modern pelecypods the ligament is composed of two distinct parts that are structurally and mechanically unlike. In some kinds of shells the ligament is truly a complex mechanism composed of different parts. These separate elements commonly are inserted along the dorsal margin of the shell in characteristic arrangements. A reconnaissance of pelecypod ligaments in general suggests that there is a limited number of types of ligament, and that certain distinctive types appear, and have appeared in the geologic past, in apparently unrelated stocks of pelecypods. It would seem that the evolution of the pelecypod ligament has at least partly been in response to mechanical stimuli, thereby permitting a given ligament type to appear in widely separate pelecypod stocks. Nevertheless, it appears probable that there is a definite sequence of evolutionary stages through which some types of ligament have passed in order to have acquired their observed degree of complexity.
The insertion of the ligament structures into the ligament area of the shell invariably has an arrangement characteristic for a given tribe of pelecypods, and the nature of the ligament can be inferred by an inspection of the ligament area of a shell from which organic ligament structures have been stripped. The discovery of Paleozoic shells having ligament areas similar to or identical with ligament areas of modern shells invites conclusions regarding the character of the ligaments in certain Paleozoic forms.
Of the two differentiated types of material in the pelecypod ligament, one is composed of fibrous, calcified conchiolin, which is elastic to compressional stresses, but tears or parts readily when subjected to tensional stresses. Naturally this structure is a compressional ligament and the functional part is invariably at or below the hinge axis. If, during growth, the hinge axis migrates ventrally, as is normally the case, part of the compressional ligament may come to lie above, or dorsal to the actual axis of movement. The material above the hinge axis is subjected to tension when the animal closes the two valves, and being weak to tensional stresses, breaks and becomes nonfunctional. These relationships were observed by me in specimens of modern Arca, Pteria, and Pinctada. The calcareous material of this fibrous ligament appears to occur in the form of fibers or "spicules" imbedded in a matrix of conchiolin. The fibers lie at right angles to the hinge axis and parallel to the growing surface (Arca, Pinctada), or at right angles to it (Yoldia).
Primitively the "resilium" or internal ligament of some pelecypods, such as Yoldia and Nucula, is composed of the calcified fibrous ligament, but the main and only functional part of the internal ligament in Pecten appears not to be homologous to the fibrous ligament. As regards the nature of the Pecten ligament I am apparently at variance with Reis (1902), who mentions the presence of very fine calcareous spicules; and I am inclined to differ with MacNeil, who regards the vertical cleavage seen in the central part of dried specimens of Pecten resilium as a heritage of a former calcareous condition.
The second kind of ligament structure is a noncalcareous lamellar layer of conchiolin which is highly elastic to tensional, compressional, and torsional stresses. In most pelecypods this material makes up the "ligament proper" of authors, as contrasted to the "resilium." In Pecten and its relatives, however, the central part of the resilium is apparently composed of this noncalcareous, lamellar conchiolin. The lamellar ligament was inappropriately called by Reis the inelastic ligament. In many kinds of pelecypods the axis of rotation of the valves is actually contained within the substance of the lamellar ligament, so that the stresses within this part of the ligament are torsional.
For the present purpose, the material that is normally calcareous and compressional in function will be termed the fibrous ligament. The noncalcareous structure that is elastic to all kinds of stresses will be called the lamellar ligament. The fibrous and lamellar elements form the ligament system.
Kinds of ligament areas—Four principal kinds of ligament areas are found in Paleozoic pectinoids. Each of these types was developed at various times in distantly related or unrelated groups of pelecypods and is represented in modern forms. It is desirable to examine the four types of ligaments as shown in living pelecypods.
Ligament of Type I—Arca. The ligament area of such Devonian forms as Pterinopecten and Lyriopecten is identical with that of the modern Arca. A distinct, flat cardinal area extends from the hinge line to the beak of each valve, comparable in appearance to the cardinal areas of the brachiopod, Spirifer. There are no resilifers, but the area of each valve is provided with several chevron-shaped grooves extending across the area with their apices just below the beak. The significance of these chevron grooves has not been understood generally by paleontologists. Their true nature may be comprehended by an examination of the living Arca.
The ligament in modern Arca transversa Say, from the New England coast, is a complicated structure, having two elements. The cardinal area of each valve is covered by a thin sheet of fibrous conchiolin and the two valves are joined at the hinge line by this material. This fibrous structure is remarkably weak to tensile stresses and would be quite inadequate in itself to hold the valves together in Arca. Indeed, it is not easy to study the structure at the hinge axis in preserved specimens because of the difficulty in keeping it intact. This layer has the physiological and mechanical properties of the "internal" ligament or resilium of such forms as Nucula or Pteria, because the functional part of this layer is compressional, and restricted to a line just under the hinge axis.
The fibrous layer is not continuous over each cardinal area, but is interrupted at the chevron grooves by the insertion of a series of separate parallel sheets, one above another, of ligamentous material quite different in appearance and function from the fibrous ligament. The conchiolin sheets are laminar in structure and are not fibrous. Each sheet of the lamellar ligament is lozenge-shaped. The last formed and smallest occurs at the center of the hinge line and the earlier ones lie at intervals above and parallel to it. Theoretically, all of the later-formed ligament sheets would be hidden from external view by the first-formed one, because new ligament bands make their first appearance only at the center of the hinge line. Growth carries the cardinal axis farther and farther away from the dorsal margin of the valves as the cardinal areas become broader. Since, however, addition to the ligament sheets occurs only at the hinge line where the mantle extends between the hinge teeth to the ligamentary structures, there is no repair of the older parts of the ligament, which eventually become disrupted if shell growth forces the beaks sufficiently far apart. In an adult Arca transversa only the small central sheet is entire and the middle portions of the older sheets have broken away, exposing the inner sheets.
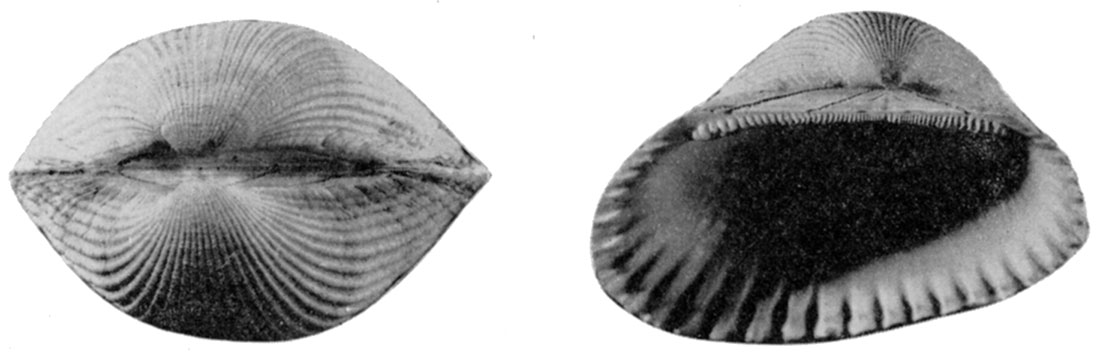
Figure 5—Arca transversa Say. X 2.3. Left, Dorsal view with ligament removed to show the cardinal area; Right, left valve showing the chevron ligament grooves of the cardinal area. [Image scaled and magnification adjusted for web presentation.]
The only points at which an individual ligament sheet grows are at its insertions along the hinge line, because only at these places is the ligament in contact with the mantle. The addition is accomplished by a small group of specialized cells of a dorsal ridge of the mantle. During growth each cluster of ligament cells migrates slowly towards the ends of the hinge and new clusters appear at the approximate center of the hinge line. There can be no lateral addition to broaden these ligament sheets, their lateral extension being accomplished solely through stretching. The ligament sheets have incredible elasticity to the tensile stresses exerted by the adductor muscles. The lamellar material will stretch to an amazing degree before it loses its resiliency and ultimately breaks. It must be kept in mind that the ligament sheets are secreted along a narrow band about a millimeter or less across. In some instances I have observed sheets of lamellar ligament stretched out to a breadth of more than 1 centimeter by the gradual divergence of the shells during growth.
I believe it is commonly assumed by some paleontologists that the hinge teeth in Arca serve as a fulcrum around which the valves rotate in the process of opening and closing. The absurdity of this notion can be quickly demonstrated by sawing a fresh specimen across, transverse to the hinge, and making direct examination of the behavior of the ligament and hinge teeth while the valves are slowly opened and closed. The axis of motion is not at the hinge teeth, but above them; the fibrous ligament and not the teeth serves as a fulcrum, and the axis of rotation of the valves is contained in this structure. The mantle extends between the hinge teeth and upward to a position in contact with the ventral surface of the ligament. When the valves are tightly closed there is ample space between adjoining teeth and sockets for a thin extension of the mantle, and it appears that the teeth and sockets are never in direct contact, but are invariably separated by an exceedingly thin layer of the mantle. The same situation occurs in Yoldia, and probably characterizes pelecypods in general.
The sheets of lamellar ligament in Arca are dark-amber colored and the lamellar structure is delicate and disposed in a horizontal direction. The material is entirely organic, containing apparently no appreciable content of calcium carbonate. In Arca transversa the first formed ligament groove is entirely posterior to the peaks. Whether this has any especial phylogenetic significance I cannot say.
In cross sections (see fig. 6) the fibrous layer appears to be laminated, but this effect is produced by the calcareous spicules which characterize this type of ligament. The fibrous ligament is iridescent through the outer third of the structure. The fibers are secreted parallel to the ventral or growing surface of the ligament and at right angles to the hinge line. That part of the fibrous layer in contact with the mantle is light-amber colored and is not iridescent, and the axis of motion lies within this part. When the cardinal axis migrates below a given part of the fibrous ligament, that part is subjected to tensile stress and soon parts along a line just above the axis. The reaction of the fibers after breaking is greater near the surface where there is more room to expand than next to the shell, and the resulting strain between adjoining fibers produces an iridescence. Addition to the fibrous ligament occurs along a midline at the inner surface between the valves where it is in contact with the mantle. There is an alternating series of lamellar ligament-forming and fibrous ligament-forming cell groups along a dorsal ridge of the mantle, and these sets of cells migrate toward the ends of the cardinal axis as new ones are introduced at the center.
Figure 6—Camera lucida drawings of Arca transversa Say, showing transverse sections through the hinge, back of the beaks. Upper, X 12, showing three elastic ligament sheets and the fibrous ligament sheet that surfaces the cardinal area. Lower, section, X 39, along a-b of the preceding diagram, showing two disconnected elastic ligament sheets inserted in the fibrous ligament; the cross shows the position of the hinge axis; stippled part is the dorsal extension of the mantle which secretes the teeth and ligament. [Images scaled and magnifications adjusted for web presentation.]
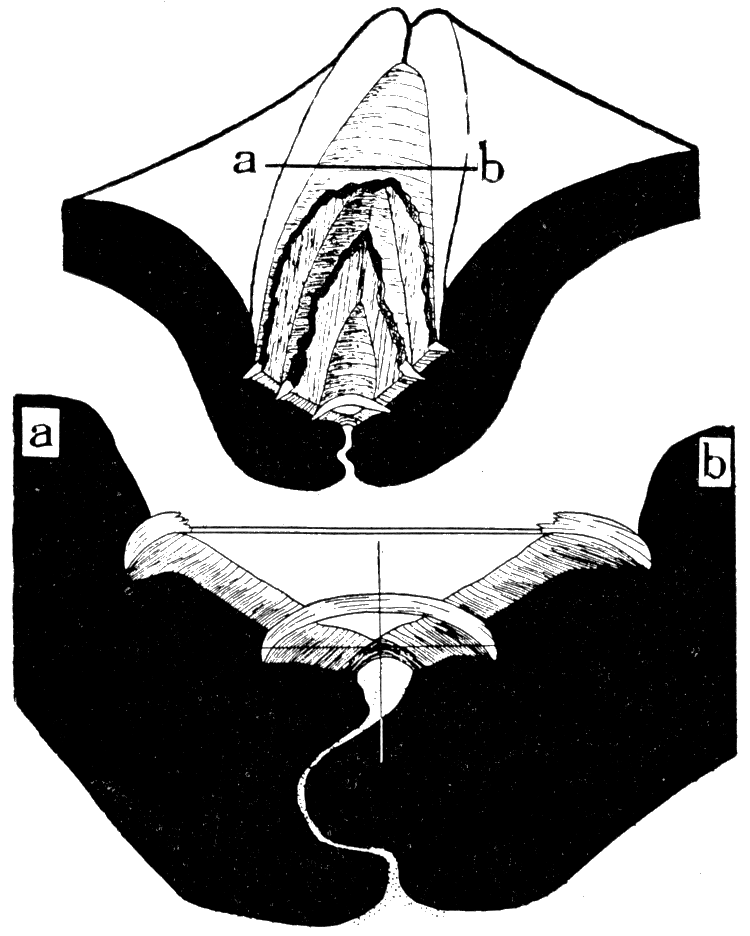
In Arca pexata Say, from Long Island Sound, there is only one sheet of lamellar ligament, therefore, only one chevron groove occurs on each ligament area, occurring at the outer margin of the area. The single ligament sheet correlates with a more simple arrangement of the ligament-forming cells of the dorsal margin of the mantle. The single ligament band is commonly entire in adult specimens because the divergence of the cardinal margins is very slight and well within the stretching limit of the ligament. Here, as in A. transversa, the fibrous ligament serves the function of a fulcrum and the action on this structure is largely compression during the closing of the valves. When the valves are in tight apposition, as in the accompanying drawings, there is no undue pressure on the mantle where it extends between the teeth. In such a ligament system as that of Arca pexata the first-formed structure is the central part of the lamellar band secreted by a cluster of specialized cells at the center of the hinge line. This cluster divides and migrates toward both ends of the hinge as the ligament grows, and a new group of cells for the fibrous ligament appears at the center.
Figure 7—Camera-lucida drawing of a section through the ligament in Arca pexata; upper, X 7.8; lower, X 23.4, showing the single elastic, lamellar ligament band above and the relatively massive fibrous ligament below; cardinal axis indicated by the cross lines; mantle by stippled area. As in the accompanying diagrams the sections were drawn while the valves were tightly closed. [Images scaled and magnifications adjusted for web presentation.]
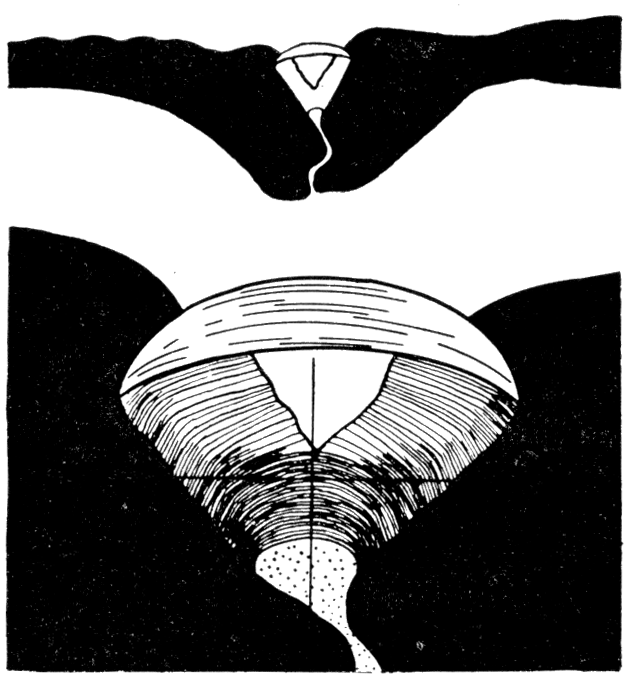
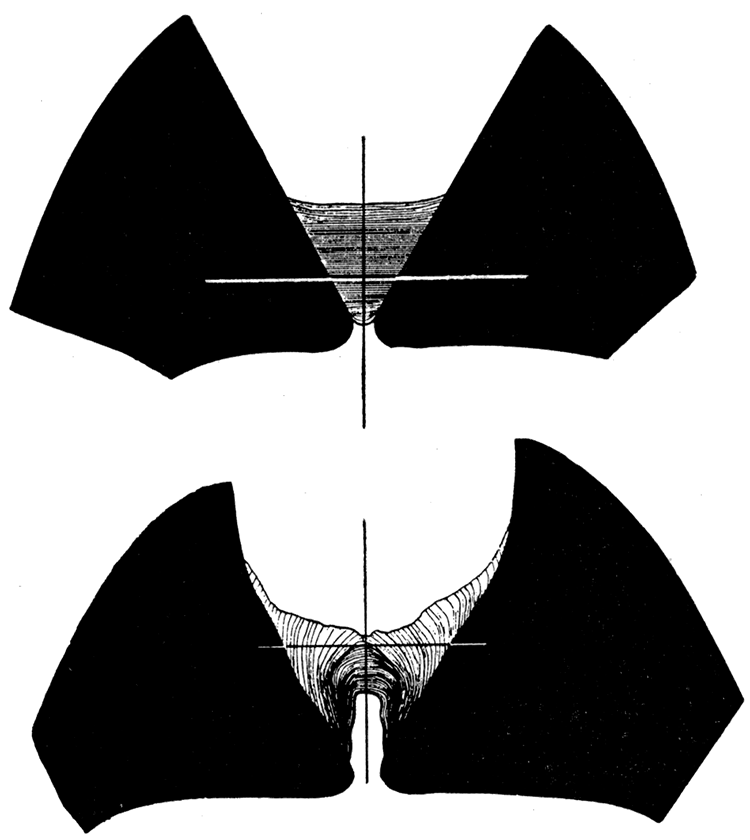
Figure 8—Left. Dorsal view of Pinctada savignyi (Monterosato), X 1.2, with ligament removed, showing diverging cardinal areas, and resilifers. Right. Cardinal view, X 1.2, of Pinctada vulgaris showing the ligament area and large oblique resilifer. [Image scaled and magnification adjusted for web presentation.]

There is no a priori reason for supposing that the type of ligament displayed by Arca is a relatively primitive one, or that it gave rise to other types of ligaments during the phylogeny of various tribes of pelecypods. There is geological evidence, however, that such is the case. Nearly all of the Devonian and Silurian pectinoids, as well as a few of the Carboniferous ones, possess a ligament area identical in plan with that of modern Arca. The assumption seems fair that these ancient shells had a ligament system like that seen in Arca. In the Carboniferous and Permian pectinoids the arcid type of ligament is suppressed overwhelmingly in favor of a ligament like that seen in Pinctada, next described. These Carboniferous and Permian forms are closely like the Devonian forms in characters other than the ligament, and I am disposed to believe that they were derived from the older forms. I regard, then, the type of ligament displayed by Pinctada and Pteria as being a second stage in ligament modification, derived from the arcid type just described.
Ligament of Type 2—Pinctada. The second type of ligament system, found in Limipecten, Aviculopecten, and a host of similar forms characterizes modern Ostreidae, Limidae, Pteriidae, and several other groups. A species of Pinctada (usually called Meleagrina), a pearl oyster of commerce, was selected for the study because the general aspect of the shell as well as the hinge is similar to that of some of the aviculopectens. Pinctada is a typical representative of the modern Pteriidae, and, except for the abbreviated posterior auricles, is very like Pteria.
In Pinctada savignyi (Monterosato), from the Mediterranean region, as in Arca, there are two kinds of ligament structures, but their distribution is quite unlike that found in Arca. A broad, flat cardinal area occurs above the cardinal axis in both valves, diverging upward at about 60 to 70 degrees. At the middle of each area there is a large obliquely triangular fossette, or resilifer, which contains the fibrous ligament.
As in Arca (figs. 7, 9), the fibrous ligament consists of a thin strap like structure which joins the two valves together along the lower margin of the cardinal areas. Transverse sections show that the appearance of the fibrous ligament is similar to that found in Arca. There is an outer iridescent zone of nonfunctional, strained and broken fibers and an inner zone of functional, unbroken layers which lie almost wholly below the axis of movement so that the stresses applied are entirely compressional. As the hinge axis migrates downward during shell growth, the fibers are progressively subjected to tension and are broken. The fibrous ligament effervesces freely in dilute acid until the ligament structure is entirely broken up. I tried to discover the nature of the calcite bodies by crushing a dried specimen and mounting the fragments in Canada balsam. Each of the crushed fragments behaved optically like individual anisotropic crystals. It is obvious, though, that there must be a greater conchiolin than calcareous content in the fibrous ligament. When the material is pulverized individual fibers can be freed. The microscopic structure of the fibrous ligament of pelecypods needs to be thoroughly investigated.
Figure 9—Pinctada savignyi. Upper. A section through the lamellar elastic ligament just back of the resilifer, camera-lucida drawing, X 9. Lower. Section through fibrous compressional ligament, camera-lucida drawing, X 9. Both valves are in tight apposition; the cross indicates the position of the cardinal axis. [Images scaled and magnifications adjusted for web presentation.]
The lamellar ligament (fig. 9, upper) is a horizontally laminated, noncalcareous triangular prism of conchiolin. The axis of movement is near the center of the ligament so that the upper layers are subjected to stretching and the lower layers are probably under slight compression when the valves are closed. The upper layers are wrinkled and exfoliate readily. Instead of breaking when their strength is exceeded, as was the case in Arca, individual lamellae draw away from their attachments at the cardinal areas, and are progressively sloughed off. The new lamellae at the inner surface of the ligament are naturally somewhat thicker than the stretched ones at the top. Except for obscure growth lines on the cardinal area, there is no apparent special provision for the attachment of the lamellar ligament to the shell.
The fibrous ligament rests in two shallow, oblique resilifers, one in each valve. Both lateral borders of the resilifers are inclined backward so that the resilium lies entirely behind the beaks. This peculiar obliquity is apparently brought about by a posterior migration of the dorsal part of the mantle during shell growth. Unlike Arca, there is in Pinctada a progressive increase in size of the cell cluster along the dorsal ridge of the mantle so that there is a gradual increase in the size of the zone of active secretion of the fibrous ligament.
Ligament of Type 3—Perna. The ligament system in Perna is like that of Pinctada or Pteria, except that instead of having only one resilifer on each valve Perna is provided with a series of resilifers which are added successively at the posterior end of the hinge during growth. Jackson (1890, pp. 327-333) has shown that young individuals of Perna have only one resilifer on each valve, and at this stage the shell is closely similar to adult individuals of Pteria. As in Pteria the single resilifer of young Perna lies wholly behind the beaks. The additional resilifers are added during shell growth at the posterior extremity of the hinge. Each resilifer contains fibrous ligament material and the flat areas between are covered by lamellar ligament as in Pteria or Pinctada.Figure 10—Perna ephippium, (modern), after Jackson. Upper. Young individual, right valve; p, prodissoconch; l, single resilifer; c, cardinal, and t, lateral teeth, X 124. Lower. Older specimen with three resilifers, and two lateral teeth, X 33. [Images scaled and magnifications adjusted for web presentation.]
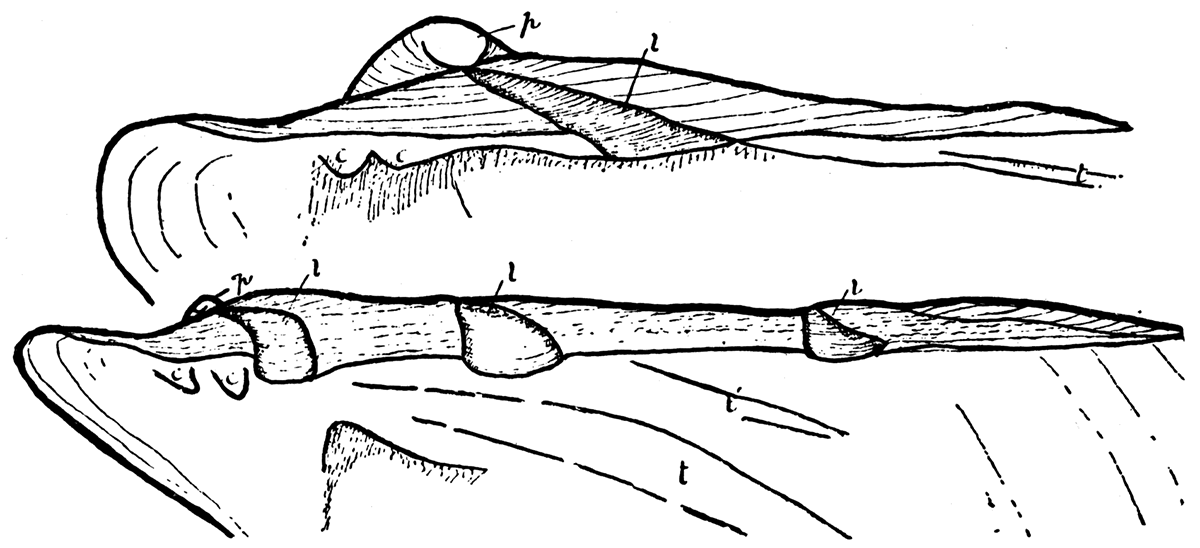
The Carboniferous genus Euchondria. and possibly the Devonian Crenipecten, had a ligament system comparable to that of Perna, with only one important difference. In Euchondria resilifers are added both in front and behind the beaks and the initial resilifer remains almost central along the hinge. Except for the supplementary resilifers in front and behind the central first resilifer, the ligament system in Euchondria is like that in other aviculopectens having but one resilifer. Apparently the hinge of Euchondria was derived from the type (pteriid) that characterized the true aviculopectens.
F. Stearns MacNeil, of the U. S. Geological Survey, a specialist in the study of pelecypod ligaments, has communicated to me that he doubts that the cardinal pits in front and behind the central large resilifers in Euchondria are actually resilifers. If these pits are not resilifers, then they are not homologous to any known structures of modern pelecypods. My reasoning is based on the following observations made on remarkably well-preserved specimens.
- Euchondria possesses triangular cardinal areas and the hinge axis is at the base of the cardinal areas, as in Arca and Perna.
- The central and subsidiary resilifers occur above, dorsal to, the hinge axis.
- Unique, nearly perfect specimens were observed having the two valves in apposition. The cardinal areas diverge and can be viewed externally when the valves are closed.
Obviously the cardinal pits of Euchondria cannot be compared with any known type of dentition because the cardinal areas diverge when the valves are closed and the cardinal structures cannot serve the function of interlocking teeth.
Ligament of Type 4—Pecten. The parts of the ligament system in modern Pectinidae are not. very much like those in the Pteriidae, and only after an inspection of modern Limopsis is it apparent how the pectinid type might have been derived from the pterioid type of ligament. (I am indebted to F. Stearns MacNeil and Hubert Schenck for calling my attention to the Limopsis type of ligament). There is no cardinal area in Pecten, except in gerontic individuals. The areas of attachment of the lamellar ligament and "resilium" are concealed, or "internal." The cardinal axis is located almost at the extreme dorsal margin of each valve and lies within lamellar ligament along the front and rear parts of the hinge. The resilium lies wholly underneath the cardinal axis, almost completely hidden from view. The resilium is attached on both sides in triangular resilifers, but the structure of the resilium is not homologous with the structure of the ligament contained in the resilifers of Pinctada, Pteria, Yoldia, Perna, etc. In these forms the resilifers are occupied only by fibrous ligament, and lamellar ligament does not extend into the resilifers. In Pecten the resilium consists of two unlike structures: (1) a pair of fibrous, tan- or cream-colored, triangular calcareous pieces which fit into the resilifers; and (2) a central mass of reddish-brown, lamellar, noncalcareous conchiolin with the lamellae parallel to the growing surface. The calcareous lateral pieces are silky in appearance, and fibrous. They are quite rigid, although they contain a considerable proportion of conchiolin, and do not aid in opening the valves, nor do they have any other obvious function. The elastic part of the resilium is bell-shaped if sectioned in the plane of the valves. In horizontal section it is transversely subrectangular, that is, flattened from front to back, with rounded corners. Upon drying, the lamellar central mass of the resilium tends to divide into vertical fibers, disposed at right angles to the lamellae.
Figure 11—Pecten (Chlamys) islandicus; (modern). Upper. Dorsal view, X 1.75, showing the tight apposition of the dorsal margins of the valves, effectively hiding the lamellar ligament and the resilium. Lower. Left valve, X 3.5, showing a kind of dentition radiating from the apex of the resilifer; the radial teeth occupy the position of the cardinal area of Pinctada or Aviculopecten. [Image scaled and magnification adjusted for web presentation.]
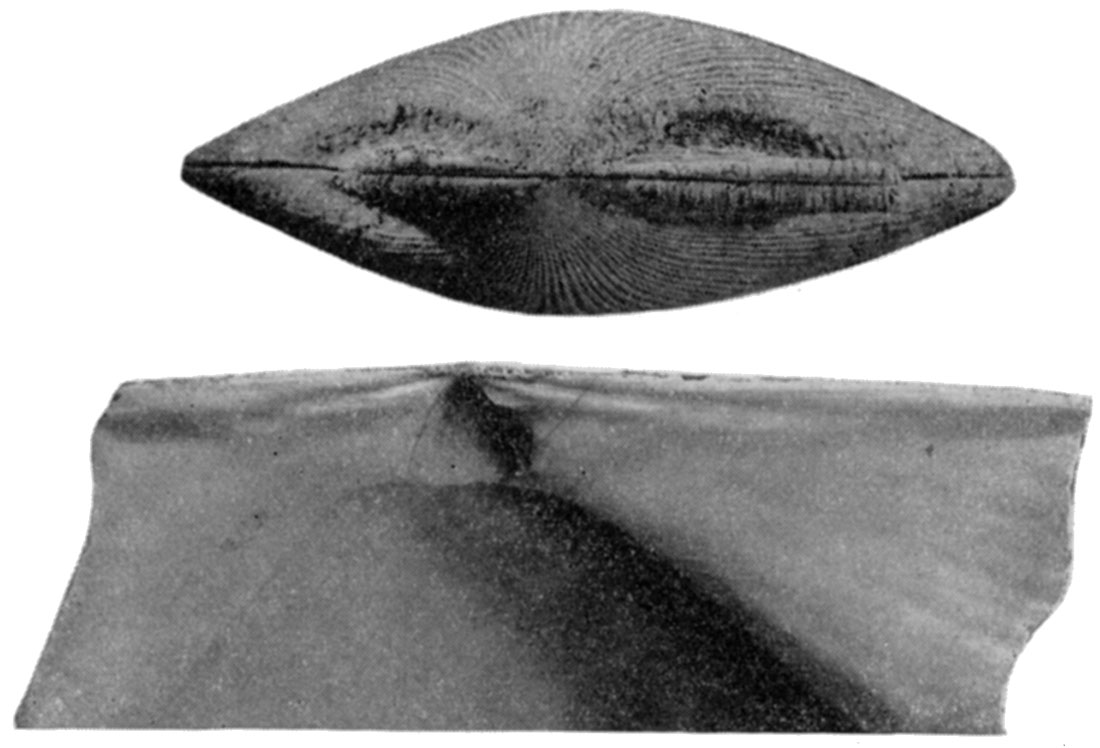
These perpendicular fibers of the dried resilium are not homologous to the fibrous structure of the ligament in the resilifers of Pinctada, or on the cardinal area of Arca. The fibers in the last two are calcareous spicules and are very small, whereas in Pecten the aforementioned fibers are coarse and irregular and contain no calcareous material that I could discern. Furthermore, they are disposed at right angels to the inner or growing surface of the resilium, whereas it will be recalled that in Arca and Pinctada the calcareous spicules are secreted parallel to the growing surface, where the mantle is in contact with the ligament.
A cup-shaped depression in the mantle fits snugly over the ventral globular end of the resilium with long processes covering the front and rear surfaces.
The lamellar ligament, along the hinge margin in front of and behind the resilium, is a light-amber colored structure secreted by a dorsal ridge of the mantle. It is not calcified and is horizontally lamellar. The lateral surfaces of the ligament are nearly parallel so there is no tendency for the older parts to cleave medially, nor is there any regular loss of older lamellae by sloughing as in Pinctada, but parting occurs along one side or the other where the ligament joins the shell. This part of the lamellar ligament outside of the resilifers is very weak, insufficient to hold the valves together if the muscle and resilium are removed. Its weakness is partly compensated for by a kind of dentition common to Pecten, Amussium , and kindred forms. There are narrow ridges on the inner surface of both valves, diverging from the apex of the resilifers, which fit into grooves in the opposite valve.
Figure 12—Pecten (Chlamys) islandicus. Upper. Section through the lamellar ligament behind the beaks, showing how the convex lamellae of the ligament are progressively straightened out as they are subjected to more and more tension above the cardinal axis; the stippled area is the dorsal ridge of the mantle, camera-lucida drawing, X 23. Lower. Section, through resilium, showing the bell-shaped lamellar ligament flanked on both sides by the calcareous vestigial fibrous-ligament, camera-lucida drawing, X 23. The position of the cardinal axis is shown by the cross, in both figures the right valve is on the right. [Images scaled and magnifications adjusted for web presentation.]
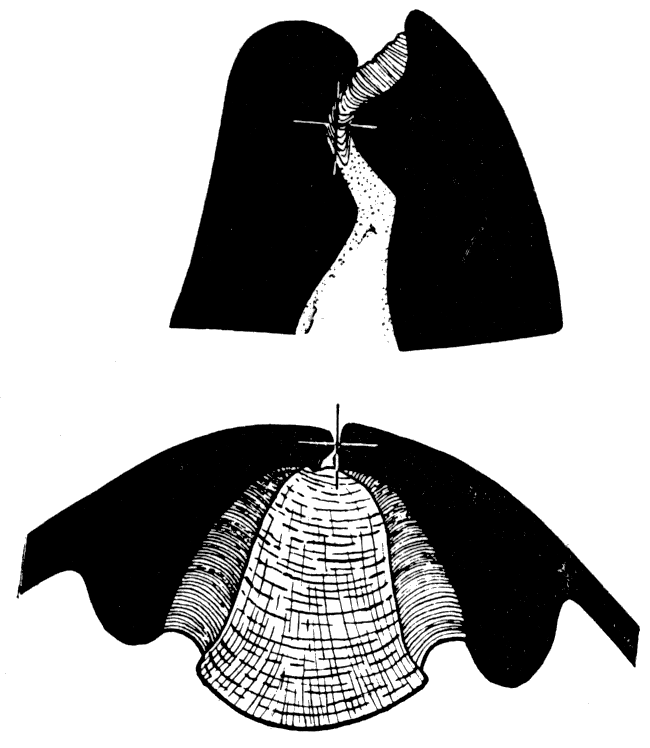
It is likely that the Pecten ligament system was derived from a pteriid one, such as that possessed by many of the aviculopectens, yet there appears to be little direct evidence that such was the case. Among the Paleozoic pectinoids apparently only Pernopecten and its relatives possessed a ligament like that of Pecten.
Evolution of the Pectinid Ligament
In figure 13 I have attempted to illustrate my hypothesis as to how the evolution of certain types of ligament systems might have proceeded. The diagrams show that the postulated sequence of stages is mechanically possible. Each stage (except 5b) can be compared to or is identical with modern or fossil types. I do not hold that the possession of identical ligament systems in different groups of pelecypods implies close relationship. For example, the close resemblance of the ligament system of some Devonian pectinoids with that of modern Arca does not suggest to me that the areas are closely related to the Devonian pectinoids. Nor does the similarity between the ligament in Lima or Ostrea with that of Pteria suggest near relationship, for we know that these genera have had a very different history. The stages of ligament development illustrated in figure 13 are stages in the modification of certain types of ligaments and were certainly developed independently in unrelated groups of pelecypods.
Figure 13—Hypothetical development of pectinid and pteriid types of ligament from the arcid type of ligament. Solid black, except 6, represents lamellar ligament; horizontal ruling, essentially the same, but is thin, relatively unimportant lamellar ligament occurring outside the resilifers; vertical ruling represents fibrous, calcified ligament.
In 1a-5c, inclusive, cardinal areas are developed and the hinge axis (a-a') is located below them, as shown in the end view of a complete shell in 6a. In 5d the hinge axis (a-a') corresponds to the dorsal margin of the shell as 6b, and there are no cardinal areas. Two diagrams, an upper and a lower, are given for each type of ligament. The upper is a plan view of the cardinal area of a right valve (anterior to the left) with ligament structures intact, or (in 5d) a view of the inner surface of the valve retaining the ligament structures. The lower diagram in each case represents a ventral (internal) view of the ligament as though seen between the partly opened valves. This view reflects the arrangement of differentiated clusters of cells on the mantle whose sole function is the secretion of new additions to the ligament. In 1a-2b there obviously is no change in the size of the clusters of ligament-secreting cells during shell growth. Growth in the dorsal margin of the mantle takes place by a curious insertion of new cells at a smgle point along the hinge line, causing previously formed parts of the dorsal margin of the mantle (and also ligament structures in the process of development) to migrate away from the single point of growth. This migration of developing ligament structures, together with the normal addition on the ventral or growing surface of the ligament, produces an oblique arrangement of the parts of the ligament, either in the form of chevrons (1a, 1b, 2a, 2b) or oblique bands (1c). In 3-5d there is no evidence that the parts of the ligament, or the differentiated clusters of ligament-forming cells along the dorsal margin of the mantle migrate during growth toward the poles of the hinge axis except inasmuch as there is a general increase in size of cell clusters during growth. Triangular resilifers, absent in the previous types, occur in 3 to 5d and ordinarily the fibrous ligament is restricted to the resilifers, but in 4, small subsidiary resilifers may be added during growth at each (or only one) end of the hinge line.
1a represents the ligament of juvenile Liopteria, Ptychodesma, and the hypothetical ancestral type; 1b represents adult Liopteria Ptychodesma, etc; 1c represents adult Myalina, characterized by the complete loss of the anterior part of the shell; 2a represents species of modern Arca, juvenile Pseudaviculopecten, and juvenile Pierinopceten; 2b represents species of modern Arca, adult Pseudaviculopecten, Pterinopecten, etc.; it is possible that the symmetrical forms like 2a and 2b were ancestral to the asymmetrical ones like 1a and 1b but the paleontologic record suggests the reverse; 3 represents Aviculopecten, Limipecten, etc., and modern Pinctada and Pteria, the first two differing from the latter two only in being a little more symmetrical; 4 represents Euchondria which differs from modern Perna in having one resilifer much larger than the others, and in the fact that new resilifers are added at both ends of the hinge instead of only at the rear end as in Perna; 5a represents the ligament of modern Limopsis which differs from Pinctada chiefly in the fact that part of the lamellar ligament is incorporated in the resilifer and is partly overlapped by fibrous ligament; 5b is a strictly hypothetical stage intermediate between the limopsid type of ligament and the pectinid type; 5c, gerontic Pecten in which there are cardinal areas; this type of ligament differs from that of Limopsis chiefly in the separation of the fibrous ligament into two nonfunctional vestigial structures; 5d, modern adult Pecten, in which the hinge axis occupies the dorsal margin of the shell; the resilifers are entirely below the hinge and there are no cardinal areas.
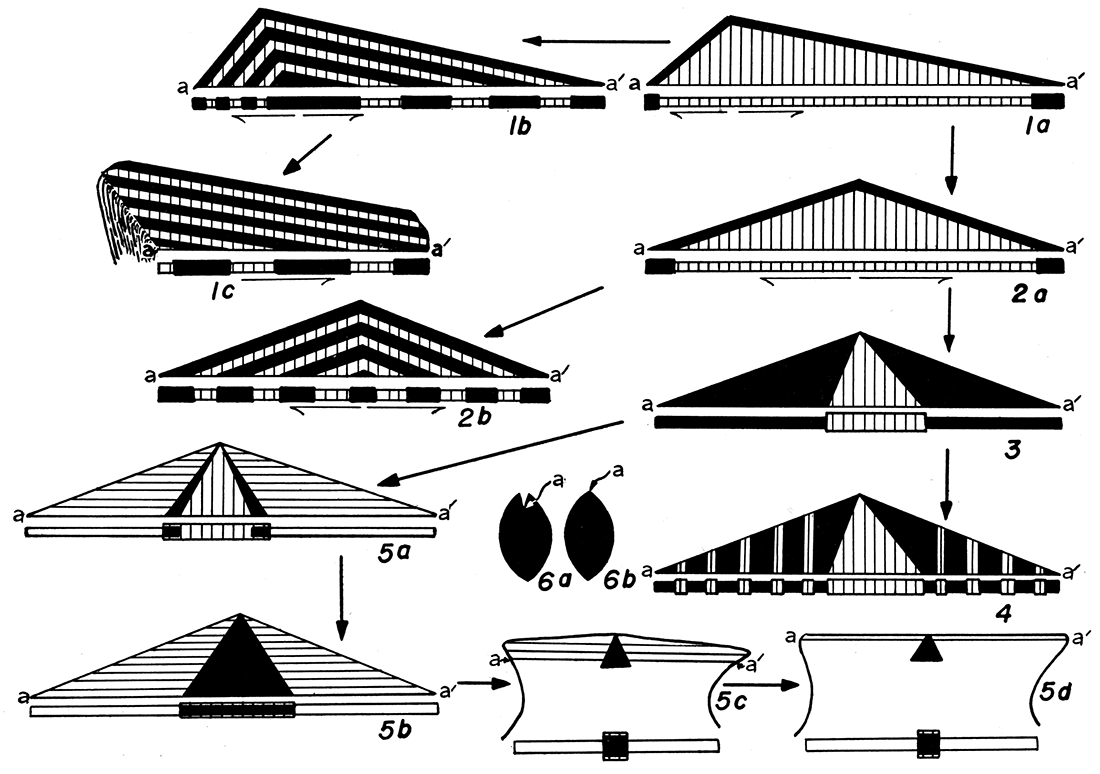
A number of Middle Paleozoic pelecypods, such as Ptychodesma, Posidonia, Liopteria, Palaeopecten, Pseudaviculopecten Newell, n. gen., and Pterinopecten, illustrate the types represented by 1a, 1b, 2a, and 2b (fig. 13). Various modern areas, Glycimeris and others illustrate 2a, and 2b. In 1a-1c the beaks are at or near the front end of the shell and there is a corresponding asymmetry of the insertion grooves for the lamellar ligament bands. Whether or not the asymmetrical type (1a, 1b) preceded the symmetrical type (2a, 2b) cannot be determined. Either condition appears equally possible.
All of the better known Devonian pectinoids are equipped with chevron ligament grooves of the arcid type. In the Mississippian rocks and the later Paleozoic, the dominant type of ligament is illustrated by Aviculopecten, Limipecten, Deltopecten (3, fig. 13): This type of ligament is possessed by modern Pteria, Pinctada, Ostrea, Lima, and many other genera. In the modern forms the type of ligament in anyone compact family appears to be constant.
Now, because of the dominance of this latter type of ligament in the Carboniferous periods and the suppression of the arcid type, which certainly had been dominant in the Devonian, I am disposed to believe that the pteriid ligament is.a natural derivative of the arcid ligament. In comparing 2a (arcid) ith 3 (pteriid) it is seen that there are several important differences which may be summarized in the following manner:
| Arcid type | Pteriid type |
|---|---|
| 1. Lamellar ligament attached in a groove. | 1. Lamellar ligament raised on a pedestal. |
| 2. Lamellar ligament length constant at hinge. | 2. Lamellar ligament length increases at hinge. |
| 3. Fibrous ligament on pedestal. | 3. Fibrous ligament in triangular pit. |
| 4. First formed ligament lamellar. | 4. First formed ligament both lamellar and fibrous. |
I do not understand why the fibrous ligament of the pteriid type should be depressed in a resilifer and that of the arcid type raised on the general surface of the cardinal area. It appears that the difference indicated in the fourth point, listed above, might be explained by acceleration of development in the pteriid type so that the initial all-lamellar stage of the arcid type might be skipped in the development of the pteriid ligament.
The most serious obstacle lies in the difference suggested in the second point above. The dorsal ridge of the mantle in the arcid type (2a, 2b lower) increases in length by inserting new cells at a single mid-point almost directly under the beaks. This peculiar serial addition causes a poleward migration of ligament structures during growth, without causing any anteroposterior increase in their dimensions. Because of this circumstance the lamellar ligament bands or sheets of the arcid ligament are uniformly the same thickness, and the chevron grooves into which they are inserted are of a uniform breadth.
The pteriid ligament system appears to be fundamentally different in mode of growth. All ligament structures progressively increase in anteroposterior dimensions during growth. Apparently new cells appear uniformly along the entire dorsal margin of the mantle so that there is a uniform increase in the size of the clusters of ligament-secreting cells.
There are no recognized forms intermediate between 2a and 3, and I admit the possibility that the pteriid ligament was not derived from the arcid type. Nevertheless the remarkable similarity between some of the Devonian pectinoids (Pseudaviculopecten Newell, n. gen.) having an arcid ligament area, with Carboniferous forms (Aviculopecten) having a pteriid ligament area, leaves little choice but to believe that the Devonian and Carboniferous forms are closely related.
There is no difficulty deriving the Perna type of ligament from the pteriid type, as has been admirably illustrated by Jackson (1890), and I believe that Euchondria (fig. 13, 4) was derived from one of the aviculopectens (3) by the acquisition of subsidiary resilifers that were progressively added during growth at each end of the hinge.
I am indebted to F. Stearns MacNeil for acquainting me with the nature of the ligament in modern Limopsis (fig. 13, 5a) and for suggesting that the Limopsis ligament may represent a key to the solution of some questions regarding the development of the pectinid ligament from more primitive kinds. The lamellar ligament outside the resilifers is thought by MacNeil not to be homologous to the lamellar ligament of the pteriid system, so we are not in accord as regards the significance of the limopsid type of ligament. It appears to me that the limopsid ligament is very nearly like the pteriid ligament, differing from it only in the fact that the lamellar ligament extends a short distance into the resilifers at their margins. The limopsid ligament appears to be intermediate between the pteriid and pectinid ligament (5c, 5d). In gerontic Pecten a cardinal area, lacking in adult Pecten, is developed. An intermediate stage between Limopsis and normal Pecten necessarily must involve the reduction of the cardinal area. In Pecten and its near relatives the lamellar ligament has invaded the entire area of the resilifers, separating into two separate halves the nonfunctional and vestigial parts of the fibrous ligament. The only Paleozoic representative of the pectinid type of ligament so far recognized is Pernopecten, which was first developed in its typical form in the Mississippian period. Because of various shell features, I regard Pernopecten as ancestral to the modern amussiums and not directly ancestral to other modern pectinoids. The latter, probably derived from the aviculopectens, apparently did not acquire the Pecten-type of ligament until the Triassic period, or less probably, the Permian.
Table III—Proposed classification of Paleozoic Pectinacea.
| Hinge | Family | Obliquity | Outer Ostracum | Relative length auricles |
Subfamily | Genus | Ornamentation | Posterior auricle |
Special features | Geologic range | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left valve | Right valve | |||||||||
| Pterinopectinidae | Prosocline | Homogeneous? | Irregular prismatic | Subequal | Pterinopecten | Intercalate costae | Intercalate costae | Subquadrate | Devonian | |||
| Pseudaviculopecten | Intercalate costae | Intercalate costae | Alate | Devonian-L. Carboniferous | ||||||||
| Lyriopecten | Intercalate costae | Intercalate costae | Alate | Anterior auricle obsolete | Devonian | |||||||
| Vertumnia | Intercalate costae | Intercalate costae | Alate | Left flat, right convex, front ear acuminate | Devonian | |||||||
| Dunbarella | Intercalate costae | Bifurcate costae | Subquadrate | Upper Carboniferous | ||||||||
| Pterinopectinella | Intercalate costae | Bifurcate costae | Alate | Costae regularly spinose | Carboniferous-L. Permian | |||||||
| Palaeopecten | Intercalate costae | Alate | Auricular crura | Silurian | ||||||||
| Rhombopteria | Lirae in quincunx | Subquadrate | Silurian | |||||||||
| Posidonia | Concentric folds | Subquadrate | Carboniferous | |||||||||
| Aviculopectinidae | Prosocline to Opisthocline |
Homogeneous | Irregular prismatic | Subequal | Aviculopectininae | Aviculopecten | Intercalate costae | Bifurcate costae | Alate | Carboniferous-Permian | ||
| Deltopecten | Large equal plicae | Alate | Permian | |||||||||
| Fasciculiconcha | Intercalate costae | Alate | Fascicled costae on left valve | Upper Carboniferous | ||||||||
| Limipecten | Intercalate costae | Alate | Pointed scale-lamellae between costae | Carboniferous-Permian | ||||||||
| Acanthopecten | Large equal costae | Small equal costae | Alate | Pointed scale-lamellae between costae | Carboniferous-Permian | |||||||
| Annuliconcha | Two ranks concentric fila | Alate | Left outer ostracum made of concentric increments normal to shell surface | Carboniferous-Permian | ||||||||
| Girtypecten | Few equal costae and coarse concentric ridges | Alate | Spines at juncture costae and fila | Permian | ||||||||
| Clavicosta | Costae intercalate in pairs | Alate | Valves equally convex | Upper Carboniferous-Permian | ||||||||
| Irregular | Homogeneous | Irregular prismatic | Posterior ear obsolescent | Pseudomonotinae | Pseudomonotis | Intercalate costae | Subquadrate | Right valve commonly cemented | Upper Carboniferous-Permian | |||
| Acline to Opisthocline |
Radial crossed lamellar |
Radial crossed lamellar |
Anterior ear longer than posterior |
Streblochondriinae | Streblochondria | Intercalate costae and concentric fila | Subquadrate | Resilifers extended anteriorly | Carboniferous-Permian | |||
| Obliquipecten | Nearly smooth | Obsolete | Right front auricle extended above hinge | Lower Carboniferous | ||||||||
| "Camptonectes" | Radial lirae in quincunx | Subquadrate | Permian | |||||||||
| Streblopteria | Smooth | Subquadrate | Carboniferous | |||||||||
| Euchondriidae | Prosocline | Homogeneous | Quadrate prisms in radial rows |
Subequal | Euchondria | Intercalate costae | Smooth | Alate | Devonian?-U. Carboniferous | |||
| ?Crenipecten | Smooth | Subquadrate | Poorly developed byssal notch | Devonian | ||||||||
| Amussiidae | Prosocline | Radial crossed lamellar |
Hexagonal prisms in concentric rows |
Subequal | Pernopectininae | Pernopecten | Smooth | Left auricles extend above hinge, byssal notch obsolete, auricular crura |
Carboniferous-Permian | |||
Prev Page--Introduction || Next Page--Systematic Paleontology
Kansas Geological Survey, Geology
Placed on web Dec. 1, 2017; originally published 1937.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/Vol10_1/03_shell.html