Kansas Geological Survey, Subsurface Geology 6, p. 56-65
by
Richard J. Robinson1, S. Chaudhuri1, and Lois M. Jones2
1Kansas State University
2Conoco Research Laboratory
An Acrobat PDF file containing the complete paper is available (684 kB).
Diagenetic processes exert major control on reservoir properties for the accumulation of economically recoverable hydrocarbons in coarse-grained siliciclastic rocks. Both reduction and increase of primary porosity may result from diagenetic processes. Although diagenetic increase in porosity is favorable to the accumulation of hydrocarbons, reduction in porosity may be desirable in some instances because the rock itself may serve as a trap for the hydrocarbon accumulation. Hydrocarbon exploration depends heavily on information from wireline geophysical logs. As diagenetically formed minerals in a reservoir affect both the physical and electrochemical properties of the rock, knowledge of the composition and distribution of diagenetic minerals is of major importance for a clear understanding of the geophysical data.
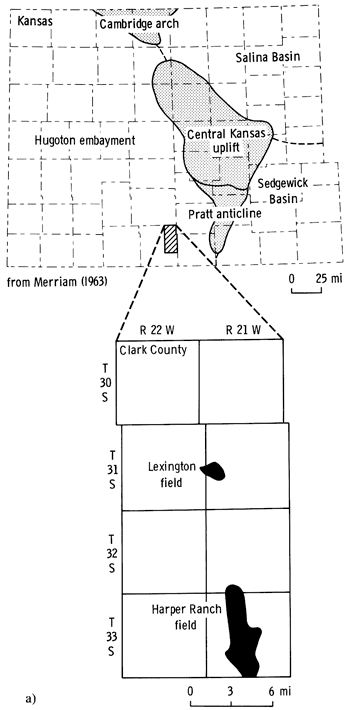
The purpose of this paper is to report the mineralogic composition, texture, and diagenetic history of the Pennsylvanian Morrowan sandstone from the Lexington oil field in Clark County, Kansas. The information can be of use in the exploitation of the Morrowan sandstone reservoirs and, in conjunction with wireline geophysical data, in the recognition of potential productive zones within the Morrowan sequence in other areas adjacent to the Lexington field. Samples analyzed in this study came from four cored wells in the Lexington field, Clark County, Kansas (fig. 1a and b).
Figure 1--a) Location of Lexington field in relation to major pre-Desmoinesian post-Mississippian structural features of Kansas. b) Location of wells in the Lexington field area.

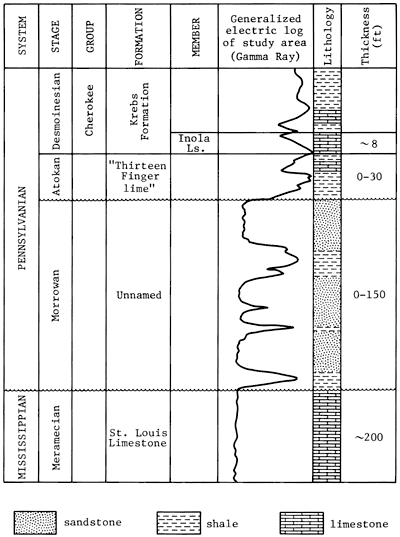
The sandstone of Morrowan Stage of the Pennsylvanian System occurs in the subsurface in Clark County and adjacent areas in Kansas. The overall thickness of Morrowan Stage rocks in the Clark County area ranges from 0 to about 150 ft. Rocks of the Morrowan Stage include sandstone and interbedded shale with minor amounts of conglomerate. The Morrowan rocks unconformably overlie the St. Louis Limestone of the Meramecian Stage and are unconformably overlain by rocks of the Atokan Stage (fig. 2). The Morrowan rocks generally are confined to paleotopographic lows on the underlying St. Louis Limestone. Following the interpretation by Mannhard and Bausch (1974), who studied the Morrowan rocks of the Harper Ranch field in Clark County, Kansas, the sandstone from the Lexington field is considered to have been deposited in a system of strike valleys developed on the truncated Mississippian strata and was related to northeastward marine transgression that was interrupted by several minor regressions.
Figure 2--Stratigraphic column for the Lexington field, Clark County, Kansas (based on correlation from Zeller, 1968; type log for Clark County, Kansas).
Samples of the Morrowan sandstone and shale were taken from several stratigraphic intervals from four cored wells. The petrographic study included identification of constituent minerals, cements, and textures of the rocks. The grain size was determined by point count using 350 points per slide. At each point the smallest projecting diameter of the grain was measured. Roundness values of the grains were determined by comparison with roundness images proposed by Powers (1953). Sorting was determined using the Inclusive Standard Deviation as described in Folk and Ward (1957). Identification of clay minerals was based on x-ray diffraction data from different fractions of clays separated from shale and sandstone. Scanning electron microscopy was used to determine the distribution and morphology of different diagenetic minerals.
Morrowan Stage sandstone consists of 70-85% framework grains by volume. Quartz comprised over 90% of the framework minerals. Chert averaged 3%, whereas glauconite, orthoclase and plagioclase feldspar, and sedimentary rock fragments (shale clasts and carbonate rock fragments) averaged 1% or less of the total volume. Tourmaline, the predominant heavy mineral, occurred in trace amounts. Conglomeratic phases contained greater amounts of rock fragments, consisting of chert, carbonate rock fragments, and shale clasts.
Petrographic details of the Morrowan sandstone are given in tables 1 and 2. The median grain size ranged from 1.70 to 2.50 or medium to fine grained. Sorting averaged 0.60 φ or moderately well sorted. Quartz grains were rounded to subangular; the decrease in roundness was partially due to the effects of etching, replacement, and interpenetration that occurred during diagenesis.
Table 1--Grain-size analysis of Morrowan sandstone, Lexington field, Clark County, Kansas.
| Sample | Grain size | Sorting | Roundness | |
|---|---|---|---|---|
| Moore 2-20 | 5172 ft | 1.89φ | ±0.65φ | 3.3 |
| 5185 ft | 2.33φ | ± 0.52φ | 3.3 | |
| Moore 2-29 | 5134 ft | 2.30φ | ±0.63φ | 3. 1 |
| 5151 ft | 2.71φ | ±0.54φ | 3. 1 | |
| Seacat 5-19 | 5245 ft | 2.64φ | ±0.59φ | 3.0 |
| 5257 ft | 2.60φ | ±0.68φ | 3.0 | |
| McPhail 1-32 | 5172 ft | 2.14φ | ±0.64φ | 3.3 |
Table 2--Point-count results of Morrowan-age sandstone, in percent by volume (Tr = trace amounts).
| Sample | Framework Grains | Cements | Pore Space | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| quartz | feldspar | chert | glauconite | shale clasts |
total framework |
clay minerals |
carbonates | quartz cement |
pyrite | total cement |
||
| Moore 2-20 | ||||||||||||
| 5172 ft | 71 | Tr | 4 | Tr | Tr | 75 | 3 | 4 | Tr | Tr | 7 | 18 |
| 5185 ft | 61 | 1 | 2 | Tr | Tr | 63 | 1 | 32 | Tr | Tr | 33 | 4 |
| Moore 2-29 | ||||||||||||
| 5134 ft | 65 | Tr | 2 | 1 | 3 | 71 | 2 | 9 | 2 | 2 | 15 | 14 |
| 5151 ft | 60 | Tr | 2 | Tr | Tr | 62 | Tr | 31 | 1 | Tr | 32 | 6 |
| Seacat 5-19 | ||||||||||||
| 5245 ft | 63 | Tr | 3 | 1 | Tr | 67 | 1 | 11 | 1 | Tr | 13 | 20 |
| 5257 ft | 70 | Tr | 5 | 1 | Tr | 76 | 1 | 10 | 1 | Tr | 12 | 12 |
| McPhail 1-32 | ||||||||||||
| 5172 ft | 73 | Tr | 6 | Tr | Tr | 79 | 2 | 4 | 3 | Tr | 9 | 12 |
The framework minerals are bonded together by ankerite, calcite, quartz, clay minerals, and pyrite, of which carbonate minerals and quartz are the most common. The proportion of quartz cements generally decreases with increasing amounts of carbonate cements.
Secondary Quartz--Interpenetration of quartz grains and secondary overgrowths are two forms of quartz cement of the sandstones (figs. 3 and 4). Many quartz grains show enlargements of secondary quartz that are in optical continuity with nucleus grain. Trace amounts of clay minerals coat the surface of the detrital quartz grains in some samples. Secondary overgrowths are an early diagenetic phenomenon. Pressure-solution effects that led to the formation of several concavo-convex boundaries among quartz grains destroyed features of earlier diagenetic events.
Figure 3--Photomicrograph of a Morrowan sandstone indicating pressure-solution effects on quartz grains.
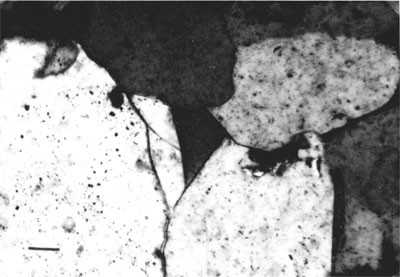
Figure 4--Photomicrograph of a Morrowan sandstone indicating quartz overgrowth on nucleus quartz grains; other grains are ankeritic cement.
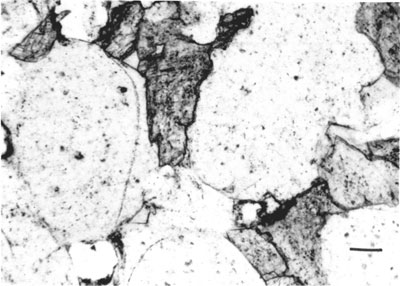
Carbonate cements--Ankerite and less commonly calcite are the dominant types of carbonate cements. Ankerite was identified by x-ray diffraction scanning at 1/4 degree per minute, indicating the (104) reflection at 2.90 0. The carbonate cements occur as discontinuous, irregular patches and lenses and as continuous groundmass of the quartz grains. Ankerite grains are commonly anhedral to subhedral but a few rhombohedral crystals of ankerite also occur. Carbonate minerals are the primary cement in nearly all sandstones comprising up to 32% of the total rock. In highly cemented rocks, the framework grains are almost completely separated from each other.
Two generations of carbonate cement occur, the earlier carbonate probably calcite and the later ankerite. The earlier cement seems to have been replaced by the ankerite leaving a "ghost" outline of the original grain (fig. 5).
Figure 5--Photomicrograph of a Morrowan sandstone indicating "ghost" original carbonate cement (G) in a predominantly ankeritic cement.
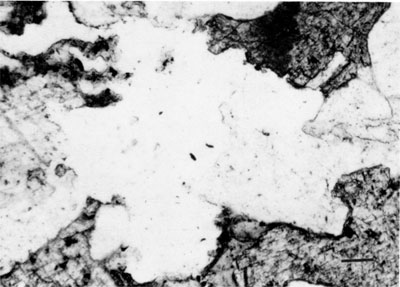
Clay minerals--Illite, randomly mixed-layer illite-smectite, and kaolinite are the major clay minerals in the Morrowan sandstone and shale. Both detrital and neoformed clay minerals occur in the sandstone. Detrital clay minerals occur as coatings on quartz grains and in some cases as a coating between the detrital quartz grain and the secondary overgrowth. Detrital clay minerals also occur as pore fillings between framework grains and the total is less than 1%. The majority of clay minerals are secondary in origin and kaolinite seems to be the dominant type among the neoformed clay minerals. Euhedral, hexagonal crystals of kaolinite often fill intergranular and secondarily enlarged pore spaces (fig. 6). Occasionally illite has grown over both kaolinite and quartz (fig. 7). Clay minerals in the sandstone account for less than 3 % of the total rock.
Figure 6--Scanning-electron microscope photograph of a Morrowan sandstone illustrating kaolinite filling a cavity in leached ankerite rhomb.
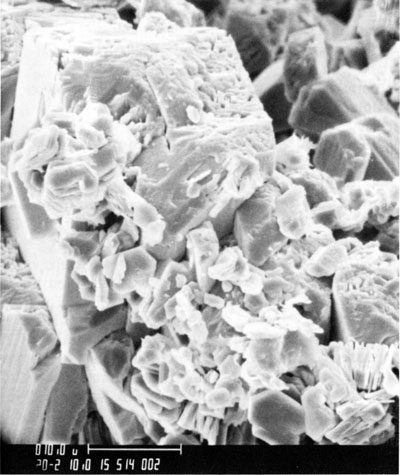
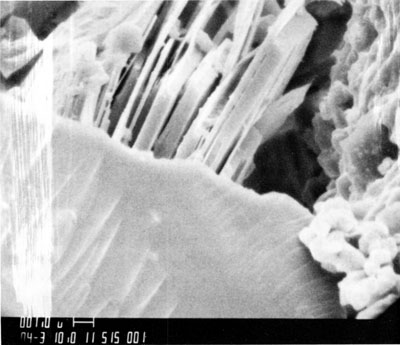
Figure 7--Scanning-electron microscope photograph showing slight etching of well-crystallized kaolinite and growth of illite bridging kaolinite and quartz.
The clay mineral composition of the sandstone is very similar to that of the interbedded shale. The composition of clay mineral is size dependent. Kaolinite is more abundant in the coarse fraction than in the fine fraction. For example, the 0.8-2.0 µm fraction of the clays consists of 54-84% of kaolinite, 13-38% of illite, 5-10% of randomly mixed-layer illite-smectite, and trace amounts of chlorite; whereas, the less than 0.2 µm fraction contains 55-79% of illite, 8-20% of randomly mixed-layer illite-smectite, and 12-35% of kaolinite.
Pyrite--Pyrite occurs as anhedral, irregular patches, and as cubic crystals. Pyrite seems to replace carbonate minerals and to occur also as pore-filling minerals. It is present in amounts of up to 2% of the total rock.
Porosity of the sandstones, estimated from thin sections, ranged from 4% to 20%, with the low porosity sandstones having increased amounts of carbonate cement. In the sandstones with higher porosities (greater than 10%), porosity is predominantly secondary due to dissolution and replacement of carbonate minerals and framework grains.
Carbonate cements from 16 core samples of Morrowan sandstone and two core samples of the St. Louis Limestone were analyzed for their Ca, Mg, Sr, and Rb contents. Atomic-absorption spectroscopy methods were used for the determination of Ca, Mg, and Sr using the method of standard addition. Solutions were analyzed using a Perkin-Elmer 305B Atomic Absorption Spectrophotometer. The instrumental parameters used for the listed elements are those recommended by Perkin-Elmer Corporation. Replicate analyses of some samples indicated maximum error of about 5% for each of the above elements.
Ca contents ranged from 9.4 to 38.9% and Mg contents from 0.2 to 10.5%. Sr contents were between 329 and 547 ppm, and Rb contents were between 1.3 and 5.3 ppm. The molar Sr/Ca ratios of the carbonate cements ranged between 1. 61 X 10-3 and 6.85 x 10-3. The chemical data of the carbonates are given in table 3.
Table 3--Chemical analyses of carbonate cements of Morrowan sandstone and carbonate rock of St. Louis Limestone (in ppm).
| Sample | Sr | Rb | Ca x 10-3 | Mg X 10-3 |
|---|---|---|---|---|
| Carbonate cements | ||||
| Moore 2-20 | ||||
| 5161 ft | 253 | 2.3 | 119 | 40 |
| 5172 ft | 518 | 3.8 | 316 | 105 |
| 5180 ft | 281 | 2.4 | 94 | 32 |
| 5185 ft | 312 | 2.3 | 196 | 76 |
| 5195 ft | 320 | 2.5 | 237 | 87 |
| Moore 2-29 | ||||
| 5127 ft | 369 | 2.6 | 152 | 52 |
| 5134 ft | 320 | 2.4 | 172 | 58 |
| 5135 ft | 269 | 2.0 | 136 | 34 |
| 5141 ft | 371 | 3.0 | 151 | 53 |
| Seacat 5-19 | ||||
| 5232 ft | 287 | 2.5 | 389 | 102 |
| 5239 ft | 321 | 2.8 | 182 | 62 |
| 5245 ft | 352 | 2.8 | 201 | 69 |
| 5251 ft | 281 | 2.7 | 160 | 56 |
| 5257 ft | 239 | 1.8 | 208 | 66 |
| McPhail 1-32 | ||||
| 5166 ft | 547 | 4.8 | 294 | 2 |
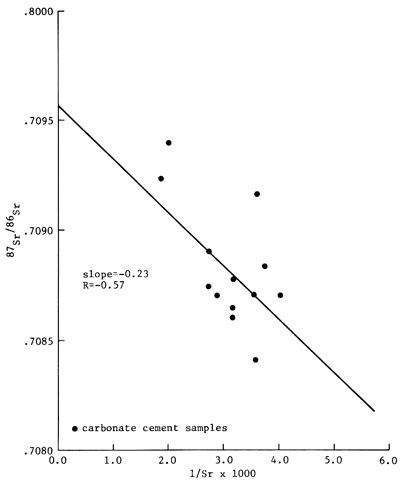
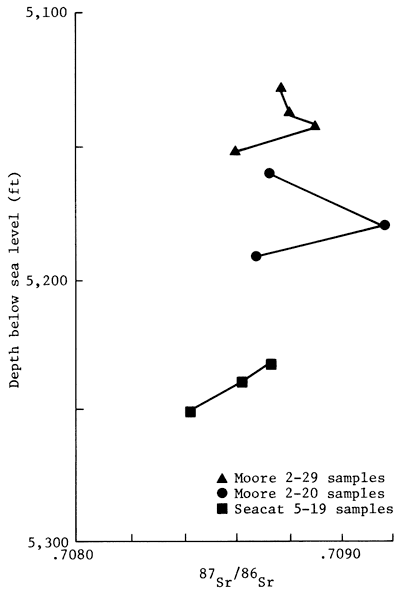
Measured 87Sr/86Sr ratios for carbonate cements are indicated in table 4. The isotopic ratio measured for the Eimer and Amend SrCO3 standard was 0.70804. The Rb/Sr ratios of the cements, ranging between 7 x 10-3 and 9 X 10-3, indicate that the Sr isotopic ratios of the carbonate cements remained essentially unchanged from the decay of 87Rb to 87Sr. In samples of the Moore 2-20 core of the Morrowan sandstone, the Sr isotopic ratios of the carbonate cements ranged from 0.70865 to 0.70904. In the Moore 2-29 core, the Sr isotopic ratios of the carbonate cements had values between 0.70874 and 0.70890. In the Seacat 5-19 core, the cements had 87Sr/86Sr ratios ranging from 0.70841 and 0.70871. An analysis of a single sample from 1-32 McPhail core indicated the Sr isotopic ratio of 0.70923. In contrast, two samples of the Mississippian St. Louis Limestone had Sr isotopic ratios of 0.76822 and 0.70856. The 87Sr/86Sr ratios of the carbonate cements from sandstones of Morrowan age are plotted against depth in fig. 8. This figure illustrates that the isotopic data vary randomly in a vertical direction.
Table 4--Strontium isotopic data of carbonate cements from Morrowan-age sandstone, St. Louis Limestone carbonate rocks and oil-field waters, from the Lexington field, Clark County, Kansas.
| Sample | 1/Sr, pp-1 | 87Sr/86Sr |
|---|---|---|
| Carbonate cements of Morrowan-age sandstone | ||
| Moore 2-20 | ||
| 5161 ft | 0.004 | 0.70871 ± 0.00010 |
| 5172 ft | 0.002 | 0.70904 ± 0.00012 |
| 5180 ft | 0.004 | 0.70916 ± 0.00009 |
| 5191 ft | 0.003 | 0.70865 ± 0.00005 |
| Moore 2-29 | ||
| 5127 ft | 0.003 | 0.70874 ± 0.00003 |
| 5135 ft | 0.003 | 0.70877 ± 0.00002 |
| 5141 ft | 0.003 | 0.70883 ± 0.00006 |
| 5151 ft | 0.003 | 0.70890 ± 0.0005 |
| Seacat 5-19 | ||
| 5232 ft | 0.004 | 0.70871 ± 0.0004 |
| 5239 ft | 0.003 | 0.70860 ± 0.0002 |
| 5245 ft | 0.003 | 0.70870 ± 0.0005 |
| 5251 ft | 0.004 | 0.70841 ± 0.00003 |
| McPhail 1-32 | ||
| 5166 ft | 0.002 | 0.70923 ± 0.00015 |
| St. Louis Limestone carbonate rocks | ||
| Moore 2-29 | ||
| 5155 ft | 0.003 | 0.70856 ± 0.0004 |
| 5158 ft | 0.002 | 0.70822 ± 0.0011 |
Figure 8--87Sr/86Sr ratio vs. 1/Sr for the carbonate cements of the Morrowan-age sandstone of the Lexington field.
The earliest diagenetic event in the cementation history of the sandstone of the Morrowan Stage was the formation of quartz overgrowths on quartz grains. This initial overgrowth probably was due to a lowering of the pH of the pore waters, possibly arising from oxidation of organic material at shallow depth. Compaction of sediments led to further reduction in porosity as is evident from the pressure-solution effects on quartz grains that resulted in the formation of several concavo-convex boundaries among neighboring quartz grains. With continued burial, quartz became unstable and was partly replaced by calcite. Upon deeper burial the increase in temperature could have caused the solubility of silica to increase. The temperature increase also can bring about precipitation of CaCO3 provided that the partial pressure of CO2 for the water remained constant and that the water composition is near that of CaCO3 saturation (Siever, 1959). Following the deep burial and replacement of quartz by the calcite cement, a change in the hydrodynamic system must have occurred that led to the ankeritization of the previously formed calcite creating secondary porosity in the rock. Because "ghost" outline of original carbonate grain can be occasionally found in the ankeritic cements (fig. 5), the conservation of volume during ankeritization must have been nearly maintained. The dolomitization reaction without any volume change may be described as:
(2 - x)CaCO3 + 0.8 Mg2+ + 0.2 Fe2+ + XCO32- = CaMg0.8Fe0.2(CO3)2 + (1 - x)Ca2+
where x is approximately equal to 0.25.
The dissolution of the ankeritic cement is the most important diagenetic event that created secondary porosity in the Morrowan sandstone. The creation of these secondary pores is a possible indication of introduction of acidic waters into the Morrowan sandstone. Data are insufficient to confidently establish whether or not the acidic water was introduced when the rocks were exposed to near-subaerial condition or when the rocks were deeply buried. Lack of evidence for any erosional unconformity at the top of the Morrowan sandstone suggests that subaerial condition was not an important factor for the introduction of acidic water. The reduction of pH of pore fluid under deep burial could have resulted either from introduction of acidic water expelled possibly from compacted mudstone interbedded with the sandstone units or from introduction of CO2 from potential hydrocarbon source beds at depth prior to the hydrocarbon maturation.
The major diagenetic changes following the ankeritization are possibly concatenate events relating to the hydrocarbon accumulation in the Morrowan sandstone. These post-ankeritization diagenetic events include partial dissolution of the ankeritic cements and occlusion of the resultant secondary porosity primarily by deposition of kaolinite and, to a much less degree, by precipitation of illite, mixed-layer illite-smectite, and pyrite. One possible mechanism by which dissolution of the ankeritic cements could have occurred was introduction of CO2 in the pore fluid of the Morrowan sandstone. Considerable amount of CO2 is generated from degradation of organic matter in source beds before peak oil generation (Moper, 1972). The CO2 generated in source beds will remain dissolved in the oil more than in the water until pathways become available for migration of the fluid from the source beds. The pressure decrease attendant with migration will facilitate release of some of the dissolved CO2 from the fluid to a separate gas phase, which will migrate ahead of the hydrocarbons and waters. In this scenario, the dissolution of the ankeritic cements of the Morrowan sandstone can be explained by the introduction of exsolved CO2 from potential hydrocarbon source beds to the pore fluids of the Morrowan sandstone.
Following the creation of the secondary porosity, precipitation of kaolinite became the major diagenetic reaction. Formation of kaolinite is favored in a slightly acid pH solution with low alkali/H+ ratio. The formation of kaolinite can be described as:2Al3+ + 2H4SiO4 + H2O = Al2Si2O5(OH)4 + 6H+
A possible source for the dissolved alumina and silica could be the CO2-rich fluids that entered the Morrowan sandstone prior to the arrival of the hydrocarbons. Alternatively, the pore water was high in Al/Si before CO2-rich fluid entered the Morrowan sandstone. Although the introduction of CO2 caused the water to become slightly acidic, kaolinite precipitation did not occur possibly because of chemical kinetics. Attempts of synthesis of kaolinite from gel or amorphous material at low temperature have encountered considerable difficulties, yet successful syntheses have been made in the presence of organic compounds (Linares and Huertas, 1971; Hem and Lind, 1974). The formation of kaolinite in the Morrowan sandstone may have been similarly catalyzed by the presence of organic compounds that entered the pore system subsequent to the introduction of CO2 in the pore fluid.
Additional reduction in the porosity occurred as a result of formation of small amounts of illite and mixed-layer illite-smectite. The growth of these minerals may be related to somewhat higher pH and higher alkali to hydrogen-ion activity ratio than that for kaolinite.
Pyrite was the last diagenetic mineral of the reservoir rock. Either bacterial reduction of sulfate in the pore water or introduction of H2O into the pore water before peak migration of hydrocarbon or both could have provided the needed sulfur species for the formation of pyrite. The iron in the pyrite could have come from either anaerobic bacterial reduction of hydrated iron oxides that may have been associated with the Morrowan sandstone or dissolution of the ankeritic cement. The overall reactions for the pyrite formation can be expressed as:
2CH2O + SO42- = HCO3- + HS- + 6H+
CH2O + 4FeO.OH + H2O = 4Fe2+ + HCO3- + 7OH-
Fe2+ + H2Saq = FeS2 + 4H+ + 2e
Fe2+ + 2HS- = FeS2 + 2H+ + 2e
The carbonate cement-clay mineral-pyrite relationship in the Morrowan sandstone indicates that major diagenetic processes continued at least through the formations of illite, mixed-layer illite-smectite, and pyrite. As the inorganic reactions become minimal after accumulation of large quantities of hydrocarbon in the reservoir, the time of hydrocarbon accumulation must post-date the period of formation of the last major diagenetic minerals. In the case of the Morrowan sandstone, the formation of clay mineral assemblage of kaolinite, illite, and mixed-layer illite-smectite is the last major diagenetic event. Therefore, the accumulation of petroleum in the Morrowan sandstone occurred after the formation of these clay minerals. The peak accumulation could have possibly happened shortly after the formation of much of the pyrite.
Foscolos and Kodama (1974) summarized several commonly occurring mineralogic features of clay diagenesis. They noted that discrete expandable clays do not commonly occur below a burial depth of about 3,000 ft and that kaolinite often disappears below 9,000 ft. Furthermore, mixed-layer illite-smectite clays commonly persist at depths between 3,000 and 12,000 ft. Considering that kaolinite is the very abundant clay mineral and that mixed-layer illite-smectite is a common clay mineral in sandstone and interbedded shale of the Morrowan sequence, the burial of the sequence was probably no deeper than about 9,000 ft.
Information on the Sr/Ca ratio of the carbonate minerals as cements for the Morrowan sandstone is very useful in determining the chemical history of the fluid that was involved in the precipitation of the cement. The molar Sr/ Ca ratio of the liquid in equilibrium with dolomite is dependent on the molar Sr/Ca ratio of the initial fluid, the molar Sr/Ca ratio of the replaced carbonate mineral (calcite or aragonite), and the distribution coefficient of Sr between the dolomite and the associated liquid. Sass and Starinsky (1979) demonstrated quantitatively that during advanced dolomitization the Sr/Ca ratio in solution approaches a limiting value which is determined by the Sr/Ca ratio of the replaced carbonate and that the limiting value is about twice that of the replaced carbonate, because of the very low value of the distribution coefficient of Sr. Veizer (1983) recently discussed the uncertainties of the value of the distribution coefficient of Sr between dolomite and liquid. The recommended values range between 0.025 and 0.07. The distribution coefficient of Sr for iron-rich dolomite (ankerite) can be expected to be slightly higher than the proposed range of values for common dolomite.
From consideration of the proposed range of values of the distribution coefficient of Sr between dolomite and liquid, the molar Sr/Ca ratios of the liquids in equilibrium with dolomitic (ankeritic) cements of the Morrowan sandstone was probably between 5.3 x 10-3 and 54.8 X 10-3. These Sr/Ca ratios of the liquids in equilibrium with the dolomite cements were undoubtedly controlled by the Sr/Ca ratios of the initial liquids that caused dolomitization and the Sr/Ca ratio of the replaced carbonate mineral. General information can be obtained concerning possible source of the initial fluid that was involved in the dolomitization process, provided the molar Sr/Ca ratio of the replaced carbonate mineral is known. The precursor of dolomite cement of the Morrowan sandstone is considered to be calcite, because of the occasional presence of "ghost" calcite (?) in the dolomite cement and presence of calcite cement found in a nonproductive unit of Morrowan sandstone from the same locality as that of the productive unit. The molar Sr/Ca ratio of the calcite cement was found to be 0.8 x 10-3, which is well within the range of 0.19 x 10-3 to 0.8 x 10-3 for commonly occurring natural calcite in sedimentary carbonate rocks (Sass and Starinsky, 1979). If the Sr/Ca molar ratio of the initial liquid was close to that of average sea water (8.6 x 10-3) and the dolomitization of the Morrowan calcite cement occurred in presence of limited amount of the liquid, that is the low liquid to solid ratio, then the molar Sr/Ca ratio of the liquid that was in equilibrium with the dolomite might have attained a value of 1.6 x 10-3, which is twice the molar Sr/Ca ratio of the original calcite in the Morrowan sandstone. However, the molar Sr/Ca ratio of the liquid in equilibrium with dolomite would increasingly become closer to that of the original dolomitizing liquid with increasing liquid to solid ratio. From the Sr/Ca values of the dolomite cements of the Morrowan sandstone, and taking into account Veizer's (1983) recommended values of the distribution coefficient of Sr between dolomite and associated liquid, the calculated molar Sr/Ca ratios of the liquids in equilibrium with the dolomite were found to be between 5.3 x 10-3 and 54.8 x 10-3. Such high Sr/Ca ratios for presumed liquids in equilibrium with dolomite could not have been derived from the original calcite mineral with molar Sr/Ca ratio of about 0.8 Δ x 10-3. The high ratios then must be a reflection of the molar Sr/Ca ratios of the original liquid that caused dolomitization of previously formed calcite cement of the Morrowan sandstone. Although some of the liquids in equilibrium with the dolomite cements had molar Sr/Ca ratios less than that of the average sea water with a value of about 8.6 x 10-3, many had molar Sr/Ca ratios that were considerably higher than that of the average sea water. The dolomitizing liquids with ratios as high as 54.8 x 10-3 is a minimum estimate of the molar Sr/Ca ratio of the original liquid, because of the reactions of the original liquid with low Sr/Ca ratio-bearing calcite. Possible natural liquids that could have such high molar Sr/Ca ratios include highly evaporated sea-water brine and liquid that was once involved in recrystallization of aragonite to calcite. Thus, in view of the very high molar Sr/Ca ratio needed for the initial liquid, cementation of the Morrowan sandstone was probably caused by a liquid that was either a highly evaporated sea-water brine or previously involved in recrystallization of aragonite to calcite.
A sample of sandstone (McPhail 1-32, 5172) from a nonproductive bed had nearly pure calcite cement. The molar Sr/Ca ratio of the cement was found to be 0.85 x 10-3. Assuming a distribution coefficient of 0.058 for the Sr between calcite and liquid (Katz and others, 1972; Veizer, 1983), the molar Sr/Ca ratios of the liquid that precipitated the calcite probably had a molar Sr/Ca ratio of about 14.7 x 10-3, which is higher than that of the average sea water. The high molar Sr/Ca ratio of the pore fluid during calcite cementation of the Morrowan sandstone suggests that at least some fraction of the pore water prior to calcite cementation was either linked to evaporation of sea water or involved in the recrystallization of aragonite to calcite.
Varied Sr/Ca ratios of the carbonate cements provide some clues as to the physical condition that governed the cementation of the Morrowan sandstone. The variation in the molar Sr/Ca ratios of the calcium-carbonate cements probably reflects that the carbonate cementation of the Morrowan sandstone proceeded progressively with varied Sr/Ca ratios of the pore fluids. Changes in the chemical character of the pore fluids are related to several pulses of fluids that were introduced into the reservoir during the formation of early carbonate cements.
The 87Sr/86Sr ratio of calcite cement from one sample (McPhail 1-32 5166) of the Morrowan sandstone was found to be 0.70923. The calcite cement seems to be the earliest among the carbonate cements. The Sr isotopic composition of the calcite cement is nearly identical to that of contemporaneous Morrowan sea water, the value of which has been given by Peterman and others (1970). The source of the Sr for this early carbonate phase, therefore, could have been the contemporary sea water Sr.
The 87Sr/86Sr ratios of the ankeritic carbonate cements ranged between 0.70841 and 0.70904. The Sr isotopic composition of the ankerite cements is distinctly different from that of the contemporary sea water. Therefore, the fluids that produced ankeritization must have derived Sr, at least in part, from a source that had 87Sr/86Sr ratio as low as 0.70841 or lower. This ratio is well within the range of value for Paleozoic marine-carbonate rocks and hence a marine source for the Sr is very likely. Although the rock or rocks from which the Sr was derived could not be positively identified, the similarity in the Sr isotopic composition between the ankeritic cement with the lowest 87Sr/86Sr ratio and the limestone and dolomite of the underlying Mississippian St. Louis Limestone makes these older rocks a possible source of Sr for the dolomitizing fluids of the carbonate cement of the Morrowan sandstone.
The Sr isotopic composition of the ankeritic cements is varied. To determine whether or not the observed Sr isotopic variation of the carbonate cements could be explained by mixing Sr of two isotopically different sources, the 87Sr/86Sr ratios of the cements were plotted against their corresponding 1/Sr values (fig. 8). The two sets of data are only very roughly linear (r = -0.57); hence, the trend is clearly suggestive of the existence of a chemical history of the cements that is more complex than derivation of Sr from two separate sources. The complex isotopic distribution is further illustrated in the vertical distribution of the Sr isotopic composition of the carbonate cements (fig. 9). As noted in the diagram, the isotopic differences can exist even among samples that are separated vertically by less than a meter. The observed vertical distribution of the Sr isotopic data indicates that diagenetic reactions that produced ankeritic cements probably proceeded in several discrete stages with randomly varied Sr isotopic composition of the pore fluids or of the replaced carbonate minerals.
Figure 9--Relationship between depth of Morrowan sandstone sample and Sr isotopic ratio of carbonate cement of Morrowan sandstone, Lexington field, Clark County, Kansas.
Core samples were obtained from the collection of Mesa Petroleum Company. This work was supported, in part, by National Science Foundation grant EAR-8019599.
Behrens, E. W., and Land, L. S., 1972, Subtidal Holocene dolomite, Baffin Bay, Texas: Journal of Sedimentary Petrology, v. 42, p. 155-161.
Folk, R. L., and Ward, W. C., 1957, Brazos River bar--a study of the significance of grain-size parameters: Journal of Sedimentary Petrology, v. 16, p. 3-26.
Foscolos, A. E., and Kodama, H., 1974, Diagenesis of clay minerals from Lower Cretaceous shales of northeastern British Columbia: Clays and Clay Minerals, v. 22, p. 319-335.
Hem, J. D., and Lind, C. J., 1974, Kaolinite synthesis of 25°C: Science, v. 184, p. 1, 171-1,173.
Katz, A., Sass, E., Starinsky, A., and Holland, H. D., 1972, Strontium behavior in the aragonite-calcite transformation-an experimental study at 40-98°C: Geochimica et Cosmochimica Acta, v. 36, p. 481-496.Kinsman, D. J. J., 1969, Interpretation of strontium concentrations in carbonate minerals and rocks: Journal of Sedimentary Petrology, v. 39, p. 486-508.
Linares, P. J., and Huertas, F., 1971, Kaolinite synthesis at room temperature: Science, v. 171, p. 896-897.
Mannhard, G. W., and Bausch, D. A., 1974, Stratigraphic trap accumulation in southwestern Kansas and northwestern Oklahoma: American Association of Petroleum Geologists, Bulletin, v. 58, p. 447-463.
Moper, J., 1978, Oil migration limitations suggested by geological and geochemical considerations; in, Physical and Chemical Constraints on Petroleum Migration: American Association of Petroleum Geologists, Short Course No. 8, p. B1-B60.
Peterman, Z. E., Hedge, C. E., and Tourtelot, H. A., 1970, Isotopic composition of strontium in sea water throughout Phanerozoic time: Geochimica et Cosmochimica Acta, v. 34, p. 105-120.
Powers, M. C., 1953, A new roundness scale for sedimentary particles: Journal of Sedimentary Petrology, v. 23, p. 117-119.
Sass, E., and Starinsky, A., 1979, Behaviour of strontium in subsurface calcium-chloride brines--southern Israel and Dead Sea rift valley: Geochimica et Cosmochimica Acta, v. 43, p. 885-895.
Siever, R., 1959, Petrology and geochemistry of silica cementation in some Pennsylvanian sandstones; in, Silica in Sediments--A Symposium: Society of Economic Paleontologists and Mineralogists, Special Publication 7, p. 55-79.
Veizer, Jan, 1983, Chemical diagenesis of carbonates-theory and application of trace element technique; in, Stable Isotopes in Sedimentary Geology: Society of Economic Paleontologists and Mineralogists, Short Course No. 10, p. 3-1-3-100.
Zeller, D. E., 1968, The stratigraphic succession in Kansas: Kansas Geological Survey, Bulletin 189, p. 1-81. [available online]
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version April 14, 2010. Original publication date 1985.
URL=http://www.kgs.ku.edu/Publications/Bulletins/Sub6/Robinson/index.html