
Kansas Geological Survey, Mineral Resources Series 4, originally published in 1976

Originally published in 1976 as Kansas Geological Survey Mineral Resources Series 4. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (12.2 MB) is also available.
Appreciation is extended to K.G.S. photographer Barbara Welter for the photographic work contained in this publication. The author also wishes to thank Helen Wolfe, K.G.S., for her assistance.
Special thanks go to the Kansas Building Stone Association for their cooperation.
This publication provides a brief historical review and discussion of Kansas building limestone. Despite a somewhat higher initial cost for a limestone building, there are real long-term savings in using limestone in preference to conventional wood construction. In view of the ominous energy supply picture, the low energy required to produce the product plus the fact that the resulting structure is relatively well insulated gives buildings constructed of limestone advantages in addition to their attractive, durable, maintenance-free surfaces. A general discussion of Kansas building limestones follows which includes the geology, properties, and the quarrying and processing of such stone.
Numerous photographs are included to show the various artistic and/or utilitarian aspects of Kansas building limestones. A short glossary of terms used in the industry can be found at the back of this publication.
This work represents one phase of a continuing Survey study of the properties and suitability of using Kansas building limestones.
There are many old buildings of Kansas building stone throughout the state which attest to its durability. Many cities and towns of Kansas have at least one old stone building, usually a church or a home, constructed before the end of the 19th century.
Settlers in eastern Kansas, despite the frequent availability of stone from many local limestone outcrops, often constructed homes, buildings, and churches of wood, because timber was readily available and easy to work. Few of these buildings remain today. By contrast, there are numerous examples of stone structures built during the 19th century that are still in use and remain in relatively good condition. The State Capitol building in Topeka (east wing, 1867), the First Baptist Church at Abilene (1876), the Pottawatomie Mission (Fig. 1) at Topeka (1848), portions of Fort Scott (1840's) and the north side of the United Church of Christ Congregational Church at Manhattan (1858) are examples.
Figure 1—The Pottawatomie Mission at Topeka was constructed in 1848 using Burlingame Limestone. It probably was the first stone church built in Kansas. No longer used for church purposes, the structure was recently renovated.

Although the western part of Kansas was settled at a later time than eastern Kansas, the lack of timber in the west made the use of stone more common. As a result, many older stone structures still exist in western Kansas. Good examples can be found in Hays and surrounding towns where the German Catholics (who settled the area) frequently relied on stone for constructing homes and churches. One of the oldest churches in that area is located in the small town of Catherine, approximately 10 miles northeast of Hays, where both the church (1890) and a few homes were constructed before the turn of the century. Examples of older churches located south of Hays include the Catholic churches at Schoenchen (1900) and Liebenthal (1902). In nearby Victoria, east of Hays, stands the St. Fidelis Church, better known as "The Cathedral of the Plains" (constructed in 1908- 1911). This church is probably the best known stone church in western Kansas. Several older county courthouses (Greeley County at Tribune, Mitchell County at Beloit) and the blockhouse and guardhouse at Fort Hays attest to the durability of the local stone. The lack of timber also gave rise to the durable limestone fenceposts which are still common in western Kansas. All of these older stone buildings were constructed from available stone, namely the Fencepost Limestone and Fort Hays Limestone and to a lesser extent the Smoky Hill Chalk.
The historical review emphasizes one of the major advantages of using Kansas building limestone, its durability. Other advantages include a maintenance free surface, well-insulated structure, beauty, and a low energy cost for producing the product.
Most people feel that stone is very expensive and they simply cannot afford such a prestige item. As far as the initial cost is concerned, stone is more expensive than wood. Much of this initial expense is not only for the stone itself but also includes the cost of laying the stone. One building stone producer recently commented that if one paid $5,000 for an all-stone exterior (three bedroom rancher with 1500 square feet of surface area), approximately 40% ($2,000) would be for the stone while the remaining 60% ($3,000) would be for the cost of construction. In general, a stonemason will charge more to lay a unit area of stone compared to a brickmason/unit area of brick and much more than a general contractor using wood.
Once the house or building is constructed, the real savings of using stone begin offsetting the initial cost. Any type of shingled or wood house must be periodically stained or painted and occasionally wood might need to be replaced. Over the years such maintenance amounts to a sizable expense which is eliminated when using stone.
Heat transfer is greater through limestone than through wood for a given thickness. However, wood exteriors are relatively thin compared to stone exteriors. The latter normally use a minimum of four inches of stone and a dead air space between the stone and sheathing. Because of their wall thickness and construction, stone buildings are usually as well or better insulated as structures finished with wood. A detailed example is given in Appendix B. In view of the potential fuel shortages and rapidly escalating costs of gas, fuel oil and electricity, the fuel conservation factor is becoming more vital.
Nearly everyone has noticed how beautiful a stone building can be. One generally thinks of this beauty in terms of a stone house they have seen. While it is true that many old stone buildings tend to be "boxy" in appearance, they possess a certain charm and character all their own. Furthermore, modernization or diversification of architectural styles has produced many beautiful buildings in recent years. In the photographs included in this publication, one can see the various utilitarian and/or artistic ways in which Kansas stone has been used in the construction of homes, churches, office buildings, etc. The photographs also support the fact that Kansas building stone is finding increased use in the interior of buildings. The durability, maintenance free surface, adequately insulated structure and beauty resulting from the use of Kansas building stone are distinct advantages that offset the initial expense.
The energy sources used to produce Kansas building stone include diesel, gasoline, propane, natural gas and electricity. Diesel and gasoline fuels are used for the operation of fork lifts (quarry and plant), front end loaders, and trucks which haul the stone from the quarry to the plant. Propane and natural gas, less significant in the total cost picture, are used to heat the plants. Electricity is primarily used to run the plant's equipment (gang saws, circular saws, etc.).
Despite these various uses of energy, the total energy cost used to produce a mixture of building stone products is less than four percent of the gross sales value. In other words, the total energy cost to produce the $2,000 of stone for the three bedroom rancher mentioned above would be less than $80.00. The low energy requirements used to produce building stone should give stone an increasingly significant advantage in the construction industry as fuel costs continue to escalate.
As mentioned, early Kansans often constructed buildings from nearby material. As a result, one can find many varieties of Kansas stone that were used for construction. Despite the local availability of limestone ledges, the economics of the situation and the consistency, extent, and properties of the limestone ledges dictate that only certain varieties of limestone are best suited for building purposes. Furthermore, the building stone industry has progressed to the point that only certain portions of some ledges are used because of their superior quality.
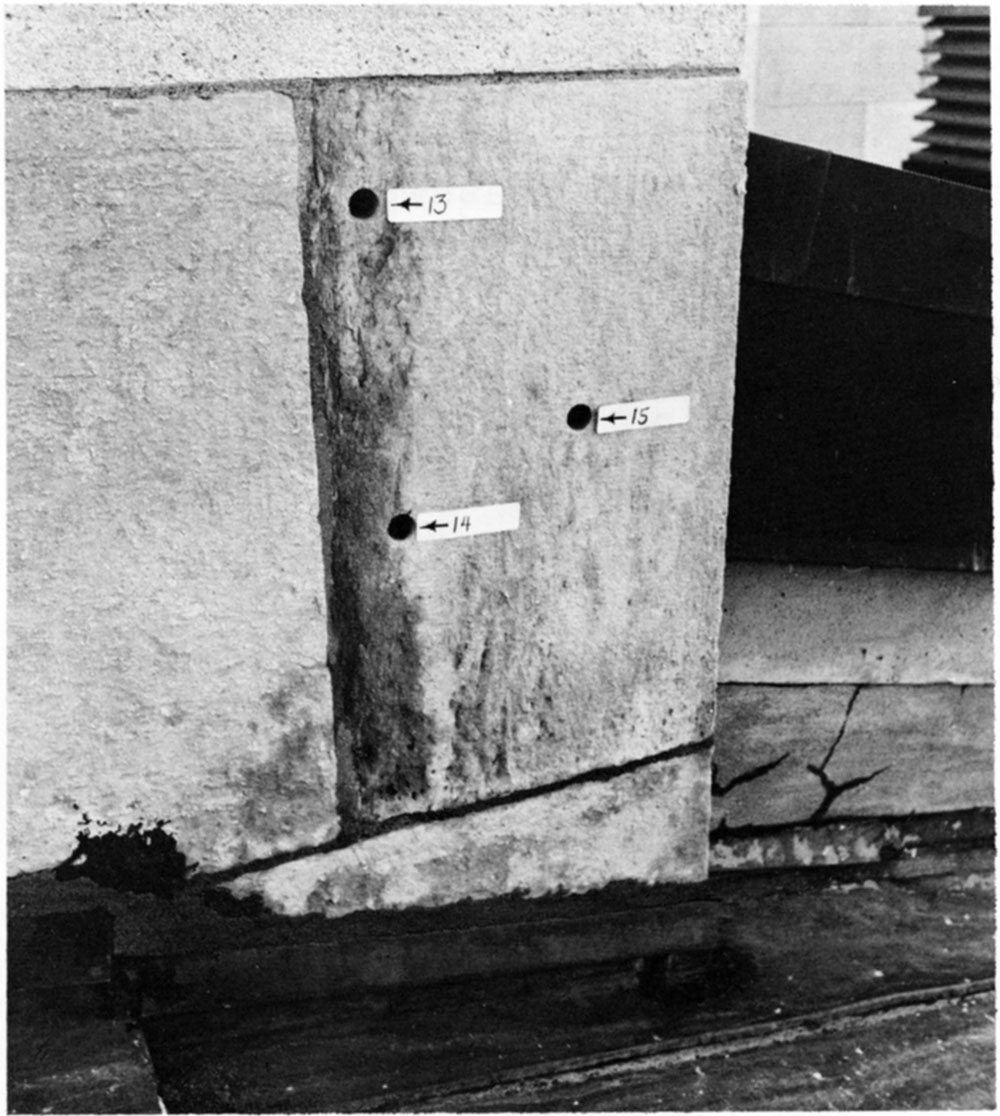
As an example, one can observe limestone blocks in the State Capitol at Topeka where zones of poor quality (presumably a transition zone between the limestone and shale at the original outcrop) were used. Analysis of such zones on the State Capitol showed that they have a high clay mineral content. Subsequently these zones deteriorated or weathered at a much faster rate than the bulk of the structure. An example is shown in Figure 2. Modern stone producers recognize these zones and cut away such sections, thus eliminating future problems.
Figure 2—A clay-rich zone in this stone which was sometimes used in early decades of stone production, has led to differential weathering in this limestone block on the State Capitol. Modern stone producers eliminate this zone.
Modern stone producers are concerned with various physical properties of stone such as its compressive and flexural (and occasionally tensile) strength, absorption, freeze-thaw stability, color, etc. These properties are used to determine whether or not a stone would be suitable for particular building purposes. A summary and discussion of these properties for Kansas building limestones is given later in the text and in Table 1.
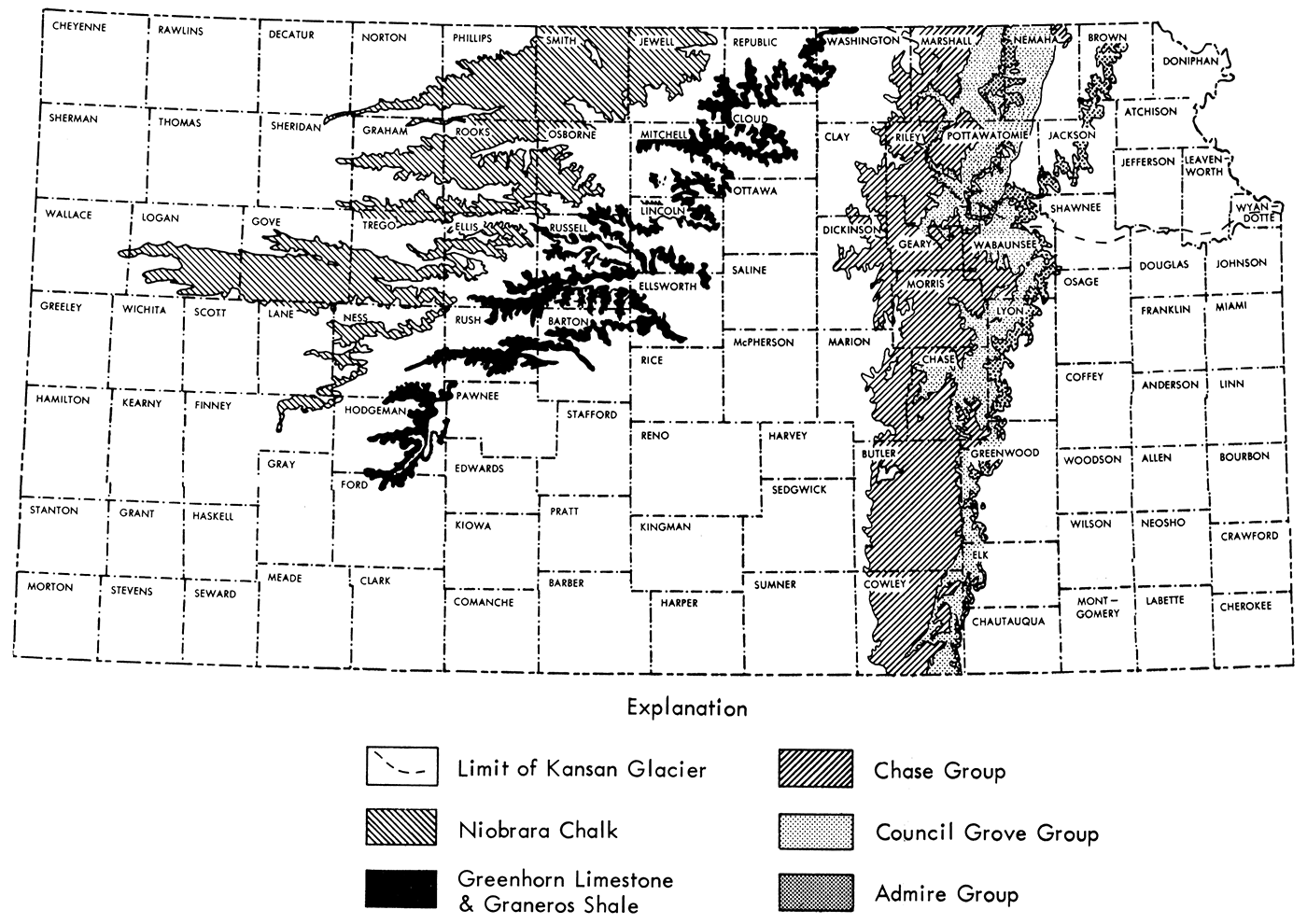
Kansas contains a very large number of limestone ledges, many of which have been used locally for construction purposes. It is beyond the scope of this report to discuss all these stones. Some of the more prominent Kansas building limestones which have been used in the past two decades include the Fort Hays and Fencepost Limestones of Upper Cretaceous age and the Cresswell, Fort Riley, Funston, Cottonwood, Neva, and Five Point Limestones of Lower Permian age. The following discussion briefly describes each of these limestones. Figure 3 shows the location of surface exposures of the different geological groups which contain these Kansas building limestones.
Figure 3—Map of Kansas showing outcrops of geological groups which contain Kansas building limestones.
The Fort Hays and Fencepost Limestones have been used extensively in western Kansas. Both stones can be seen on several buildings at Fort Hays State College in Hays, Kansas. Other examples of Fort Hays Limestone include the Greeley County Courthouse at Tribune and older buildings at Fort Hays. The commonly observed limestone fenceposts, the Cathedral of the Plains at Victoria, numerous churches in the vicinity of Hays, and the Mitchell County Courthouse (1901) at Beloit are constructed from the Fencepost Limestone. The latter was particularly popular because it was easily accessible and had a thickness of about eight inches. It was an ideal ledge to quarry and lay before the days of modern machinery.
Geologically, the Fort Hays Limestone and Fencepost Limestone are part of the Upper Cretaceous sequence of Kansas rocks (see Figure 4). The Fort Hays Limestone member forms the lower part of the Niobrara Chalk Formation and is a relatively soft stone. As such, it can be easily worked but does not have quite the load-bearing capability of most other Kansas building limestones. The Fort Hays Limestone should only be used in relatively arid climates because the stone is very susceptible to deterioration by moisture. Hence, its use is restricted to the dry western portions of Kansas. It is still produced on occasion from a small quarry near Fort Hays College. A relatively recent example of its use is the Russell County Courthouse at Russell.
Figure 4—Stratigraphic succession of a portion of the Upper Cretaceous sequence in Kansas showing relative locations of the Fort Hays and Fencepost Limestones.
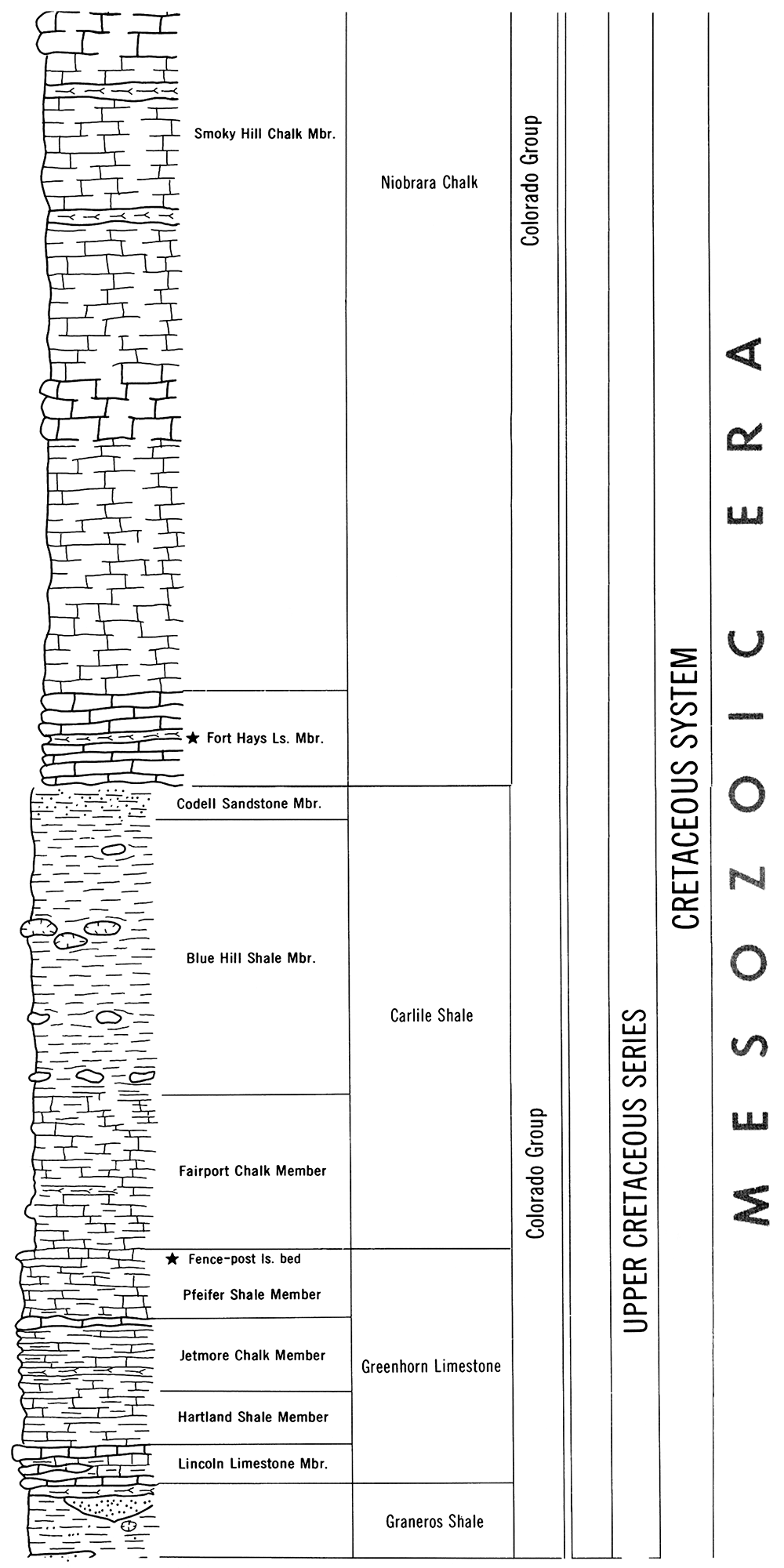
The Fencepost Limestone Member lies above the Pfeifer Shale Member in the upper part of the Greenhorn Limestone Formation and below the Fairport Chalk Member in the lower part of the Carlile Shale. The Fencepost Limestone has several unique features. It occurs over a large area in a relatively uniform thickness of approximately eight inches. The uniform thickness, small amount of overburden, and relatively flat terrain made it a natural choice for building stone and fenceposts around the turn of the century. It is also reported that the stone was used at one time to make telephone poles.
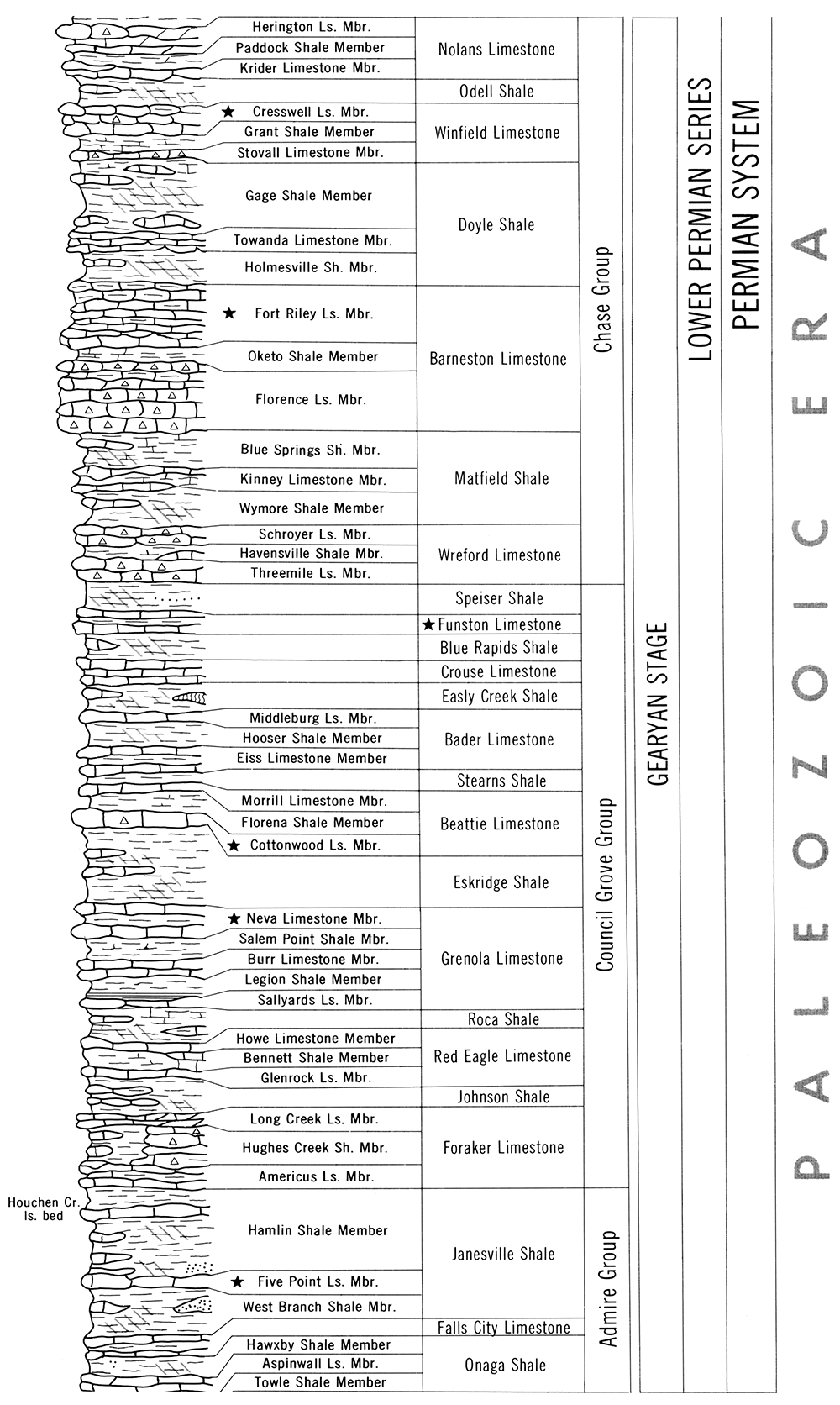
Except for the Cretaceous ledges, the remainder of the Kansas limestones used extensively in recent years for building are found in the Gearyan Stage of the Lower Permian Series. This stage is divided into three groups, the Chase Group (upper), Council Grove Group, and Admire Group (lower).
Good building limestones are found in all three groups. By contrast, no quality building stone is present in the Upper Permian rocks in Kansas. Figure 5 illustrates the stratigraphic succession of the Lower Permian series.
The majority of limestones in this group often contain pockets or zones of chert which make the stone unsuitable for building. Such chert zones are particularly common in the Three Mile and Schroyer Limestones and are especially noticeable in the thick Florence Limestone which is the cap rock on many of the Flint Hills. The Florence Limestone was at one time used locally for building stone.
The Cresswell Limestone Member is the uppermost of three members comprising the Winfield Limestone. Separated by the Grant Shale, the bottom unit of the Winfield Limestone is designated as the Stovall Limestone. The latter often contains pockets of chert that make it unsuitable for building stone. Use of the Cresswell Limestone has generally been restricted to the Winfield and Wichita areas. Because of its thin-bedded or laminated nature, it is commonly used for residential structures or other structures that do not require large blocks. An example of its use is the All Saints Church in Wichita. In some areas of Kansas the most massive portion of the Cresswell Limestone, the basal ledge, contains gray zones with abundant chert that make it unsuitable for building. In at least one area of the state the Cresswell Limestone becomes a dolostone and is too soft and fractured for use as a building stone.
The Fort Riley Limestone has been and continues to be one of the major Kansas building limestones. One of the best known locations of the Fort Riley is in the vicinity of Junction City where it is called the "Junction City limestone." This stone was used to construct the east wing (oldest part) of the State Capitol Building in Topeka and numerous buildings at Fort Riley (Geary County). Quarried extensively at one time, active production for building stone purposes has since ended around Junction City.
The other notable occurrence of the Fort Riley Limestone is in Cowley County where it is called "Silverdale limestone" after the small town where it is extensively quarried. The Silverdale stone is denser and stronger than the Junction City stone. Silverdale stone is well known by those familiar with the industry and its use is widespread in Kansas. Typical examples include the Cowley County Courthouse and the Church of Saint Jude in Wichita.
Figure 5—Stratigraphic succession of a portion of the Lower Permian sequence in Kansas. Stars indicate Kansas building limestones.
Unlike the Fort Hays and Fencepost limestones, the Fort Riley Limestone contains a thick ledge suitable for building stone so that large blocks can be quarried. The Fort Riley Limestone is a much harder and stronger limestone and cannot be cut directly from the ledge. Drilling and blasting are used to produce large blocks from selected ledges which are then hauled to the mill for further processing.
One of the two building limestones quarried in Pottawatomie County is the Funston Limestone. Locally, because this stone is quarried in the vicinity of Onaga, Kansas, the Funston used for building stone is normally called the "Onaga limestone." This location is unique since the ledge is approximately four to five feet thick compared to the hard, thinner (two feet) Funston ledge found elsewhere in the state. In addition to its frequent use for residential construction, "Onaga limestone" has been used in constructing the Eisenhower Museum (Abilene, Kansas) and the Topeka Public Library.
One of the most extensively used building stones in Kansas is the Cottonwood Limestone Member which forms the basal unit of the Beattie Limestone Formation. The Cottonwood Limestone is quarried in Chase County by the three major Kansas building stone producers. In this locality, the ledge is approximately six feet thick with the densest portion of the stone in the lower half of the unit. The upper half of the Cottonwood often contains large pores that are considered to make it more resistant to freeze-thaw deterioration than the lower portion of the ledge. This resistance is particularly important when the stone is frequently exposed to moisture.
Because the Cottonwood Limestone is quarried by all the major stone producers, it is not surprising that numerous examples of Cottonwood can be found in many parts of the state. One of the oldest structures is the Chase County Courthouse constructed in 1872. The bridge on the cover of this publication (1891) and most of the Capitol Building in Topeka are examples of older Cottonwood stone construction.
The uppermost member of the Grenola Limestone Formation is the Neva Limestone which is used as a building stone, particularly in the vicinity of Riley County where it is quarried. Despite its thickness of six feet, it is difficult to use all of the ledge because of its inconsistency. As a whole, the ledge is a hard stone and portions are relatively laminated. A zone towards the middle of the ledge at a quarry south of Manhattan is particularly hard. This zone has the highest known compressive strength of the Kansas building stones with a modulus of rupture greater than 20,000 psi. Unfortunately, this zone is both extremely hard and highly laminated in certain locations, making it completely unsuitable for building purposes. Above this zone is more suitable rock and three to four feet of the ledge can be used.
The "newest" and perhaps the most unusual building limestone in Kansas is the "Chestnut Shell limestone." Despite the fact that the stone was used locally as early as 1870, it was only recently quarried on a commercial scale. This unique stone is known only in northeastern Pottawatomie County where a two-foot ledge near Wheaton is quarried. The "Chestnut Shell" is a local name used for the layer of coquina which constitutes the Five Point Limestone in this vicinity. The natural bedding planes eliminate the need for blasting and allow the stone to be picked up from the ledge with a front end loader. In addition to residential construction, the stone has been recently used in eastern Kansas on several buildings including the new library and country club in Lawrence, Kansas and the McCall Pattern Co. building in Manhattan, Kansas. In other areas of the state, the Five Point Limestone becomes a typical limestone and in the past was used in Wabaunsee County for building purposes.
Despite the relatively low load-bearing strength of this stone due to its high porosity and structure, it offers good resistance to freeze-thaw action and the extremely high shell content comprises a most attractive and unusual texture.
As previously mentioned, modern stone producers utilize only certain ledges or parts of ledges for building stone purposes. Certain key properties must be examined to determine which limestones are best suited for building stone. There is no perfect building stone; i.e., stone which can be used for all purposes.
The most important limestone properties considered for building stone are color, texture, strength, specific gravity or bulk,density, and absorption. A summary of these properties for Kansas building limestones is presented in Table 1.
Table 1—Pertinent Physical Properties of Kansas Building Limestones. Properties were determined by Clayton Crosier, Civil Engineering Department, University of Kansas and by personnel at the Kansas Geological Survey, University of Kansas.
| Limestone | Texture | Color | Percent Absorption |
Specific Gravity |
Compressive Strength (psi) | |
|---|---|---|---|---|---|---|
| Perpendicular to bed |
Parallel to bed |
|||||
| Fort Hays | Med. to fine grain | Buff | NOT AVAILABLE | |||
| Fencepost | Fine grain | Buff | 5-6 | 2.2 | 10,200 | 11,500 |
| Cresswell | Fine grain | Buff | NOT AVAILABLE | |||
| Fort Riley | Med. to fine grain | Lt. Buff | 9 | 2.1 | 6,200 | 8,500 |
| Funston (Onaga) |
Fine grain | Lt. Buff | 7.5 | 1. 96 | 9,600 | 9,800 |
| Cottonwood | Med. to fine | Gray | 6.2 | 2.2 | 11,300 | 11,500 |
| Neva | Dense to fine | White | 3.1 | 2.44 | 22,600 | 18,800 |
| Five Point (Chestnut Shell) |
Coquinoid | Buff to pink | 5.4 | 2.1 | 4,800 | 5,600 |
Color
All of the Kansas building limestones currently produced are relatively uniform shades of buff or cream to "white" (off-white) or gray. The one exception is the "Chestnut Shell" which varies from buff to pale chestnut to pink depending on the location. The most important aspects of color to a stone producer are uniformity and, equally important, the effect of weathering on the color. A building constructed of limestone blocks from the same ledge in which there are slight visible differences in the color of individual blocks is sometimes unacceptable. In contrast, there are structures built from two or three stones with distinct color and texture differences which look quite attractive. Examples can be seen in the Photographic Review Section. Equally important are color changes which result from several years of exposure. As a general rule (disregarding the discoloring effects of dirt, algae growth, metal stains, etc.) buff, pink and reddish colored stone are relatively stable because the iron present in the stone is in the ferric state and not subject to oxidation. If the iron is present in the stone as ferrous iron, a slow oxidation to the ferric state can occur with a resulting change of color.
The staining due to airborne particles collecting on the surface of building stones (darkening the stone) is generally a slow process and is not always noticeable. It can be observed on old buildings on areas protected from rainfall, e.g., directly under large protruding window sills. By contrast, the darkening effect of algal growth occurs on highly exposed areas. Little information exists on why the latter phenomenon occurs and why many buildings never show signs of such discoloration. Information on weathering of different stones can be readily obtained from a stone producer. Kansas building stone producers can supply a list of buildings constructed from their material so that an interested individual can look at buildings constructed from the various limestone ledges.
Texture
In simple terms, texture refers to the arrangement, size, and shape of individual grains or fossil remains that make up the stone. Kansas building limestones are generally medium to fine-grained but unusual textures of the Cottonwood Limestone and "Chestnut Shell limestone" are the result of fossil remains. Although the Cottonwood Limestone contains several fossil types, abundant fusulinids with their wheat grain shape produce an interesting texture.
This is particularly true after many years of exposure. Weathering removes a very thin surface layer of limestone and causes the more resistant fusulinids to stand out in relief. The "Chestnut Shell" is basically a coquinoidal layer and the extremely high fossil concentration gives rise to a most interesting and unusual texture.
Strength
There are three types of strength moduli associated with building stone: compressive, transverse, and tensile. All three are measured in terms of the pounds per square inch that cause failure. The most important of the three is the compressive strength of the stone which is also referred to as the crushing or load-bearing strength. Obviously, stone used in the structure of a building must be able to withstand a load without failing. In the case of a home, the load-bearing capability need not be very large.
By contrast, large buildings may require stone with a higher compressive strength. Most Kansas limestones and certainly the building limestones more than satisfy the compressive strength requirement. Despite the size of the structure, building stone is seldom subjected to a load of more than 500 psi (pounds per square inch). The compressive strength modulus of currently produced Kansas building limestones is rarely less than 4000 psi and it can be as high as 20,000 psi.
The transverse modulus of rupture of a material is also called its flexural strength. It refers to the load (applied perpendicularly to the long axis of a piece of stone supported only at its ends) required to break the stone when subjected to flexure. For certain applications this measurement is important, for example, stone used in the lintels which form or span the tops of doorways and windows.
Least considered of the strength moduli is the stone's tensile strength. Normally stone is not used in applications where tensile stress is involved since the tensile strength of limestones are characteristically low (hundreds of psi). The simplest way to describe this modulus is to think of the weight or load that will cause failure when hung from the end of a vertical limestone rod.
Bulk Density
The bulk density value (g/cc, lbs/cu. ft.) is primarily determined by the chemical composition and porosity of the stone. Normally, a high density implies a limestone of high strength. Limestones usually contain impurities such as clays, soluble salts, iron and magnesium. The latter two may replace small amounts of calcium in the calcium carbonate lattice or may occur as distinct minerals, such as pyrite, limonite, or dolomite. Such impurities affect the bulk density of the limestone. The effect of porosity on the bulk density is probably obvious: the greater the porosity, the lower the density. The bulk density is important to a stone producer from an economic standpoint. The higher the density or weight the more it will cost to ship the stone per unit distance.
Weatherability or Durability and Absorption
One of the most important properties of a good building limestone is its ability to withstand the elements over a long period of time. A highly absorbent stone filled with fractures or "seams" is unsuitable. The load bearing (as well as flexural and tensile) strength is very low in such rocks. Such seams are susceptible to rapid weathering due to the easy access of solutions that promote dissolution, reaction, or failure through freeze-thaw action. Magnesium from clay minerals or solutions in the seams may cause dolomitization which is accompanied by a decrease in rock volume, thus promoting fractures. The same dissolution and reaction mechanisms occur in stone with a high porosity and permeability.
There is no absolute rule governing the relationship of porosity to a stone's resistance to weathering. One standard method of testing involves determining the dry, saturated, and suspended weights of a piece of limestone to determine its percent apparent porosity. Using this method, a stone with low absorption will show a low apparent porosity but may actually have a high porosity. This method only accounts for accessible pore spaces. The relationship of absorption, porosity and durability is complex as shown by the following example. One would suppose that a limestone with a large amount of connected pores (high porosity, permeability and absorption) would undergo dissolution by rainwater. However, one generally desires some porosity in a building stone so that it can "breathe." Otherwise, absorbed moisture, particularly at ground level, will be retained and may prove to be detrimental during periods of freezing (water to ice is accompanied by an increase in volume). Hence, while a stone may indeed have lower strength and be more susceptible to solution action (usually a longterm process) due to connected pores, these pores can make the stone more durable by imparting "flexibility" to the limestone structure. This flexibility may increase the stone's resistance to damage caused by volume changes associated with freeze-thaw action.
Before the advent of modern machinery, the quarrying and processing of building stone was a slow process. Building stone was typically obtained from exposures on hillsides. With limited means of removing much of the overburden, the stone producer had no choice but to work the stone in this manner. With luck, this exposure contained bedding plane partings which made separation of the limestone blocks much easier.
Often the processing was done on site. Blocks were hauled by horse and wagon to the site where they were cut or dressed by hand. This is the manner by which the stone used in the State Capitol in Topeka was processed.
As described earlier, relatively flat terrain, good exposures and a relatively uniform thickness of approximately eight inches made the Fencepost Limestone an ideal building stone around the turn of the century. Hand augers were used to drill a line of holes into which wedges were inserted. Hammering the wedges caused the rock to separate along the desired line. Blocks were then hauled to the site and dressed by masons. The quarrying marks are still visible on many of the early Fencepost limestone buildings such as the Cathedral of the Plains at Victoria.
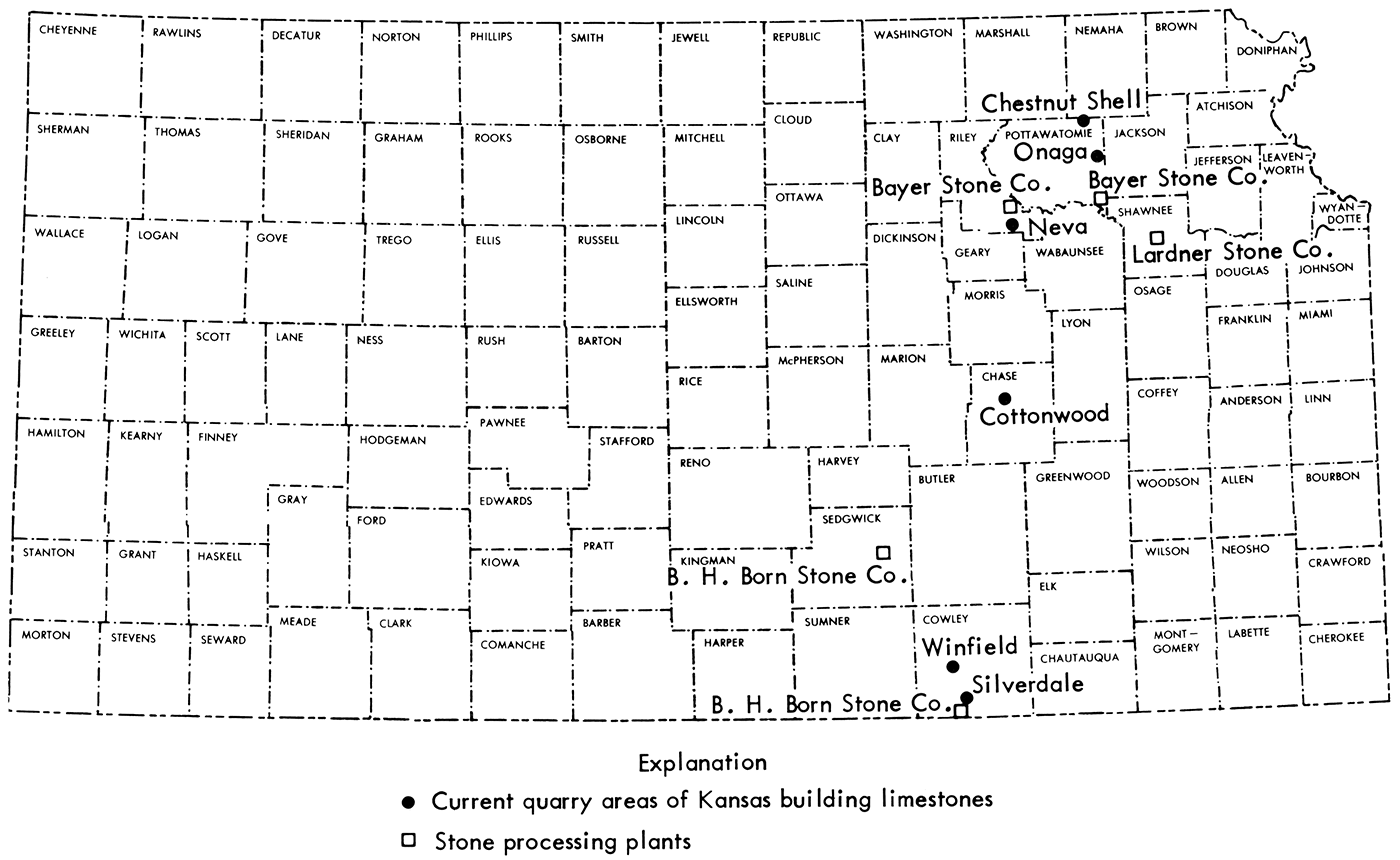
Modern quarry practice involves the stripping of overburden with earth moving machinery. If the overburden becomes too thick or the limestone ledge becomes too thin, economic principles dictate that the stone producer move to another quarry site. The locations of current building stone quarries and production facilities are shown in Figure 6.
Figure 6—Map of Kansas showing locations of sources and processing plants of Kansas building limestones.
For thicker ledges, drilling (to approximately three-quarters the depth of the ledge) and blasting are generally used to remove large blocks from the ledge. Thin ledges do not require blasting and wedging can be used to separate the blocks. As in the past, the stone producer makes the best use of natural partings in the ledge to facilitate removal of blocks. If sufficient fractures or partings are present, it is possible that a front end loader can be used to separate and remove blocks from thin ledges. An example of a modern stone quarry is shown in Figure 7.
Once the blocks are obtained, they are hauled to a plant where they are first sawed into slabs on a gang saw (Fig. 8). Basically, a gang saw consists of a series of long horizontally mounted saw blades in a parallel arrangement which slowly cut through the large quarry blocks. The slabs are then processed in different ways depending on the desired end product. Often the slabs are cut into desired sizes by a diamond coated circular saw such as the one shown in Figure 9. Afterwards a splitter (Fig. 10) may be used to produce split face stone of desired sizes. Finally, a planer (Fig. 11) can be used to further shape the block or give it a smooth face. As its name implies, the planer is a mechanized plane that progressively shaves thin layers from the stone until the desired shape or smoothness is obtained. Other methods of processing include stone grinders and a stone-working lathe which is used for turning columns and also does fluting if desired.
Figure 7—Typical building stone quarry.

Figure 8—A gang saw cuts quarry blocks into large slabs.

Figure 9—A diamond coated circular saw used to cut slabs. Water is used to facilitate cutting and control dust.

Figure 10—A splitter produces rough textured split-face stone.

Figure 11—A planer adds the finishing touches to a surface to form a smooth face finish.

Figures 12 and 13—Two photographs showing the positioning of a fluted Neva Limestone panel on the new Engineering building at Kansas State University.


Figure 14—Saint Patrick's Church, south of Atchison, was built during the early 1860's using Kereford Limestone.

Figure 15—The Capitol Building of Kansas at Topeka as viewed from the southwest. The east wing was constructed in 1867 from Fort Riley (Junction City) Limestone. The remaining wings, rotunda, and the dome were primarily constructed from Cottonwood Limestone.

Figures 16 and 17—Fort Hays Limestone was used to construct the blockhouse at Fort Hays (Hays, Kansas). The second photograph clearly shows the saw marks on the individual blocks.


Figure 18—Typical limestone fenceposts made from the Fencepost Limestone. Lack of timber led to the widespread use of limestone fenceposts, still commonly observed in central and western Kansas.

Figure 19—A portion of the First Southern Baptist Church at Abilene constructed in 1876 from Cottonwood Limestone.

Figure 20—Saint Joseph's Church at Liebenthal (south of Hays) was built in 1902 from Fencepost Limestone

Figure 21—This stone home near Elmdale was constructed in 1891 from Cottonwood Limestone.

Figures 22 and 23—Two views of the Saint Fidelis Church, better known as the Cathedral of the Plains, at Victoria. The church was constructed between 1908 and 1911, mainly from Fencepost Limestone. The church and adjacent buildings typify the pioneer spirit and the importance of the church in the area around Hays, Kansas near the turn of the century. Approximately 17 million pounds of stone were hauled from the quarry by the settlers to construct the church.


Figure 24—The interior of Our Redeemer Lutheran Church at Arkansas City shows a tasteful use of Silverdale limestone.

Figure 25—The Cresswell member of the Winfield Limestone was used to build Trinity Lutheran Church at Winfield.

Figures 26 and 27—Exterior and interior views of the Church of Saint Jude in Wichita constructed of smooth face Silverdale limestone.


Figure 28—The Peace Lutheran Church at Manhattan utilizing Neva Limestone in a modern architectural style.

Figure 29—Winfield Limestone was used on the exterior of the All Saints Catholic Church in Wichita.

Figure 30—Cottonwood Limestone columns along a portion of the Trinity Episcopal Church at Lawrence.

Figure 31—A large stone cross (Onaga limestone) highlights the First Baptist Church at Manhattan.

Figure 32—Kansas building limestone is used by sculptors. Kansas sculptor Peter Felton is creating this work from Silverdale limestone (photo by Craig Dexter).

Figure 33—Fort Riley (Silverdale) Limestone was used to form a waterfall at the Sedgwick County Zoo.

Figure 34—Picnic table and stools made from Onaga limestone.

Figure 35—The Chestnut Shell limestone provides an attractive background for the artistry on the waterworks at Manhattan.

Figure 36—Traditional all-stone house in Wichita made from Silverdale rubble.

Figure 37—An attractive mixture of Cresswell stone and other building material is exhibited at this home in Wichita.

Figure 38—A good example of limestone use for landscaping, as well as the residence, is shown by this photograph. Located at Wichita, this riverfront view illustrates the use of Cresswell and Silverdale trim.

Figure 39—An interior view at the previous home utilizing the Cresswell ledge of the Winfield Limestone.

Figure 40—An attractive appearance is provided by this blend of Neva, Onaga, and Chestnut Shell limestones.

Figure 41—Another residence shows a blend of Neva, Onaga, and Chestnut Shell limestones on an interior wall.

Figure 42—Fluted columns and circular pieces of Onaga limestone create an extremely attractive fireplace in this residence at St. Marys.

Figure 43—Cottonwood cut stone and Silverdale split-face stone were used to form part of the veterinary medicine building at Kansas State University in Manhattan.

Figure 44—Cottonwood Limestone and a lesser amount of Fort Riley Limestone was used to form the Memorial Campanile at the University of Kansas in Lawrence.

Figure 45—Fort Hays State College is unusual in that practically all buildings are constructed of limestone. Typical of the campus buildings is McCartney Hall, constructed from Fort Hays Limestone.

Figure 46—Garvey Art Center, at Washburn University, constructed from Cottonwood Limestone.

Figure 47—The Lawrence Country Club in Lawrence is covered with Chestnut Shell limestone.

Figure 48—A close-up of the block of Chestnut Shell limestone illustrating its unusual coquinoidal texture.

Figure 49—Cottonwood Limestone enhances the appearance of the Eisenhower Library, the first building constructed at the Eisenhower Center in Abilene.

Figures 50 and 51—Two examples of how limestone and wood are attractively combined include the dining hall at the 4-H Rock Springs Ranch (Onaga limestone) and the Chisholm Trail State Bank in Wichita (Silverdale rubble).


Figure 52—Fluted Onaga limestone panels add an interesting texture to the Hackler Office building in Olathe.

Figure 53—Cottonwood Limestone was used when building the Eisenhower Chapel at the Eisenhower Center in Abilene.

Figure 54—Silverdale stone was used to form the unique map and Cowley County courthouse at Winfield.

Figure 55—A local limestone was used to form the columns at the Greyhound Hall of Fame in Abilene.

Figures 56 and 57—Exterior and interior views of the impressive Salina Country Club at Salina constructed from Neva Limestone.


Figures 58-61—Cottonwood Limestone has been used extensively in large buildings in the Topeka, Kansas area. Examples include the Municipal Auditorium, State Office Building, Kansas Power and Light Building, and City Hall.




ashlar—a general term used to described rectangular blocks of stone (squared units) formed by sawing, planing or splitting. When blocks of each horizontal layer (course) are of equal height, it is called coursed ashlar. Split ashlar refers to blocks that have rough but relatively straight faces. If the exposed face protrudes from the wall (edges are chiseled back) it is termed pitched face ashlar. Such stone differs from cut stone since ashlar (1) can be in uniform or random lengths, and (2) is not accurately sized.
bed—a relatively uniform layer or ledge of rock which lies between two bedding planes or between different rock types or units. The bedding plane is a general term describing the surface formed by two adjacent ledges or strata.
calcite—a mineral name for the most common form of calcium carbonate, CaCO3, the major constituent of limestone.
chert & flint—one of the naturally occurring forms of silica, SiO2. It is so dense and extremely fine-grained that it exhlbits a conchoidal fracture. Generally, light colored material is called chert while the dark (grayish) form is termed flint.
cleavage—generally a plane (or parallel planes) of division in a mineral as determined by the arrangement of ions and their bond strengths in the mineral.
cobble (cobblestone)—a more or less rounded piece of rock, often formed by water and/or wind erosion. In terms of size classification, the diameter of a cobblestone varies from about 10 inches (25.6 cm) to as small as 2 1/2 inches (6.4 cm). Larger sizes are called boulders while smaller sizes are called pebbles.
coquina—a relatively coarse textured limestone composed of one or several types of cemented shells and/or shell fragments. The shells are usually bonded by calcite cement and the resulting limestone is extremely porous and attractive. The Chestnut Shell Limestone in northeastern Pottawatomie County, Kansas is an example of this type of stone.
curing—the process of allowing freshly quarried blocks of stone to dry before using. This is especially important in freezing weather since moisture will freeze, expand and possibly damage the stone.
cut stone—any building stone cut to a specific shape and size in accordance with building plans.
dimension stone—stone occasionally selected but usually fabricated to a desired shape and/or size for a large variety of purposes. All cut stone is dimension stone but the reverse is not true. For example, a sculpture or flagstone can be types of dimension stone but not examples of cut stone.
dolomite—the mineral name for calcium-magnesium carbonate, CaMg(CO3)2. Rock composed of dolomite is correctly called a dolostone and not limestone. A mixture of calcite and dolomite is called a dolomitic limestone.
efflorescence—the deposition of a film of soluble salt(s) on the surface of stone or clayware as a result of moisture migrating from the interior to the surface where the moisture evaporates leaving behind the soluble salt(s). Efflorescence commonly occurs when stone or clay contains a significant amount of soluble sulfates, especially calcium sulfate.
flagstone—a relatively thin, flat stone commonly used for walkways, patio floors or as stepping stones.
gangsaw—a series of parallel saw blades used to cut the large stone blocks from the quarry into slabs (tabular pieces) .
limestone—a sedimentary rock composed mainly of the mineral calcite.
milling—a catch-all term describing the processing of stone blocks from the quarry into a finished product by one or more methods.
planer—a machine using a tool which shaves off successive thin layers of stone until the desired shape and/or smoothness is obtained. Although frequently used to produce a smooth finish, the planer tool or blade may be set to "cut" deeply and the resulting plucking action yields a rougher or plucked finish.
quarry sap—the moisture present in freshly quarried stone.
riprap—large irregularly shaped pieces of rock (usually a mixture of sizes) used for various construction purposes such as facing of dams and levees, retaining walls, etc. Note that riprap can be composed of any type of rock, not just limestone although the latter is common in Kansas.
rubble—similar to riprap, rubble is also irregularly shape~and sized broken stone used in constructing walls, columnS), etc. Usually rubble is of smaller size than riprap.
spalling—generally used to describe the flaking off of pieces of stone as the result of weathering (freeze-thaw action or thermal expansion and contraction) and/or pressure.
splitter (split-face machine)—a machine that splits large stone pieces into smaller, more usable pieces. The rough finish produced in this manner is called a split-face finish.
Calculations of the heat lost or transferred through any given material or wall structure is derived from an equation developed by the French mathematician Fourier.
dQ/dt = kA (dT/dx)
dQ/dt = heat transfer (Q) per unit of time (t)
k = coefficient of thermal conductivity
A = area through which the heat is flowing
dT/dx = temperature difference causing the heat flow per unit thickness (path length through material in direction of heat flow)
In the case of equilibrium or steady state conditions, heat transfer is constant so that dQ/dt = q. Solving for k yields the equation
k = (g/A) (dx/dT)
where k is commonly expressed in units of
[(BTU) (inch) / (hour) (sq.ft.) (°F)] or [(BTU) (in.) / (hr.)(ft2)(°F)]
Although k is called a proportionality constant, in reality k will vary for a given material with the temperature and density. In general, the k of a rigid material (depending on the material) slightly decreases or increases with increasing temperature and decreases with decreasing density. The k value is not appreciably affected by small temperature changes. Since we are considering a relatively narrow temperature range, k will be considered a constant for our purposes. Certainly, decreasing density (increasing porosity) will change a measured k value but an average value can be used with a reasonable degree of accuracy.
In considering the heat flow through a wall composed of a single material:
q = kA [(T1 - T2) / x]
where T1 and T2 are the temperatures on each side of the wall of thickness x. Introducing R = x/kA simplifies the equation to:
q = (T1 - T2) / R
where R is called the thermal resistance.
Considering the case of a wall constructed of n materials, each material has a q value and the total effect is:
Q = (Ti - To) / (R1 + R2 . . . + Rn) = (Ti - To) / (Rt)
where Ti and To correspond to inner and outer wall temperatures respectively.
If we consider only one square foot of wall area, then U, the overall coefficient of heat transfer through a wall in BTU/hrft2 °F is given as
U = 1 / Rt
If we recall that R = X/kA and that A = one square foot then
Rt = 1 / U = (x1 / k1) + (x2 / k2) + . . . (xn / kn)
The above equation includes solid materials used in wall construction. There are three other terms which should be included.
In the construction of buildings, there are often dead air spaces. For example, air spaces exist between components of a wall or between regular doors and windows and storm doors and windows. If the air space has a thermal conductance of a, then the thermal resistance of an air space, Ra = 1/a. Normally, the thermal conductance of an air space of 3/4" or more is considered constant (a = 1.10).
The two other terms are based on film effects, specifically a thin layer or film of air next to the inner and outer surfaces of a wall with respective surface conductances of fi and fo and with film or surface resistances of Ri = 1/fi and Ro = 1/fo for A = 1 ft2.
Considering the effects of air spaces and films now yields:
Rt = (1 / fi) + (1 / fo) + (1 / a1) + (x / k1) + . . . + (1 / an) + (xn / kn)
= (1 / fi) + (1 / fo) + (1 / C1) + . . . + (1 / Cn)
where C is the thermal conductance20f a material of a specified thickness in BTU/hr. ft2 °F. This means that Rt = ∑1/C = ∑x/k.
The surface coefficients vary with surface area and wind velocity. A rough exterior has a greater surface area which transfers more heat than a smooth exterior. A greater wind velocity also increases the heat transfer. As the inside walls of buildings are normally smooth and the air is considered still air, fi is normally considered constant. By contrast, fo varies from approximately fo = 1.4 + 0.30v for smooth surfaces to fo = 2.1 + 0.5v for rough surfaces where v is the wind velocity in miles per hour. It is a standard practice to use fo = 6.0 and 4.0 for winter and summer calculations respectively assuming v = 15 mph during the winter and 7.5 mph during the summer. Note also that different authors may use slightly different coefficients for fi and fo.
We now have the means and calculate U values for various wall constructions. Normally only outside and inside surface coefficients are considered. The basic procedure is to consult tables for k or C values and calculate the R for each component material. The U value is the reciprocal of the sum of the R values.
Consider two houses with the following wall structures in the winter (see Table 2). These are examples of composite walls currently being used by local contractors.
Table 2—Calculation of the Over-All Coefficient of Heat Transfer (U) Through Typical Composite Walls of Conventional and Stone Structures
| Conventional Structure | k or C | R |
|---|---|---|
| fo (outside film) (15 mph wind) | 6.0 | 0.17 |
| 5/8" plywood sheathing and exterior | 1.28 | 0.78 |
| 3 1/2" mineral wool | 0.27 | 12.95 |
| 1/2" gypsum board | 2.25 | 0.45 |
| fi (inside film) | 1.46 | 0.68 |
| 15.03 | ||
| U = 1/R = 6.65 x 10-2 BTU/hr ft2 °F | ||
| Stone Structure | k or C | R |
| fo | 6.0 | 0.17 |
| 4" Stone | 12.5 | 0.32 |
| 1" Air space | 1.03 | 0.97 |
| Vapor-proof building paper | ||
| 3/8" plywood sheathing | 2.12 | 0.47 |
| 3 1/2" mineral wool | 0.27 | 12.95 |
| 1/2" gypsum board | 2.25 | 0.45 |
| fi | 1.46 | 0.68 |
| 16.01 | ||
| U = l/R = 6.24 x 10-2 BTU/hr ft2 °F | ||
| For the same two structures during the summer, the same values are used except fo is calculated assuming a 7.5 mph wind so fo = 4.0, R = 0.25 and the resulting U values are: | |
| Conventional Structure: | 6.61 x 10-2 BTU/hr ft2 °F |
|---|---|
| Stone Structure: | 6.21 x 10-2 BTU/hr ft2 °F |
The very small difference in the U values may at first seem insignificant. However, the heat loss in BTU/hr would be
q = UA (Ti-To)
which includes the effect of the total wall area and the inside and outside temperatures. The following example (Table 3) is calculated for winter conditions assuming an average monthly temperature of 20 °F for a 3 bedroom rancher with 1500 ft2 of wall space and an inside temperature of 70°F.
Table 3—Calculation of Total Kilowatt Hours (kwh) Lost Through Walls of Conventional and Stone Structures During a Winter Month
| Conventional Structure |
Stone Structure |
|
|---|---|---|
| U in BTU/hr ft2 °F | 6.67 x 10-2 | 6.24 x 10-2 |
| 1. Multiply by 1500 ft2 | ||
| 2. Multiply by 50 °F | ||
| q in BTU/hr = | 4,988 | 4,680 |
| 3. Multiply by 24 hr/day | ||
| 4. Multiply by 30 day/mo. | ||
| Total heat lost through walls in BTU/mo. |
3.591 x 106 | 3.369 x 106 |
| Kilowatt hours lost through walls using 3,412 BTU/kw |
1,052 | 978 |
These figures show that 6.6% more kwh are required to heat the conventional structure. Using a vapor-proof building paper with R = 0.08 would lower the U value for a stone structure to 6.21 x 10-2 BTU/hr ft2 °F. The kwh lost in this case would be 982 and the wood structure would require 7.1% more energy to heat than to the stone structure. One may conclude that very small changes in the U value for a wall have a significant long term effect on energy consumption.
To further illustrate the merits of a well-insulated wall, some contractors will use a building paper of R = 0.12 and in place of 3/8" plywood sheathing, a 1/2" insulating board is used with R = 1.6. These two modifications and an 8" stone thickness would produce a U of 5.72 x 10-2 BTU/hr ft2 °F. For the above winter conditions, only 905 kwh would be lost through the walls during a month. Using this value, a conventional wood structure would require over 16% more kwh than the stone structure.
These calculations show beyond a doubt that it is worthwhile to spend the extra money in order to obtain a well-insulated wall structure. This statement is especially true at a time when we face potential energy shortages and continued increases in fuel costs.
Kansas Geological Survey
Placed on web Oct. 12, 2018; originally published 1976.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/MRS4/index.html