Prev Page--Installation || Next Page--Site 7
Site 6
Soil profile and physical and chemical characteristics
The soil profile at site 6--an Ost soil, a fine-grained loamy, mixed, thermic Typic Argiustoll--consists of grayish-brown silt loam down to 40 cm (16 in. or 1.3 ft) (the A horizon); the upper portion of this horizon is characterized by a medium granular structure, and the lower part by a weak, medium subangular, blocky structure. Underlying that layer is an 8-cm (3-in.) thick grayish-brown E horizon with a silt loam texture and a weak medium-platy structure. A relatively hard brown to yellowish-brown silty clay loam layer underlies the E horizon down to 77 cm (30 in. or 2.5 ft), making up the Bt horizon, which is characterized by a moderate, medium prismatic and subangular, blocky structure with many fine platelike soft masses of lime and common fine irregular salt masses; this Bt horizon is strongly effervescent. Underlying this soil is another light yellowish-brown soil of moderate, medium subangular, blocky structure; this soil is characterized by only a Bt horizon, which can be subdivided into three Bt subhorizons down to 147 cm (57.9 in. or 4.82 ft). This second soil has a clay loam texture and is characterized by many rounded carbonate nodules that are strongly effervescent. A third light-brown buried soil is also present, consisting of a Bt horizon of subangular blocky structure and a transitional BC layer down to the maximum analyzed depth of 230 cm (90.5 in. or 7.5 ft). This third soil also has a clay loam texture and is characterized by many rounded carbonate nodules that are strongly effervescent.
The soil horizons and their grain-size distribution (texture), as determined by the Soil Conservation Service (SCS), are shown in fig. 5. It can be seen from the figure that the clay fraction increases significantly in the 45-90-cm (1.5-3.0-ft) depth interval, whereas the sand fraction decreases. As the fine-grained fraction increases, the surface area per unit volume of soil increases markedly, thus making the soil more chemically active because of its greater surface charge per unit volume and its capacity to hold greater amounts of water by adsorption. The significance of these observations as they relate to organic chemical movement through the soil will be shown later.
Figure 5--Grain-size distribution and soil horizons for site 6.
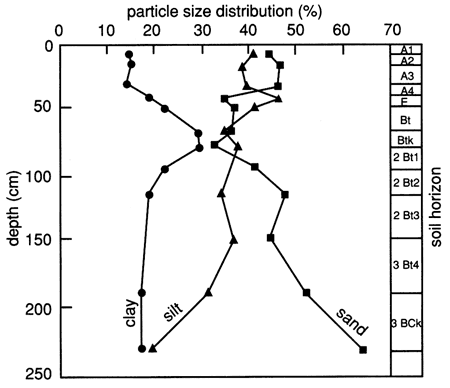
According to the SCS, the clay fractions of all analyzed soil horizons (A1, A3, Bt, 2Bt, 2Bt3, and 3BCk) exhibit only medium mica and montmorillonite peaks, with kaolinite and quartz peaks being in the small and very small range.
The organic carbon content of the site 6 soil profile (fig. 6) is much higher in the upper 40 cm (1.3 ft) than in the rest of the soil profile. Organic carbon makes up over one-half of the organic matter, and therefore the organic carbon content is commonly used to characterize the amount of organic matter in soils. Generally, the percentage of organic matter in a soil is considered to be 1.72 times the percentage of organic carbon (Birkeland, 1984). Soil organic matter considerably increases both the water-holding capacity and the CEC of soils. The organic acids produced from organic matter form chelating compounds that increase the solubility of some ions in the soil environment. The carbon dioxide gas that is evolved during humus formation (the bulk of the soil organic matter) reaches concentrations higher than those in the atmosphere and ultimately forms carbonic acid, which lowers the soil pH.
Figure 6--Organic carbon content and cation-exchange capacity profiles for site 6.
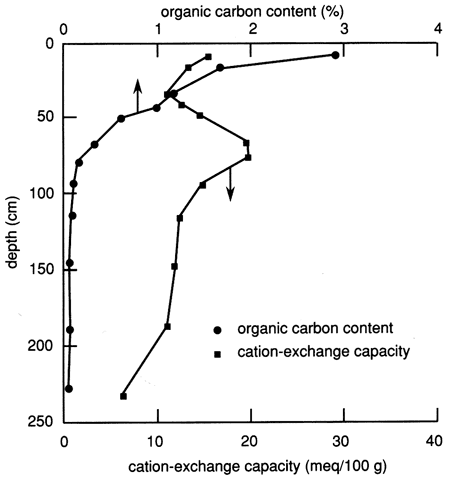
The CEC of the soil profile is also shown in fig. 6. In addition to a relatively high proportion of organic matter, this figure shows that the near-surface soil has a relatively high CEC, which gradually decreases toward the bottom of the upper 30 cm (1 ft) of soil. In general, the CEC profile follows the clay content profile (fig. 5). Most soil colloids (inorganic and organic) have a net negative surface charge and thus attract cations. The strength of cation attraction varies with the colloid and the particular cation, and some cations may exchange with others. The total negative charge on the surface is the CEC. It is expressed in milliequivalents per 100 g oven-dried material.
The soil bulk density, determined by the core method using a Madeira sampler and by the clod method (analyzed by the SCS), is shown in fig. 7. An increase in bulk density with depth is evident and apparently results from the lower organic matter content (fig. 6), less aggregation and root penetration, and compaction caused by the weight of the overlying layers.
Figure 7--Bulk density distributions determined by the two methods indicated for the soil profile at site 6.
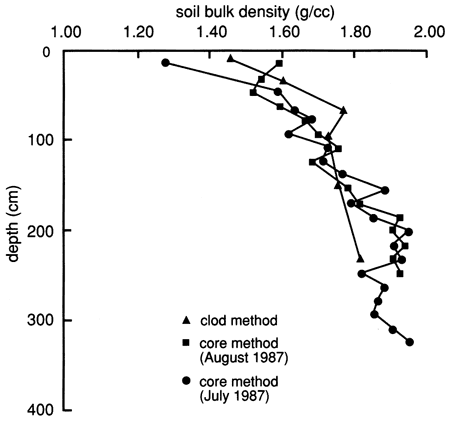
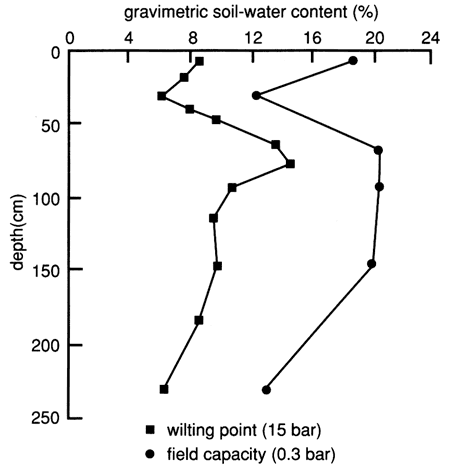
Figure 8 depicts the available water-holding capacity of the soil profile of site 6. The available water-holding capacity is the difference between the field capacity (specific retention) and the permanent wilting point (the point at which the moisture is so tightly held by the soil particles that the roots can no longer extract it). Water and chemical retention and movement in soil are strongly related to the surface area per unit volume of the soil mass, and this in turn is related to the clay fraction (fig. 5) and the organic matter content (fig. 6).
Figure 8--Available water-holding capacity of the soil profile at site 6.
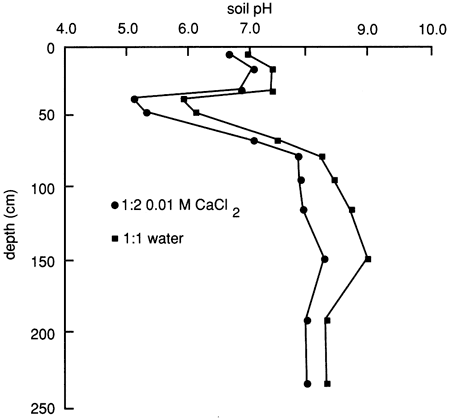
The soil solution pH distribution of the soil profile based on a 1:1 soil to water extract and a 1:2 soil to 0.01 M CaCl2 solution is depicted in fig. 9. The soil approaches definitely acidic conditions just below the upper 30 cm (1 ft), whereas below the 60-cm (2-ft) depth it approaches moderately alkaline conditions. Soil pH is often related to dissociation, adsorption, and chemical alteration of organic chemicals, the concentration of inorganic ions in the soil solution, and the mineral character of the soil. The two principal controlling factors are the organic matter content and the type and amount of carbonate minerals and dissolved carbonate anions. A large amount of organic matter induces acidity, except when counterbalanced by a high concentration of soluble carbonates.
Figure 9--Soil solution pH at site 6.
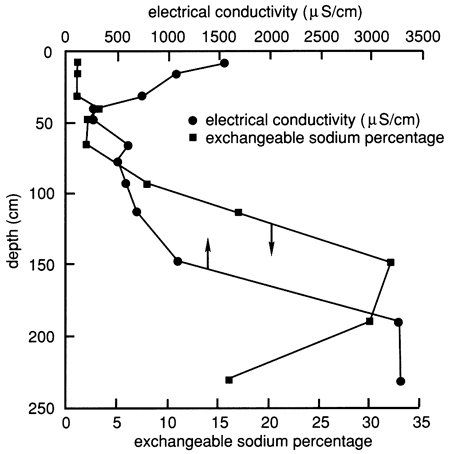
The soil profile contains an appreciable amount of salts, especially in the lower horizons below 120 cm (4.0 ft) and in the upper 30 cm (1.0 ft), as shown by the electrical conductivity of the water extracted from saturated soil pastes (fig. 10). The exchangeable sodium percentage, which indicates the degree of saturation of the soil-exchange complex with sodium, is also shown in fig. 10. The lower part of the soil profile below 120 cm (4.0 ft) contains a significant amount of sodium, enough to influence hydraulic conductivity. However, in the 2Bt1 horizon [77-93 cm (2.5-3.1 ft)] and the 2Bt2 horizon [93-113 cm (3.1-4.82 ft)] the salt content is low enough to keep the clay in a dispersed state, given the relatively high sodium content of these horizons (fig. 10).
Figure 10--Electrical conductivity and exchangeable sodium percentage for the soil profile at site 6.
Water chemistry
Site 6 is located in Stafford County at the edge of a flood-irrigation field used for growing corn during the period of study. The average precipitation in the area is 61 cm/yr (24 in/yr) (Sandyland Agricultural Experiment Station, 1983). The site has a zone of concentrated salts in the upper 6.1 m (20 ft) of the soil, as shown by the background specific conductance measurements collected from the 6.1-m, 10.1-m, and 13.7-m (20-ft, 33-ft, and 45-ft) wells before the test (table C. 1), the observed salt accumulations within the soil profile (below the A and E horizons), and the electrical conductivity of the water extracted from the soil pastes (see fig. 10). The specific conductance measurements from the 6.1-m (20-ft) well are considerably higher than those from either the 10.1- or 13.7-m (33- or 45-ft) well (table C. 1). Drilling logs of these wells indicate clay layers between the 6.1-m and 10.1-m (20-ft and 33-ft) wells (see fig. 4). These layers may prevent movement of salts to the lower aquifer zones.
A definite source of the salts in the sampled soil solutions and ground water is the paleosols at depth. Evapotranspiration losses may be responsible for concentrating salts in the upper soil zone of the field. Plants are capable of absorbing fairly fresh water but leave most of the dissolved solids from the water in the soil zone (Bower, 1978). Continuous flood irrigation for a considerable length of time may also result in movement of salts from the upper root zone into the deeper lower permeability horizons. The bromide/chloride ratios for the soil background solutions (collected from the suction lysimeters) and for the ground water at the site were generally too low to be from oil-brine disposal in the Great Bend region (Whittemore, 1984).
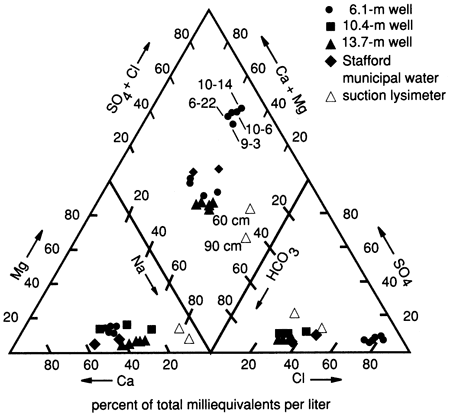
The dominant water type at this site is Ca-Na-HCO3-Cl water (fig. 11). As stated previously, this composition is probably due to the movement of a slug of salts from the soil zone downward to the shallower water table. The location of the 60- and 90-cm (2- and 3-ft) lysimeters on the trilinear plot (fig. 11) illustrates the effects of salt movement through the soil zone. The lysimeters show a Na-Ca-HCO3 water, which may indicate concentration processes by plants and cation-exchange processes with clays in the soil zone. The presence of carbonate nodules below a depth of 50 cm (20 in.) appears to substantiate the idea of concentration and precipitation of constituents from the soil solution.
Figure 11--Trilinear diagram showing water chemistry data for three wells, two suction lysimeters, and Stafford municipal water (used for flooding) at site 6.
At site 6 the low level of nitrate in the soil zone is probably due to the lack of nitrogen fertilizer application on the test plot (table C.1). The concentrations of nitrate in the 20- and 33-ft (6.1-m and 10.1-m) wells are similar to the levels recorded for the 38-ft (11.5-m) GMD5 ground-water quality network well (26 mg/L nitrate in the quarter section east of our test site) (table C.1). This implies that the water in the monitoring wells reflects the overall ground-water flow from the farm.
Water and chemical flooding
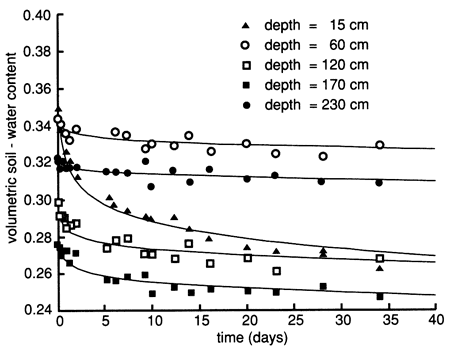
The soil-moisture profile at site 6 just before flooding indicated dry conditions throughout most of the soil profile (fig. 12). To reduce lateral water movement from the area to be flooded to the surrounding area and to increase the soil water content, we flooded the perimeter strip of the area with 3.79 m3 (1000 gal) of Stafford municipal water on September 9, 1987. We covered the wetted perimeter area with plastic immediately and then flooded the enclosed area with 9.52 m3 (2250 gal) of Stafford municipal water at a rate that ponded the water to a depth of 5-10 cm (2-4 in.) for 6 days (September 9-15, 1987). We then flooded the area with 1.89 m3 (500 gal) of chemical solution (atrazine and sodium bromide) for approximately 1 day. The chemical flooding was identical with water flooding with respect to application rate and ponding depth. The flooded area was hexagonal in shape and enclosed 15.4 m2 (166 ft2) of soil. As soon as the chemical slug seeped into the soil (September 16, 1987), we covered the site with heavy black plastic and a thin layer of soil to minimize evaporation losses. The soil-moisture profiles before and during flooding show that the soil became nearly saturated down to approximately 270 cm (8.9 ft) (fig. 12). Soil-moisture drainage curves for 15-cm (6-in.) depth intervals after covering the site with plastic are shown in fig. 13, where soil-water drainage down to the 230-cm (7.5-ft) plotted depth can be recognized.
Figure 12--Soil-water content profiles before and during flooding in 1987 at site 6.
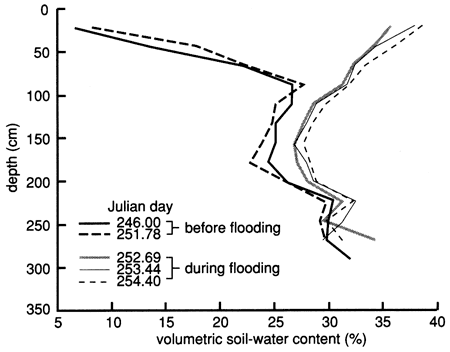
Figure 13--Soil-water drainage curves in 1987 for five depths at site 6. The curves were derived by means of a least-squares fit using a drying model.
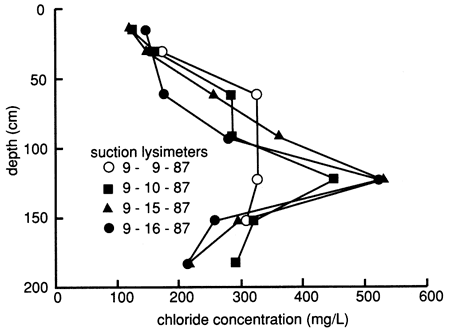
During water flooding but before chemical flooding, we sampled the suction lysimeters on various dates and analyzed the samples for chloride, bromide, and sometimes other dissolved inorganic constituents. The distribution of dissolved chloride with depth, plotted in fig. 14, suggests that the flooding freshwater (with a chloride content of 157 mg/L) displaced naturally existing salts downward within much of the soil profile (see section on the soil profile, especially fig. 10). However, there also is evidence of some preferential movement of floodwaters through the profile. Flood solutions appeared to dissolve salts at the 120-cm (3.9-ft) depth, but the high chloride contents were not displaced to soil horizons immediately below this depth. Instead, the results suggest that the variations at the underlying depths are caused by water bypassing the 120-cm level. The high content of chloride in samples from the 120-cm depth could have accumulated at the base of the root zone by evapotranspiration. The presence of carbonate nodules below a depth of 60 cm (2 ft) appears to substantiate the idea of concentration and precipitation of constituents from the soil solution. The salts would then be available for dissolution and deeper penetration during prolonged wet periods or excessive irrigation, as simulated by the flood experiment.
Figure 14--Profiles of dissolved chloride sampled from suction lysimeters during flooding at site 6.
The source of the high-conductivity water is probably the abundant irregular salt masses and rounded carbonate nodules, which increase with depth throughout the soil profile below the A and E soil horizons (see fig. 5). The measured electrical conductivity of water extracted from saturated soil pastes before the flooding experiments generally increased with depth and ranged from 2000 µS/cm to >3000 µS/cm below the 120-cm (3.9-ft) depth; the specific conductance of suction lysimeter samples ranged from 2300 µS/cm to 4300 µS/cm over the sampled depth intervals of 90-150 cm (3.0-4.9 ft) during flooding (table C.1). Data from drillers' logs indicate increasing caliche amounts from the near-surface down to almost 9 m (30 ft), whereas the unconsolidated sediments become sandier with depth below 1.5 m (4.9 ft). Although the upper 75 cm (2.5 ft) of the soil profile was dry before the flooding experiment, the profile below 2 m (7 ft) was relatively wet (see fig. 12). The sandier and wetter nature of the deeper sediments would facilitate transmission of saline solutions to the shallow water table [3.4 m (11-ft) deep]. To saturate the unsaturated soil profile within the diked area at site 6, 3.83 m3 (1010 gal) of water would be required, assuming no displacement of native pore water and no lateral flow. Thus the remaining 6.58 m3 (1740 gal) of chemical solution and flooding water (representing 38% of the pore water within the flooded pore volume above the water table) would have displaced an equal amount of pore water. Assuming a combination of displacement and mixing with the 2.7-m (8.9-ft) water column [based on the 6.1-m (20-ft) well and a 3.4-m (11-ft) depth to water], the concentration of the leaching solution needed to raise the ground-water conductance from 2500 µS/cm to 4200 µS/cm (see fig. 8) would be 5800 µS/cm, a plausible estimate given the abundance of salt masses and carbonate nodules in the deeper soil profile.
After several days of suction lysimeter sampling, the performance of the lysimeters deteriorated. Many lysimeters, especially those at shallower depths, could not hold a vacuum long enough to collect sufficient pore fluid for analysis. Careful cleaning of the rubber stoppers in the lysimeters (as supplied by the manufacturer) and wrapping with Teflon tape did not markedly improve the performance. Subsequent freezing conditions rendered all suction lysimeters inoperable during the winter.
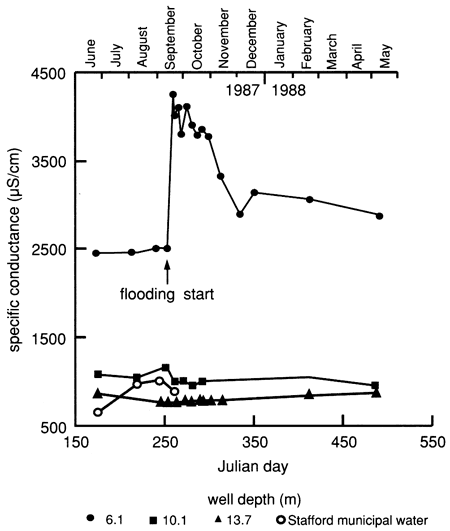
The displacement of more mineralized soil water by the fresher flooding water is also reflected in the specific conductance and chloride concentration in the 6.1-m (20-ft) observation well, as shown in fig. 15, whereas the deeper observation wells remained practically unaffected. The dissolved solids content increased markedly in the ground water, as indicated from the shallow observation well [6.1 m (20 ft)] after the onset of water flooding at the surface. Thereafter the amount of dissolved solids decreased steadily, but to levels appreciably higher than before the experiment. The salt movement to the water table is probably the result of piston-like displacement of more-saline soil solutions in the soil profile.
Figure 15--Specific-conductance time-series distribution of observation wells and of Stafford municipal water used for flooding at site 6.
The significance of the observations from site 6 to agricultural chemical movement is that, during periods of unusually high rainfall and ponding in the area or during excessive flood irrigation, downward movement of chemicals concentrated in soils could be triggered, thus affecting the quality of the shallow ground water.
We used the instantaneous profile technique (Rose et al., 1965; Watson, 1966; Hillel et al., 1972) to analyze the results of the flooding experiment. We wrote a set of FORTRAN 77 computer programs to analyze and graphically display the field data. To summarize the results of the flooding experiment, we depict in figs. 16 and 17 the field-obtained water characteristic or water-retention curves (which express the ability of the soil to retain water as a function of its energy status) and the hydraulic conductivity curves (which express the ability of the soil to transmit water as a function of its degree of water saturation) for various depths. Because of the clayey nature of the soil profile, the observed water characteristic and retention curves show only a minor decrease in water content with an increase in capillary pressure or suction (fig. 16). The resulting Darcy water fluxes for various depths as a function of elapsed time since the site was covered with plastic (to prevent evapotranspiration losses) are shown in fig. 18, in which an exponential decrease with time can be readily recognized.
Figure 16--Field-measured water-retention curves for site 6.
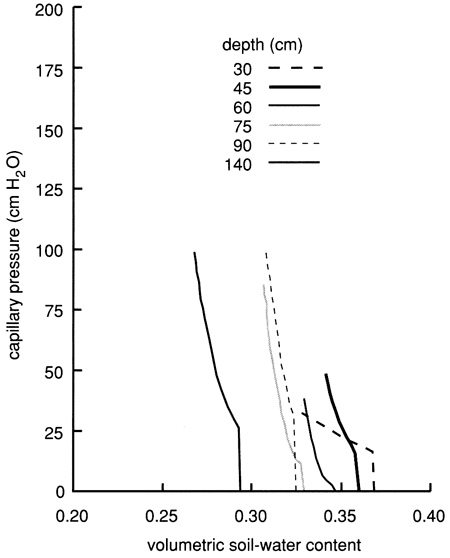
Figure 17--Hydraulic conductivity curves derived from the instantaneous profile technique for site 6.
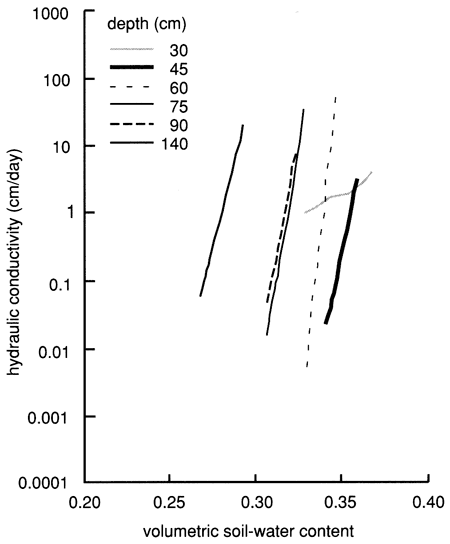
Figure 18--Soil-water flux time distributions derived from the instantaneous profile technique for site 6.
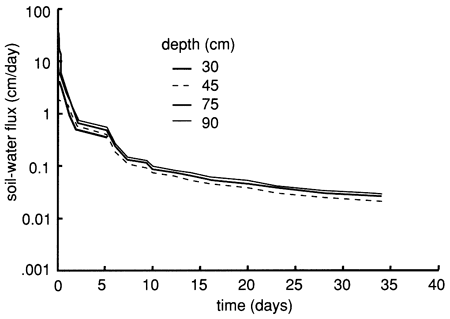
The soil profile temperatures during the flooding experiment (September to November 1987) are shown in fig. 19. A progressive soil temperature decrease with time at all measured depths is evident. The figure also shows that during September there was a progressive temperature decrease with depth. However, from October onward there was a temperature-depth reversal; that is, deeper temperatures were higher than shallower temperatures. It is well established that the temperature of a soil greatly affects the physical, biologic, and chemical processes occurring in that soil. In low-temperature (cold) soils chemical and biologic rates are slow, with absorption and transport of water and chemical species adversely affected.
Figure 19--Soil-temperature time-series distribution for six depths at site 6.
Bromide
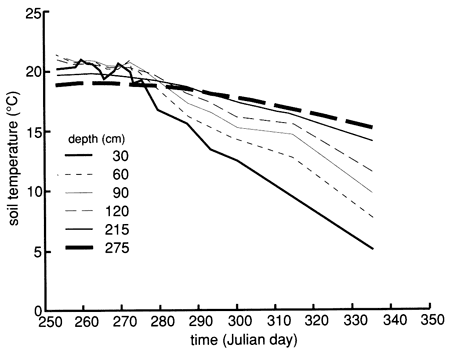
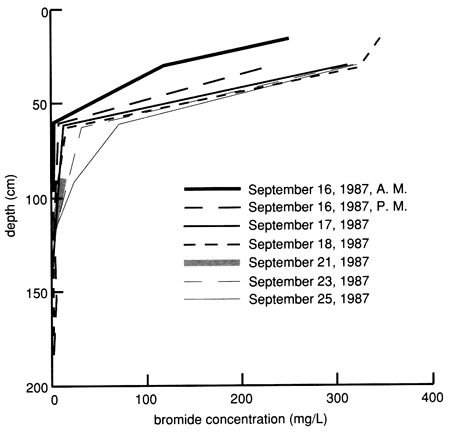
Bromide concentrations versus time after the start of chemical flooding are shown by the tracer breakthrough curves through the soil profile based on suction lysimeter sampling (fig. 20). Bromide concentrations were corrected by subtracting the small background values at each depth (mostly in the range of 0.1-0.2 mg/L). The actual bromide content of the chemical solution applied was 376 mg/L. The added bromide penetrated to the 150-cm (5-ft) level in concentrations appreciably above background within 2-3 days after the beginning of chemical flooding. Conclusive penetration of tracer bromide to levels substantially higher than the highest background content occurred after 2 weeks at the greatest lysimeter depth of 180 cm (6 ft). However, conclusive penetration of tracer bromide to the 120-cm (4-ft) depth was not detected until 9 months after addition, corroborating the observation of some preferential flow based on chloride movement. The bromide concentration distribution in the soil profile after chemical flooding was initiated (September 15, 1987, 11:00 A.M.) is shown in fig. 21; the bulk of the bromide solution is indicated in the top 30 cm (1 ft) of the soil profile. Before chemical flooding, the bromide concentration in the soil profile was within the thickness of the 0 bromide concentration line and thus not plotted in fig. 21. Lack of complete data at later times prevented the study of the bromide pulse decay. The bromide persistence observed at the 30-cm (1-ft) lysimeter is caused by the substantial decrease in hydraulic conductivity and water flux in the soil profile after flooding (see figs. 17 and 18) and the existence of a clayey underlying soil zone (see fig. 5). The greater apparent penetration of the applied water is due to the infiltration of floodwater without tracer before initiation of the chemical flood.
Figure 20--Bromide concentration breakthrough curves based on suction lysimeter sampling for site 6. Bromide is expressed as the ratio of bromide corrected for background levels (Brc) to bromide tracer concentration in the flooding solution (Br0).
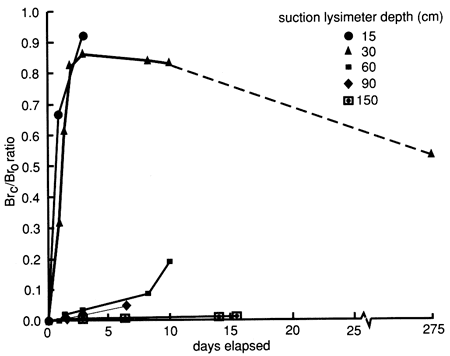
Figure 21--Bromide concentration profiles after chemical flooding was initiated at site 6.
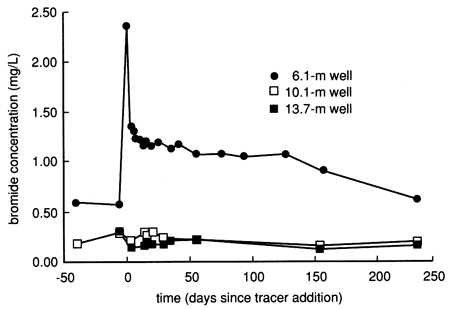
The bromide time-series distribution from the shallow 6.1-m (20-ft) observation well has a pattern similar to the specific conductance and chloride time-series distributions for the same well (see fig. 15). The water and chemical flooding displaced naturally existing bromide in the soil profile down to the water table, thus creating the relatively elevated bromide levels in the shallow ground water after the start of flooding (fig. 22). The displaced bromide is interpreted to exist naturally in the deep soil because the accompanying rise in chloride in the well water kept the bromide/ chloride ratio in the range expected for concentration of natural soil waters by evapotranspiration (Whittemore, 1988). The deeper observation wells were not affected by the bromide displacement process (fig. 22). More than a year after chemical flooding, no bromide concentrations related to tracer breakthrough to the water table were observed in any of the wells. All bromide data are tabulated in tables C.1 and C.4.
Figure 22--Bromide concentration time-series distribution from site 6 observation wells.
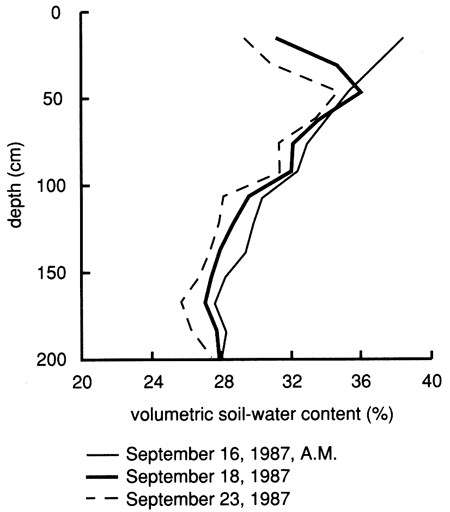
Figures 15 and 22, referring to the upper (shallow) saturated zone, and fig. 14, referring to the vadose zone, demonstrate vertical displacement of pore fluids by the flooding water. Comparisons of water content with depth on the dates of bromide sampling indicate that the relative distribution of the bromide tracer with depth does not correspond to the water content distribution in the soil profile (fig. 23). This observation supports the deduction from figs. 14, 15, and 22 that the applied chemical flood primarily displaces the initial pore fluids during the infiltration and percolation process; percolation through the soil profile by bypassing the existing pore fluid is minor. As a consequence of this displacement, the bromide tracer remains near the soil surface, thus explaining its persistence in the upper foot of soil.
Figure 23--Soil-water content profiles since chemical flooding was initiated at site 6.
Atrazine and atrazine metabolites
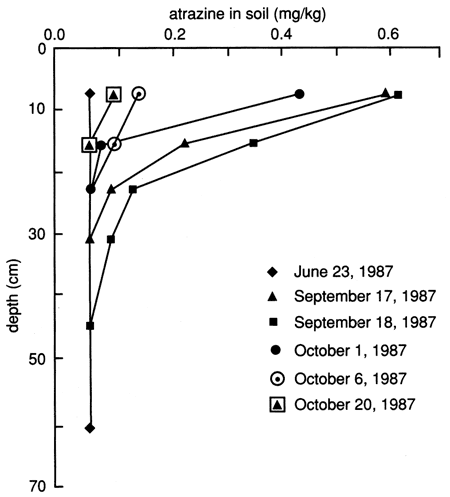
The atrazine versus time breakthrough curve based on suction lysimeter sampling is shown in fig. 24. The atrazine breakthrough curve based on soil core extraction is shown in fig. 25. Because each core sample comes from a different (random) location within the flooded area and because of natural soil heterogeneity, the atrazine breakthrough curve based on soil cores shows wide fluctuations, which result from random noise. Otherwise the breakthrough curves are similar to the ones based on suction lysimeters. The vertical distribution of atrazine in the soil decays exponentially with depth (fig. 26). Atrazine did not penetrate below the top 30 cm (1 ft) of soil until December, approximately 3 months after flooding. However, during the February and May 1988 soil core samplings, some atrazine was detected to a depth of 75 cm (30 in.). However, this apparent deeper penetration of atrazine occurred after the plastic cover had been removed. Since then, the field has been plowed and chemically treated with additional atrazine.
Figure 24--Atrazine breakthrough curves for site 6 based on suction lysimeter sampling.
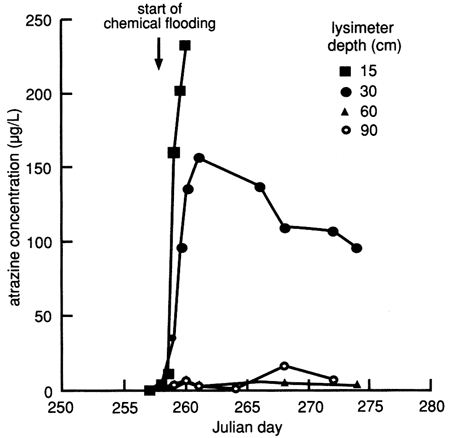
Figure 25--Atrazine breakthrough curves for site 6 based on soil cores. Detection limit is 0.04 mg/kg.
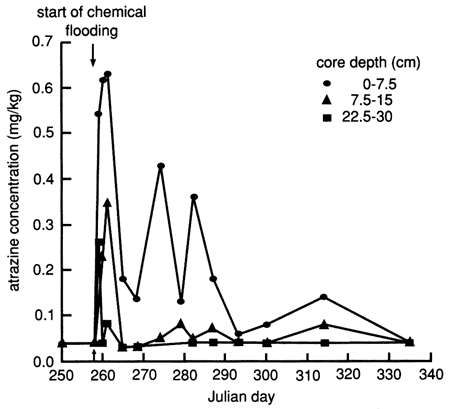
Figure 26--Atrazine profiles at site 6 based on soil cores.
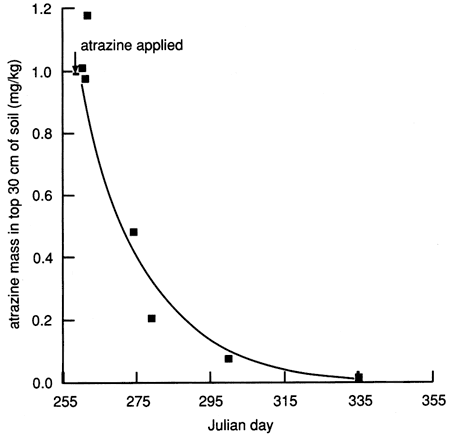
Given that the applied atrazine did not penetrate below the top 30 cm (1 ft) of soil as of December 1987 and given that the calculated soil-water fluxes decreased drastically to a fraction of 1 cm/d within a few days of covering the site with plastic (see fig. 18), we calculated a mass balance for atrazine to check whether the atrazine measured in the soil cores before December 1987 accounted for the amount of atrazine applied at the surface. During the first 3 days after chemical flooding, the mass balance accounted for 97-100% of the applied atrazine. However, after 3 days, only 10-50% of the atrazine mass could be accounted for (fig. 27).
Figure 27--Atrazine mass in the upper 30 cm of soil at site 6, plotted on an arithmetic scale.
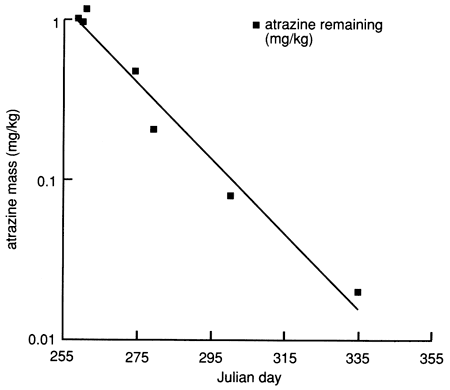
Physical and chemical properties of the soil profile essential for biologic and chemical degradation of atrazine to daughter products could explain this apparent mass imbalance. Figure 6 indicates that the content of organic carbon is relatively high in the top 30 cm (1 ft) of soil and decreases rapidly with depth. The CEC of the soil in the top 30 cm (1 ft) also is the highest in the soil profile, and the soil pH reaches its minimum value of 5.9 just under the top 30 cm of soil, below which it rapidly increases to greater than 8.5 just below the 100-cm (3.5-ft) depth. The soil changes from silty loam to silty clay loam below 48 cm (19 in.), with a corresponding decrease in hydraulic conductivity. The high organic carbon content of the top 30 cm is conducive to high biologic activity, which may result in biodegradation of atrazine (dealkylated metabolites). The low pH at just below the top 30 cm induces atrazine hydrolysis (to hydroxyatrazine),which is catalyzed by the presence of clays of high CEC (chemical degradation). The high soil-moisture content (caused by flooding) and the relatively high soil temperatures would also stimulate microbial degradation of atrazine in soils. Figure 27 indicates that atrazine degradation at site 6 may have followed first-order kinetics; that is, the logarithm of the atrazine concentration decreases linearly with time, as shown in fig. 28. The half-life of atrazine (i.e., the time for one-half of the applied atrazine to disappear from the application site) was approximately 13 days at site 6 (based on fig. 28). The field-estimated half-life of atrazine is much shorter than the half-life of more than two months reported in other studies [e.g., Jury et al. (1987)]. This may be one reason why atrazine was not detected in any of the 1988 surveyed chemigation wells in the Great Bend Prairie by the Kansas State Board of Agriculture (Anderson, 1989).
Figure 28--Atrazine mass in the upper 30 cm (1 ft) of soil at site 6, plotted on a logarithmic scale. The data points (filled squares) indicate the amount of atrazine remaining (mg/kg).
Core samples of the 0-30-cm (0-1-ft) and 60-90 cm (2-3 ft) intervals of site 6, collected on February 25, 1988, were analyzed for atrazine and atrazine metabolites; the results are tabulated in appendix E. The hydroxyatrazine concentration (0.124 mg/kg) present in the 0-15-cm (0-6-in.) depth interval was 1.6 times the amount of parent atrazine present. For the 15-30-cm (6-12-in.) depth interval, the hydroxyatrazine concentration (0. 116 mg/kg) was 0.4 times the amount of parent atrazine present. The hydroxyatrazine concentration for the 60-75-cm (24-30-in.) and 75-90-cm (30-36-in.) intervals (0. 167 mg/kg and 0. 185 mg/kg, respectively) were 3.1 and >4 times the amount of parent atrazine present, respectively, although the parent atrazine was below the detection limit (0.05 mg/kg) for the 75-90-cm (30-36-in.) depth. Therefore an appreciable portion of parent atrazine degraded into hydroxyatrazine in the upper 90 cm (3 ft). The dealkylated metabolites of atrazine at the 0-30-cm (0-1-ft) and 60-90-cm (2-3-ft) depth intervals were below the detection limit (0.05 mg/kg). Such concentrations seem low, given the high organic matter content of the upper 30 cm (1 ft) of soil, but cold soil temperatures during the winter sampling period may have limited biologic activity. Additional soil samples collected in May and August 1988 were sent for atrazine metabolite analysis, especially to verify biologic activity (appendix E). The results show significant amounts of biodegradation by-products [ethylatrazine (G-28279) and isopropylatrazine (G-30033)] and hydroxyatrazine (G-34048) for the 0-15-cm (0-6-in.) depth interval from the August 1988 sampling and the 30-45-cm (12-18-in.) depth interval from the May 1988 sampling.
No atrazine was observed in any of the observation wells, an observation that is consistent with the retention and decomposition of atrazine in the upper 75 cm (2.5 ft) of soil and the lack of significant concentrations of tracer bromide in the shallow ground water. All atrazine data are tabulated in appendix D.
Prev Page--Installation || Next Page--Site 7
Kansas Geological Survey, Geohydrology
Placed on web Aug. 23, 2010; originally published 1990.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/GW12/04_site6.html