Prev Page--Occurrence || Next Page--Wells
Water Quality
Water quality is a general term used to refer to the amount and type of dissolved or suspended material in the water. When water first contacts the earth as rain or snow (or frost or dew), it is usually quite pure--very close to distilled water in terms of its lack of dissolved materials. As it moves over or through soils, rocks, and sediments, it dissolves or picks up small quantities of the materials with which it comes in contact. These materials may be inorganic or organic, natural or human-made, or even living (such as bacteria and viruses). The total dissolved solids content (often abbreviated TDS, and usually reported as milligrams per liter or parts per million) in a water sample is often used as a general indicator of water quality. Each type of dissolved substance raises different issues with respect to water quality, and scientists and health authorities have established regulations for drinking water. Some regulations are maximum permissible contaminant levels (primary regulations) for public water supplies, while others are recommended levels (secondary regulations). Table 1 shows some examples of the maximum and recommended levels in Kansas for selected natural and artificially introduced contaminants. These are U.S. Environmental Protection Agency values that are also adopted by the Kansas Department of Health and Environment. Sources of additional or more detailed information can be found in Appendices B and C.
Table 1--Maximum and recommended levels in Kansas for selected natural and artificially introduced contaminants (KDHE). *Treated-water standard.
| Constituent | Source Concentration, mg/L n = natural h = human |
Concentration, mg/L Maximum Contaminant Level |
Recommended Contaminant Level |
|---|---|---|---|
| Arsenic | n, some h | 0.05 | |
| Lead | n, h | 0.015* | |
| Mercury | n, some h | 0.002 | |
| Nitrate (as N) | n, h | 10 | |
| Fluoride | n | 4 | |
| Sodium | n, some h | 100 | |
| Sulfate | n, some h | 250 | |
| Iron | n | 0.3 | |
| Benzene | h | 0.005 | |
| Trichloroethylene (TCE) | h | 0.005 | |
| Atrazine | h | 0.003 |
Because ground water is relatively inaccessible, has sources that are diffuse or not well known, and moves very slowly compared to surface water, recognition of a water-quality problem may be separated in both time and space from the events that cause the problem. One example of this is the occurrence of increased nitrate concentrations in ground water as a result of agricultural fertilizer applications. Decades have elapsed between the first use of commercial fertilizers and the recognition of a widespread problem. The result is that large volumes of soil and ground water now contain high nitrate concentrations for which there is no effective removal technique. Remediation of such problems may be difficult or impossible. We have thus come to place greater and greater value on the prevention of problems before they occur. This in turn requires an improved understanding of ground-water processes and their relationship to the hydrologic cycle, which increases the need for hydrologic research.
Natural factors and controls
Under natural conditions, ground-water quality depends primarily on the nature of the materials the water has been in contact with, and on the length of time it has been in contact. Other factors also play a role: evapotranspiration increases TDS because the dissolved solids are left behind and become more concentrated as the volume of water is reduced by loss of water vapor. Higher recharge during wet periods can, in turn, dilute shallow mineralized waters.
Most salts are quite soluble, which is why salt deposits at surface or salt outcrops are found only in very arid environments. Water flowing through salt deposits or salty soils often becomes saline very quickly because the salt dissolves easily. Salt contamination is a significant problem in Kansas, where thick deposits of salt, deposited during the Permian Period of geologic history, underlie much of central Kansas. Where those salts are near the surface, they may be dissolved by freshwater and naturally contaminate water supplies. Although most salts are not particularly toxic, human physiology cannot tolerate very saline water. Table 2 shows the levels of salt tolerable for drinking water, and presents some natural concentrations for comparison. Since some of the brines in Kansas' salt-bearing formations are highly concentrated, it's easy to see why natural mixing of different waters can cause water-quality problems. Freshwater is defined as having less than 500 mg/L chloride and less than 1,000 mg/L TDS.
Table 2--Levels of salt tolerable for drinking water and some natural concentrations for comparison.
| Type of Water | Chloride (mg/L) | TDS (mg/L) |
|---|---|---|
| Rain (typical) | 0.1-1 | 5-10 |
| Fresh surface water in Kansas | 3-500 | 100-1,000 |
| Fresh ground water in Kansas | 3-500 | 160-1,000 |
| Drinking water, maximum recommended |
250 | 500 |
| Livestock consumption, satisfactory limit |
--- | 3,000 |
| Saline to brine waters in Kansas |
500-190,000 | 1,000-330,000 |
| Seawater | 19,400 | 35,800 |
Carbonate minerals (such as are found in limestones) are not as soluble as the salts found in brines, but water that stays in contact with carbonate rocks for long periods tends to become hard. Hardness is caused primarily by dissolved calcium and magnesium carbonates, and some of its symptoms are failure of soaps to lather and the formation of scale deposits in pipes and cooking utensils. Hard water generally presents no major health hazard, but its use can be unpleasant and costly. By contrast, rocks such as granite (common in the Rocky Mountains) are very unreactive and usually have only a small effect on the TDS of water even over long periods of time.
Water that flows through rich soils or is in contact with productive biological communities or buried organic deposits may pick up significant concentrations of natural organics. These are seldom a major problem in themselves, but they may co-occur with microbes or parasites that affect humans or other animal hosts. Although these disease-producing processes have a natural origin, they are discussed below under the human-effects and water- treatment categories.
Human effects
When we think of human (sometimes called anthropogenic) effects on water quality, we often focus on the addition of artificial chemicals to the environment. Although this can be important, humans have far greater effects through the modification, acceleration, and redirection of natural processes. Here we provide examples of activities that affect water quality, both by modification of natural processes and by artificial activities and procedures.
Storage and irrigation
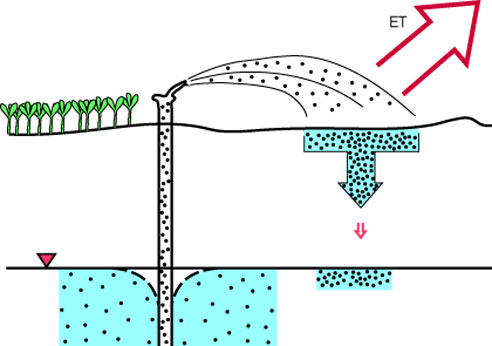
When a large surface area of water is exposed to the atmosphere, either as free water or as moist soil, evapotranspiration results in water loss and an increase in the TDS of the remaining water. Turning small flowing streams into large, static reservoirs enhances this effect, but normally not to an extent great enough to cause problems, at least in Kansas. Irrigation, however, can have noticeable effects. Most irrigation water applied to crops is lost to evapotranspiration, leaving behind both the salts that were originally contained in the water and soluble materials that may have been mobilized from the soil. This can result in a buildup of salts in the soil, and when irrigation return water or natural flow through the system results in recharge or runoff, the resulting water may be high in TDS (illustrated in figure 16). For example, shallow ground waters in alluvium of the Arkansas River in western Kansas are saline in some locations where irrigation water from the river was used in the past.
Figure 16--Irrigation causes the evaporation of water, but the natural salts remain in more concentrated form. Soils and ground water gradually become more salty as a result of prolonged irrigation.
Fertilizer application
Accelerating the natural cycle of plant and animal productivity through intensive agriculture requires modifying the natural nutrient cycle as well as the natural water cycle. When fertilizers are added to soils in quantities sufficient to maximize crop production, some fraction of the excess may be transported to surface or ground water. This is particularly a concern with respect to nitrate, which is critical for plant growth, but which can also cause the potentially fatal "blue-baby" syndrome in infants. Elevated nitrate concentrations are found in shallow ground water in many parts of Kansas and throughout the Midwest. Although some of this comes from point sources of contamination such as septic tanks or localized areas such as feedlots, at least some of the additional nitrate is believed to result from the non-point source of large-scale fertilizer application.
A feedlot near Dodge City.

Resource extraction
This is a catch-all term that includes mining, oil and gas production, and even ground-water withdrawal. All of these activities have the potential for causing at least one of two types of ground-water quality problems. In the terminology used above, these tend to be point-source contamination problems, although the points may be so many, so old, and so ill-defined that the problem is now indistinguishable from a non-point problem.
One problem common to all of these activities is the creation of artificial short-circuits between different hydrologic compartments. Boreholes, wells, pits, and mine shafts can all connect different aquifers by penetrating intervening confining layers, or can provide a direct pathway from the surface to the ground water. This can permit poor-quality ground water to mix with and degrade usable water in other aquifers, or it can provide a direct pathway for surface contaminants to reach the ground water without passing through the protective filter of the unsaturated zone. This is an important process in contaminating domestic well waters pumped from poorly constructed wells.
A Flint Hills oil well, Greenwood County.

Another potential problem, particularly in mining and oil production, is the production and disposal of toxic or highly contaminating byproducts. Oil in Kansas co-occurs with brine, so large quantities of saltwater are pumped to the surface with the oil; that brine must be disposed of in ways that will not adversely affect surface-water or ground-water quality. In the early days of oil production in central Kansas, that brine was pumped into ponds, with the intent of evaporating the water and storing the remaining salt. Instead, the saltwater moved underground, sometimes contaminating freshwater aquifers. For example, in central Kansas, near the town of Burrton, disposal of oil-field brine in shallow ponds has created a plume of underground saline water in the Equus Beds aquifer, which is slowly moving toward the Wichita water-well field. While wells can be drilled to intercept that saltwater and slow its progress, such solutions are costly, only marginally effective, and point out the importance of preventing water-quality problems. Evaporation ponds have since been banned and now the brine is pumped back underground into deep geologic formations, often into the same rocks that produced the saltwater and oil originally.
Certain ore bodies and the processes used to extract them generate toxic or corrosive wastes in large quantities, and their safe and economical storage or disposal has become a major problem. For example, in southeastern Kansas, water entering underground lead and zinc mines sometimes is contaminated by the weathering of sulfide minerals, including pyrite, in the mine walls. Weathering of pyrite in coal mines in southeastern Kansas can also lead to contamination of surface and shallow ground waters by acid mine drainage. This water can become highly acidic; where it exits from the mines, it can create problems for plants and animals. As is often the case with ground-water contamination, cleaning up the contamination is extremely expensive; the contaminated ground-water remains as a hazard and a warning for future generations.
Pits where coal was strip-mined, now filled with water in Cherokee County.

Industrial and agricultural chemicals
Synthetic chemicals, such as solvents and pesticides, are relatively new additions to both ecosystems and human diets. They tend to be the focus of concern because they are toxic or else we have no good basis for assessing the long-term effects for many of them, and organisms (including people) have not evolved methods of coping with their possible toxicity. As the sensitivity of analytical methods improves, we find traces of these artifacts of technology virtually everywhere at or near the land surface. The public debate about acceptable levels and the relative risks, costs, and benefits of pollution prevention or clean-up will be with us for a long time. From the standpoint of the hydrologic sciences, dealing with this type of contamination has taught us two major lessons. One is that remediation or cleanup of ground-water pollution is usually slow, extremely costly, and incomplete--so prevention, prediction, and containment are the practically preferable alternatives. The second is that two factors are critical to understanding and preventing ground-water pollution. The primary factor is how water moves through both the unsaturated and saturated zones, and a secondary factor is how the contaminant interacts with soil, aquifer, and water in ways different from water itself.
Population, urbanization, and concentration
We discussed issues of scale with respect to the hydrologic system; they also play an important role in water-resource problems. The population and transportation patterns of Kansas were firmly established in an era when domestic water consumption was measured in buckets per day per household, virtually all agriculture was dry-land farming, and a community of more than 10,000 people was a major metropolis. Now, populations are vastly larger and more concentrated, and water demand is enormously greater. Some Kansas cities are not located in places where water is abundant. It is not surprising, then, that communities such as Hays are having trouble meeting present water demands, or that the Wichita area is looking farther and farther afield for possible future water supplies.
In terms of water quality, population and economic concentration also have a potentially enormous effect. The absolute amounts of wastewater, industrial contaminants, and solid waste per unit area have risen phenomenally in urbanized areas. It thus becomes harder and harder to protect water supplies from becoming unacceptably contaminated. The high cost (relative to present market value) of either purifying or transporting large volumes of water explains why it is considered possible that the price and availability of water may ultimately be a more stringent limit to growth than the price and availability of energy.
Water-supply treatment
Municipal water systems typically treat surface-water supplies by processes such as coagulation and settling, aeration, and chlorination to remove distasteful or harmful materials. Ground-water supplies have traditionally required less treatment because recharge moves through soil and aquifer materials, and the natural filtering processes eliminated most solids and organisms. This perception is still valid in many areas, but increasingly stringent water-quality standards and concerns about ground-water contamination are bringing more attention to ground-water treatment. Regardless of their source of water, concerned citizens are increasingly turning to the use of bottled water and to home purification devices. Ironically, the quality of as-delivered municipal water can be substantially better than water passing through poorly maintained water dispensers or home purification equipment.
Prev Page--Occurrence || Next Page--Wells
Kansas Geological Survey, Kansas Ground Water
Comments to webadmin@kgs.ku.edu
Web version Jan. 2005. Original publication date August 1993.
URL=http://www.kgs.ku.edu/Publications/Bulletins/ED10/05_qual.html