
Kansas Geological Survey, Bulletin 96, part 3, originally published in 1952

Originally published in 1952 as Kansas Geological Survey Bulletin 96, part 3. This is, in general, the original text as published. The information has not been updated.
Shale oil can be produced from a number of black shale deposits in Kansas. This report presents the results of testing of samples of some of these black shales from 37 locations. Kansas oil shales are in general black platy to fissile shales composed of fine silt and clay-sized minerals. They contain a relatively large percentage of black to light-brown bituminous matter. When viewed in thin section normal to the bedding plane the organic matter appears as thin laminations, but when viewed from a section cut parallel to the bedding planes it appears as blotches. Obtainable yields of shale oil range from a slight trace to a maximum of 22.8 gallons per ton, and the average yield from favorable localities is from 6 to 12 gallons per ton. Most of the shales tested yield a large volume of gas concurrent with the oil. The average yield is about 1,230 cubic feet per ton of gas that shows from 30 to 45 percent hydrogen and a very high B.t.u. content. The average specific gravity of the shale oil ranges from 0.53 to 0.73 with the majority of the samples from 0.61 to 0.63. Determination of the gross heating value of the shale as a bulk fuel showed that much of the material contains from 1,000 to more than 3,000 B.t.u. per pound.
The quantity of recoverable shale oil known to occur in Kansas, calculated by using only shales that yield 5 or more gallons per ton and using the area of those shales lying under 100 feet or less of overburden, is about 3 billion barrels. Some experiments were conducted to increase the obtainable yield of shale oil. Socony-Vacuum Oil Company, Inc., bead catalyst "A" increased the obtainable yield and Wyoming bentonite increased the yield when as much as 50 percent by weight was mixed with the charge of shale being distilled. Both procedures are worthy of more study.
Oil shale was defined by Gavin (1922) as follows: "Oil-shale is a compact, laminated rock of sedimentary origin, yielding over 33 percent of ash and containing organic matter that yields oil when distilled but not appreciably when extracted with the ordinary solvents for petroleum."
Oil-shale deposits (Pls. 1, 2) constitute a large reserve of potentially usable fuel in Kansas. The State Geological Survey, as a part of its inventory of the State's fuel resources, undertook a study to determine, in a reconnaissance manner, the extent and quality of the State's oil-shale resources. The results of this study are presented in this report. Present economic conditions do not indicate that an oil-shale industry will develop immediately in Kansas. The availability of basic data on oil shale is important, however, because of the potential future use of this material as a source of liquid and gaseous fuels on a competitive basis, its possible use in the near future as a low-cost, solid fuel, and its ready availability as an emergency fuel source.
Plate 1--A, Quarry of the Ft. Scott Natural Cement Company showing the Little Osage shale in the foreground. B, A thick exposure of black shale in the Pleasanton shale, SW NE sec. 7, T. 32 S., R. 19 E. This shale is used as road material in Labette County.

Plate 2--A, An exposure of black shale below the Ardmore limestone in a coal pit. The black shale is seen as the dark area in the wall of the pit above the man (in circle). B, An exposure of the black shale below the Blackjack Creek limestone and above the Mulky coal. The man is stepping up onto the coal; the shale and limestone are seen in the upper left-hand corner of the picture.

Current extensive oil-shale studies are being conducted in the United States by the U.S. Bureau of Mines, the most spectacular research being at the extraction plant at Rifle, Colorado, and at an experimental refinery and laboratory at Boulder, Colorado, under cooperative agreement with the State of Colorado. On the basis of results thus far obtained, Kansas oil shales, when compared with the Rocky Mountain deposits, do not have a high yield. Furthermore, they differ from the Colorado shales in several important aspects, such as the ratio of gross heating value to oil yield, stratigraphic occurrence, and physical appearance.
Attempts to extract oil from shale date back to the 17th century, but the founding of an oil-shale industry was first reported in the middle of the 19th century with the initiation of the Selligue retorting process in France in 1838 and the Young process in Scotland in 1850. Since that time industrial oil-shale operations have been established or attempted in Australia, Canada, New Zealand, Switzerland, Sweden, Estonia, England, Ireland, Wales, Manchuria, and the U.S.S.R. The most extensive known deposits occur in these countries and in Germany, Yugoslavia, Norway, Africa, Syria, Mongolia, Arabia, Argentina, Chile, Brazil, Uruguay, and the United States (Prien, 1951). Oil-shale industries in operation today are found only in areas where little or no natural petroleum is available (Kraemer and Thorne, 1951; Guthrie and Klosky, 1951).
Industrial oil-shale activities in the United States began in 1857 in an Ohio River Valley plant which distilled 3,000 gallons of oil daily from the Devonian shale of that area, but since 1925 there has been no active commercial shale-oil production in the United States. However, now that research in this field has been stimulated by the Federal government, particularly by the current program of the U.S. Bureau of Mines, production of oil and oil products from shales in this country may not be too far in the future. During the last war three new processes for oil retorting were developed in Germany. One of these is similar to the underground coal gasification process now under investigation by the Bureau of Mines at Gorgas, Alabama.
It has been known for some time that some black shale deposits in Kansas contain obtainable oil. This background information, partly from other unrelated Geological Survey projects, was used as a guide in field sampling. Samples for testing were taken from 37 locations of black or dark-colored shale deposits occurring throughout the State. Not all the samples collected contained recoverable oil. During various testing stages some of the samples were eliminated on the basis of physical examination and some were eliminated because the bed sampled was too thin.
None of the samples tested showed exceptionally high yields, although some deposits were found to have better characteristics than others. Of the samples collected only those which fit the following limits were considered in estimating reserves: (1) shale deposits at least 3 feet thick, (2) deposits from which samples showed a yield of at least 5 gallons of oil per ton of shale, and (3) shale deposits under less than 100 feet of overburden. Most of the beds that may be properly classed as oil shale are concentrated in the eastern part of the State, although samples were collected as far west as Wallace County, where the Sharon Springs shale locally shows small yields. These dark shales occur stratigraphically throughout the Pennsylvanian System in eastern Kansas and the Cretaceous System in central and western Kansas.
The Kansas shales capable of producing hydrocarbon oils are in general black platy to fissile shales composed of fine silt and clay and contain a relatively large percentage of organic matter. This organic material occurs finely disseminated throughout the shale and as occasional coaly fragments. Obtainable yields from the shale samples tested range from a slight trace to about 22.8 gallons per ton of shale. However, samples from shale beds that meet both yield and mining thickness specifications generally fall in the range from 6 to 12 gallons of oil per ton of shale.
Most of the Kansas shales give a large volume of gas concurrent with the distillation (average gas yield being about 1,230 cubic feet per ton), and in view of the high heat content in the gas most of the present oil shale work includes the utilization of the gases. The specific gravity of the shale oil ranges from about 0.53 to 0.73 with the average being about 0.61 to 0.63. Some laboratory experiments have been carried out to increase the yield but no methods have yet been found that are cheap enough to warrant economic development at the present time,
Total recoverable oil contained in the Kansas oil shales studied is estimated at about 3 billion barrels. This does not include the possible obtainable oil from shales on which testing is incomplete or from many of the tested shales which indicated a yield of below 5 gallons per ton. These and many thin shales (3 feet thick or less) that occur in the upper Pennsylvanian rocks were not considered in the current investigation.
Many theories have been advanced as to the geologic origin of the world's oil shales. The one now most generally held is that oil shale is the end product of a process involving the deposition of predominantly vegetable matter, together with sedimentary silt and mud, at the bottom of shallow fresh or saline lakes, followed by the subsequent decay of the organic constituents and the lithification of the inorganic sediment. The organic material (kerogen) is the part of the shale that yields oil when distilled and the characteristic trait of kerogen to resist solution in common solvents has been used to define oil shale. However, in relation to the organic matter these shales contain a great quantity of inorganic matter, which consists generally of clay, silt, and calcium carbonate. Therefore, in conjunction with the oil-shale studies, both in Kansas and elsewhere, efforts are being made to convert this inorganic matter into useful products. In Scotland, nitrogen from the shale has been converted to ammonium sulfate, with a yield of about 30 pounds per ton of shale. The nitrogen compounds from shale oil are excellent solvents for varnishes, paints, and lacquers (Prien, 1951).
Black or dark-colored shale deposits occur extensively throughout much of Kansas. It was not within the scope of this study to sample all these occurrences; therefore, some of the shale beds not tested may contain an appreciable amount of oil. Ceramic tests on many of the black shale deposits not included in this study had shown that there was little possibility of obtaining oil from them. Also, as sampling and testing proceeded, experience was gained which permitted evaluation of shale beds in the field on the basis of their physical characteristics and many more deposits were eliminated by field examination. Still other deposits were eliminated because they were too thin. In some cases, nevertheless, sampling was selective and arbitrary and there is a strong possibility that there are deposits of oil shale in Kansas not covered in this report.
From previous experience with black shale it was known to lose its more volatile components upon long exposure to the atmosphere. This has been confirmed recently by the Bureau of Mines (Stanfield and others, 1951). For this reason all samples collected were sealed in glass fruit jars. Active coal pits, shale pits, and quarries were used wherever possible to obtain fresh material. Wherever the shale had been exposed for a long time, a trench was dug into the shale until fresh material was reached and then sampled. It is our judgment that all samples used in the study are representative of the deposits.
Localities for sampling were picked from several sources, including field reconnaissance. The stratigraphic files of the State Geological Survey and published reports by Pierce and Courtier (1938), Jewett (1940, 1945), Elias (1931), Latta (1948), Moore (1949), and Runnels (1949) were used for preliminary locations. W. B. Howe gave valuable assistance in the selection of stratigraphic zones and locations to be sampled in the Cherokee shale.
The samples collected in the field were removed from the sealed jars and spread out in trays to dry. One to three days were necessary to dry many of the samples. As soon as the shales had dried to equilibrium in air, they were crushed to pass an 8-mesh sieve and replaced in the sealed fruit jars. The samples were not further treated prior to distillation.
The primary purpose of the investigation was to record the yield of shale oil. A mercury retort was formerly used by the U.S. Bureau of Mines for retorting oil-shale samples. However, this method is cumbersome, time consuming, and apparently unreliable for low-yield shales. Recent work by the Bureau of Mines has shown that a Fischer retort for low temperature carbonization of coal could be modified for oil-shale distillation (Stanfield and Frost, 1946). This method is much more compact and overcomes the four main objections to the mercury retort. (1) Higher yields are obtainable than with the mercury retort; (2) there is more control over the procedure; (3) more of the organic matter is converted to shale oil; and (4) the total time is approximately 2 hours compared to a minimum of approximately 6 hours per assay when using the mercury retort (Stanfield and Frost, 1946, p. 2). Also, from our point of view, the large saving of space made the Fischer retort process the most practical method.
Details on the assay procedure are given by Stanfield and Frost (1946); therefore only an outline of the procedure is given here. An oil-shale sample of 100 grams, air dry, is charged into the retort using five perforated aluminum disks to provide separation of the particles and to allow more heat transmission. The retort is heated by a gas burner and a maximum of 500° C. is attained in 50 minutes. This temperature is then maintained for 20 minutes. The shale oil distills into a weighed centrifuge tube that is maintained at O° C. with ice. A reflux condenser is attached so that all gases pass through it. The coolant is water maintained at 0° C. by an ice and salt mixture packed around a series of copper coils. After completion of the distillation the total loss of weight is determined by reweighing the retort. The shale oil is centrifuged and measured. From these data weight percent yield of shale oil, water, and gas loss are obtained. Specific gravity of the shale oil also was determined approximately.
The assay is completed by determining the loss on ignition at 900° C. on the spent shale. This ignition loss represents organic matter and volatilized inorganic matter. The percentage of ignition loss subtracted from 100 gives the percentage of ash in the spent shale. Table 1 presents the complete data for shales assayed in this study which had more than 1 gallon of shale oil per ton of shale.
Table 1--Yield of products from Kansas oil shales. Table 3 gives a complete list of samples.
| Loc. no. |
Sample no. |
Gallons of oil per ton |
Specific gravity of oil at 25° C |
Distillation products as weight percentages | ||||
|---|---|---|---|---|---|---|---|---|
| Oil | Water | Gas and loss |
Nonvolatile organic* |
Ash | ||||
| 6 | 1 | 9.6 | 0.60 | 2.4 | 3.8 | 0.7 | 78.14 | 14.96 |
| 7 | 1 | 4.31 | 0.61 | 1.10 | 3.8 | 1.6 | 84.05 | 9.45 |
| 8 | 4 | 1.68 | 0.57 | 0.40 | 5.2 | 0.5 | 88.39 | 5.51 |
| 8 | 5 | 8.39 | 0.54 | 1.90 | 5.5 | 1.1 | 79.42 | 12.08 |
| 8 | 6 | 7.19 | 0.53 | 1.60 | 6.4 | 1.3 | 77.95 | 12.75 |
| 9 | 1 | 2.64 | 0.67 | 0.20 | 3.3 | 0.3 | 83.04 | 13.16 |
| 9 | 3 | 3.83 | 0.66 | 1.05 | 3.5 | 1.3 | 78.05 | 16.10 |
| 11 | 1 | 12.2 | 0.61 | 3.50 | 3.9 | 1.4 | 76.51 | 14.69 |
| 12 | 1 | 2.16 | 0.67 | 0.6 | 3.6 | 0.6 | 87.76 | 7.44 |
| 12 | 2 | 1.68 | 0.57 | 0.40 | 4.2 | 0.7 | 87.39 | 7.31 |
| 14 | 2 | 2.64 | 0.64 | 0.70 | 3.3 | 0.8 | 80.12 | 14.58 |
| 14 | 3 | 6.47 | 0.63 | 1.75 | 5.1 | 1.65 | 91.5 | |
| 15 | 1 | 9.35 | 0.71 | 2.75 | 4.1 | 0.85 | 75.75 | 16.55 |
| 17 | 1 | 11.98 | 0.72 | 3.60 | 3.1 | 1.9 | 76.43 | 14.97 |
| 20 | 1 | 1.2 | 0.60 | 0.30 | 3.2 | 0.10 | 89.97 | 15.43 |
| 21 | 1 | 2.64 | 0.64 | 0.90 | 7.2 | 0.20 | 82.64 | 8.96 |
| 24 | 4 | 6.95 | 0.62 | 1.80 | 8.1 | 1.9 | 78.34 | 9.86 |
| 24 | 5 | 5.75 | 0.54 | 1.30 | 10.1 | 1.2 | 77.58 | 9.82 |
| 26 | 1 | 4.55 | 0.58 | 1.10 | 8.1 | 2.6 | 76.15 | 12.05 |
| 27 | 1 | 1.44 | 0.67 | 0.40 | 4.0 | 0.1 | 88.84 | 6.66 |
| 27 | 2 | 9.83 | 0.78 | 3.20 | 2.5 | 2.2 | 80.76 | 11.34 |
| 27 | 3 | 1.92 | 0.63 | 0.50 | 2.7 | 0.4 | 83.85 | 7.55 |
| 28 | 1 | 2.88 | 0.67 | 0.80 | 3.3 | 0.9 | 80.25 | 14.75 |
| 29 | 1 | 7.43 | 0.61 | 1.90 | 4.9 | 1.8 | 80.29 | 11.11 |
| 30 | 1 | 3.12 | 0.54 | 0.70 | 6.6 | 2.6 | 77.24 | 12.86 |
| 31 | 1 | 3.36 | 0.57 | 0.80 | 4.5 | 4.0 | 82.16 | 8.54 |
| 31 | 2 | 2.88 | 0.58 | 0.70 | 7.6 | 1.9 | 76.43 | 13.37 |
| 33 | 1 | 7.43 | 0.71 | 2.20 | 3.4 | 1.5 | 83.46 | 9.44 |
| 34 | 1 | 4.31 | 0.56 | 1.0 | 8.2 | 2.2 | 78.77 | 9.83 |
| 35 | 1 | 19.65 | 0.66 | 5.4 | 3.6 | 1.6 | 76.46 | 12.94 |
| 36 | 1 | 5.03 | 0.52 | 1.1 | 4.9 | 1.5 | 80.96 | 11.54 |
| 37 | 1 | 9.11 | 0.63 | 2.4 | 6.0 | 2.5 | 72.79 | 16.31 |
| Spec. lump sam. from 33 |
22.77 | 0.73 | 6.9 | 5.5 | 2.1 | 71.28 | 14.22 | |
| AR | 1 | 12.46 | 0.79 | 4.1 | 0.3 | 1.5 | 94.1 | |
| AR | 2 | 8.15 | 0.85 | 2.9 | 0.1 | 0.2 | 96.8 | |
| *Determined by burning spent shale to 900° C. | ||||||||
Three special tests were conducted in order to obtain data on possible methods of increasing the yield. First investigated was the possibility of increasing the yield of shale oil by an additive. This was aimed at releasing more distillation products during the regular retorting process. The kerogen decomposes at about 420° C. and yields shale oil and other products. The yield of shale oil is somewhat dependent upon several physical conditions as well as the chemical characteristics of kerogen. In the process of distillation the rate of heating and maximum temperature attained have an important bearing on the ratio of liquid shale oil to non-volatile bituminous material derived from the kerogen (Hubbard and Robinson, 1950). It was determined that bentonite increases the yield of shale oil from Kansas oil shales when mixed with the charge in the retort. There are two possible reasons for this increase: (1) the water of hydration contained in the montmorillonite (bentonite) molecules produces steam that has a scrubbing action and "opens" the shale, allowing a larger percentage of kerogen to convert to shale oil; and (2) the very large surface areas of the clay could have a catalytic action.
The second test of a method for increasing the yield consisted of utilization of the gas from the retorting process. The gas yield of Kansas oil shales is relatively high and the percentage of hydrogen contained in the gas is large. It was possible that this hydrogen in the gas would increase the yield of oil from the shale if contacted with more shale. A double retort system was set up with the gas yield from the first retort passing into a long tube containing the second charge. This tube was heated to the same temperature as the first retort. Both spent shale and fresh shale were tested by this method. No further yield could be obtained using fresh shale. Apparently the best use for the gas to date is its utilization for fuel to reduce the cost of retorting. Experiments along this line are being conducted by the Bureau of Mines.
The third series of tests was made using a commercial cracking catalyst of the Socony-Vacuum Oil Company. This catalyst apparently cracked some of the heavier fractions that are not usually recovered with the resultant total yield showing slightly higher density and slightly higher gas losses. A relatively large amount of distillate was absorbed into the beads. This action tended to cause difficulty in observing any possible increase in yield. The shale oil obtained after retorting with these beads had a much better appearance than shale oil obtained using only the Fischer retorting procedure. The shale oil from retorting alone had a very dark color with a large portion of tars or asphalts, while that from retorting with the beads was a clear light-green liquid.
Petrographic examination of the black shales was restricted to selected samples from which an appreciable yield of shale oil had been obtained; however, not all locations that yielded oil were examined. Most thin sections prepared for microscopic study were cut across the bedding, and almost all the sections thus cut exhibited extremely thin bedding (Pl. 3). This thin bedding can be attributed to compression of the shales after deposition or alternation of lithologies.
Plate 3--Photomicrographs of Kansas oil shales. A, Little Osage shale and B, Anna shale. Inorganic material (light areas) contains quartz and muscovite-type mica; the organic material is opaque. C, Unnamed black shale below the Ardmore limestone. Large light area is a piece of calcite. Plane polarized light, [x 100 in published version, enlarged to show detail on web page, about x 225],
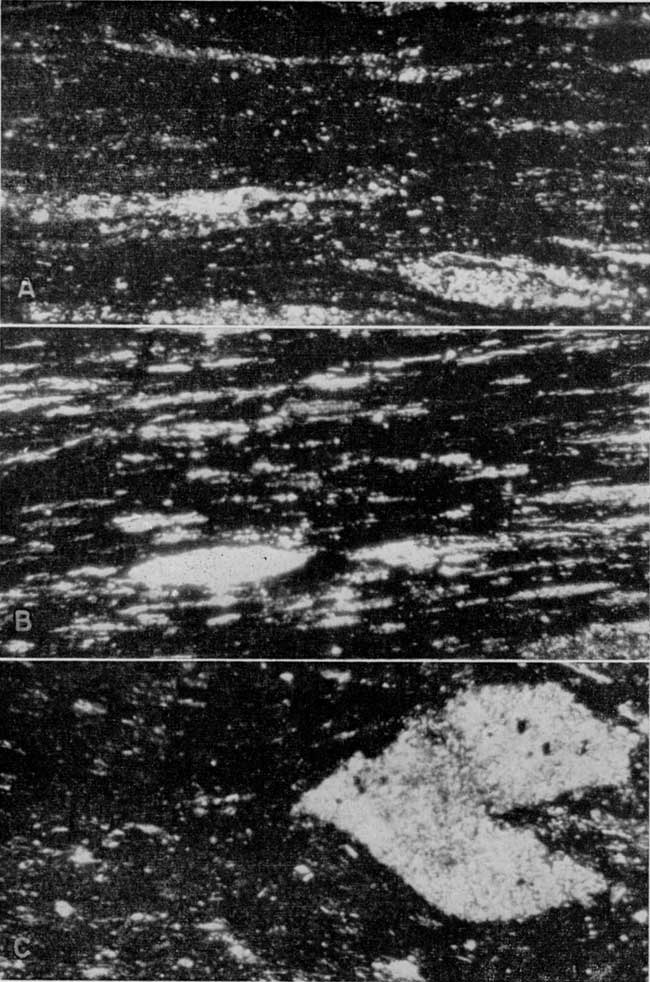
According to W. W. Hambleton of the Department of Geology of the University of Kansas (personal communication), the organic material of the shale strongly resembles coaly material. It is for the most part opaque, but contains variable amounts of translucent material. This translucent material looks like the translucent attritus of coal. Material resembling spores and resin bodies was observed. There is some suggestion that the greater the amount of translucent material the higher the yield of oil from the shale.
The minerals other than the organic material and clay are, with a few exceptions, very fine. The organic material often obscures these minerals so that they cannot be identified, but for the most part the minerals that can be identified are quartz and muscovite type of mica.
Some sections contain nodules. Those observed are made up of both calcite and collophane, and when seen on the outcrop occur along one bedding zone. Calcite also occurs in some sections as fracture fillings and disseminated throughout the shale.
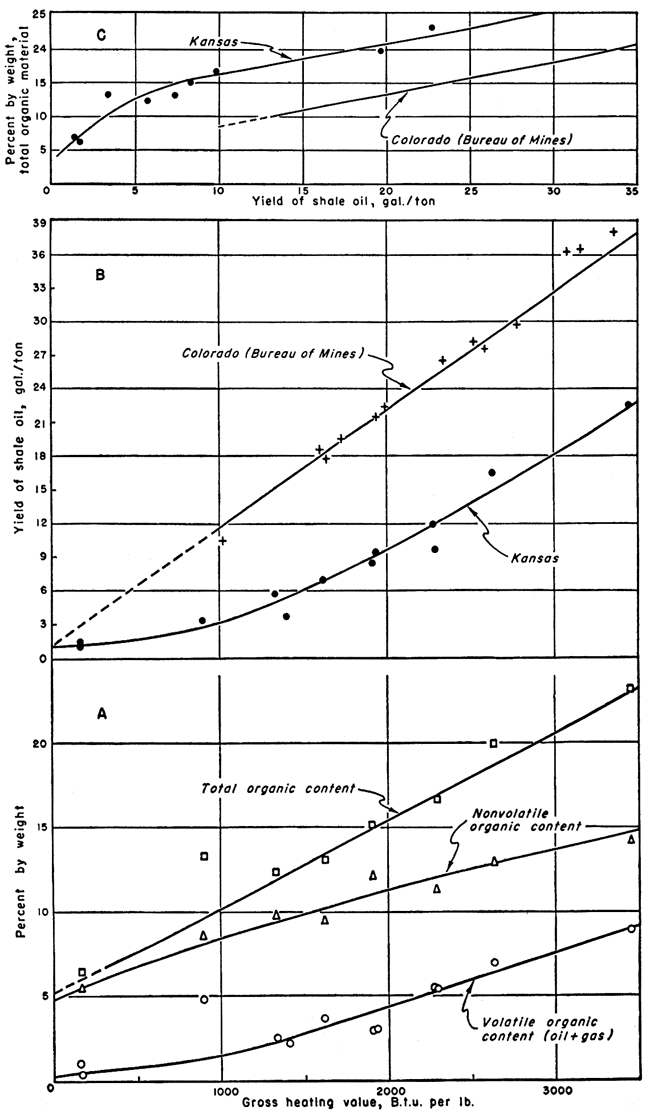
Gross heating values were determined by use of a Parr peroxide bomb calorimeter for a number of Kansas shales. The samples were selected both for yield of shale oil and for the amount of nonvolatile organic material present. Recent studies of Colorado oil shale have shown high gross heating values on samples with high shale-oil yields (Stanfield and others, 1951), and a direct relationship between gross heating value and oil yield. It was judged that the Kansas shales, being of a bituminous type, had good possibilities as a solid fuel and the results of this study confirm this judgment. The gross heating values of the Kansas shales are much higher per gallon of shale-oil yield than the values for the Colorado shales. For instance, a Colorado shale with a yield of 36 gallons per ton has a gross heating value of 3,100 B.t.u. per pound while a Kansas sample with a yield of 22.8 gallons per ton has a gross heating value of 3,450 B.t.u. per pound. Another Kansas shale with a yield of 12 gallons per ton tests 2,276 B.t.u. per pound compared to about 1,700 B.t.u. per pound for a Colorado shale sample which yielded 20 gallons of oil per ton (Stanfield and others, 1951, fig. 7).
The gross heating values of the Kansas shales are summarized in Table 2 along with other pertinent data. Graphs showing the relationship between gross heating values and the other data are presented in Figure 1. The three graphs in Figure 1 show (A) weight percent of volatile organic, nonvolatile organic, and total organic constituents plotted against gross heating value of the Kansas shales; (B) yield of shale oil versus gross heating value for both Kansas shale and Colorado shale (Stanfield and others, 1951); and (C) total organic content versus yield of shale oil for both Kansas shale and Colorado shale.
Table 2--Gross heating values of Kansas shales and related data.
| Sample no. |
Gross heating value, B.t.u. per pound |
Volatile organic, weight percent |
Nonvolatile organic, weight percent |
Total organic, weight percent |
Ash | |
|---|---|---|---|---|---|---|
| Oil | Gas | |||||
| 6-1 | 1,935 | 2.4 | 0.7 | 14-96 | 18.06 | 78.14 |
| 17-1 | 2,276 | 3.6 | 1.9 | 15.79 | 21.29 | 76.43 |
| 8-4 | 167 | 0.4 | 0.5 | 5.51 | 6.41 | 88.39 |
| 9-3 | 1,397 | 1.05 | 1.3 | 16.11 | 18.46 | 78.05 |
| 20-1 | 167 | 0.3 | 0.1 | 15.43 | 15.83 | 80.97 |
| 24-5 | 1,332 | 1.3 | 1.2 | 9.82 | 12.32 | 77.58 |
| 33-1 | 1,618 | 2.2 | 1.5 | 9.44 | 13.14 | 83.46 |
| 31-1 | 896 | 0.8 | 4.0 | 8.54 | 13.34 | 82.16 |
| 27-1 | 0 | 0.4 | 0.1 | 6.66 | 7.16 | 88.84 |
| 33-lump | 3,453 | 6.9 | 2.1 | 14.17 | 23.17 | 70.43 |
| 27-2 | 2,288 | 3.2 | 2.2 | 11.36 | 16.16 | 80.94 |
| 35-1 | 2,630 | 5.4 | 1.6 | 12.94 | 19.94 | 76.46 |
Figure 1--Graphs showing relationships of gross heating values to assay data and total organic content to oil yield. Data for Colorado shales from Stanfield and others (1951).
It is not possible in a reconnaissance such as this to obtain complete data on Kansas oil-shale reserves. Nevertheless, enough information is available for some beds to permit the calculation of the total amount of oil available within certain arbitrary limits. It should be kept in mind that all reserves figures reported here are minimum figures. The arbitrary limits chosen for calculation of reserves are as follows: all shale beds included are at least 3 feet thick, yield at least 5 gallons of oil per ton of shale, and are under less than 100 feet of overburden. The results obtained from these calculations should be interpreted with discretion, as they are not an attempt to predict what might be minable shale, but rather they show how much shale oil is present in Kansas within these stated limits. Some of the deposits are shown in graphic form in Figure 2. The basis on which they were selected was not only the yield of shale oil obtained but also their areal extent and proximity to operating quarries and mines.
Figure 2--Graphic representation of some of the oil-shale deposits tested. Numbers at the base of the sections refer to Table 3 where complete data for each section may be found. The initials WBH and JMJ at the bottom of some of the sections refer to Wallace B. Howe and J. M. Jewett who measured those particular sections prior to sampling.
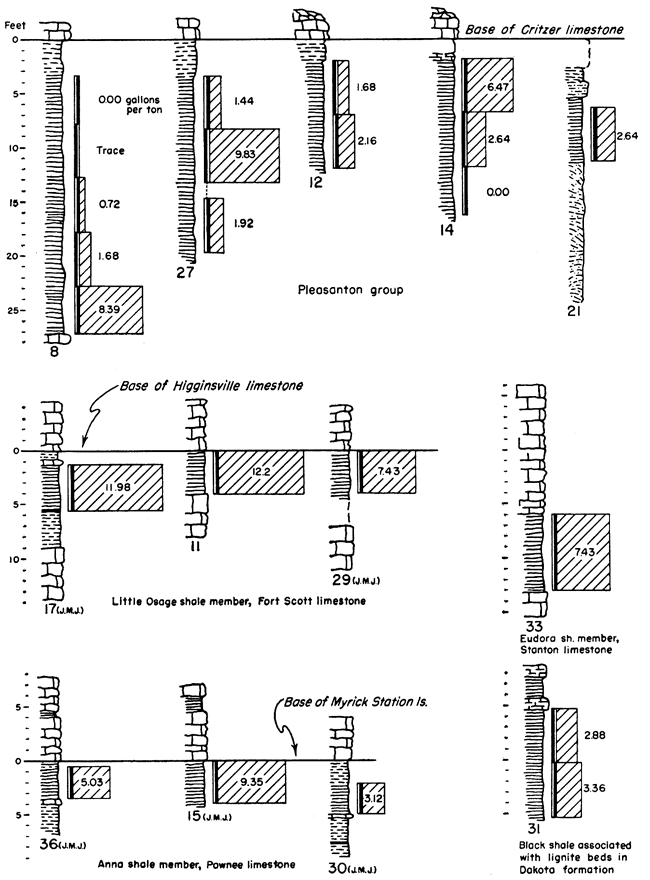
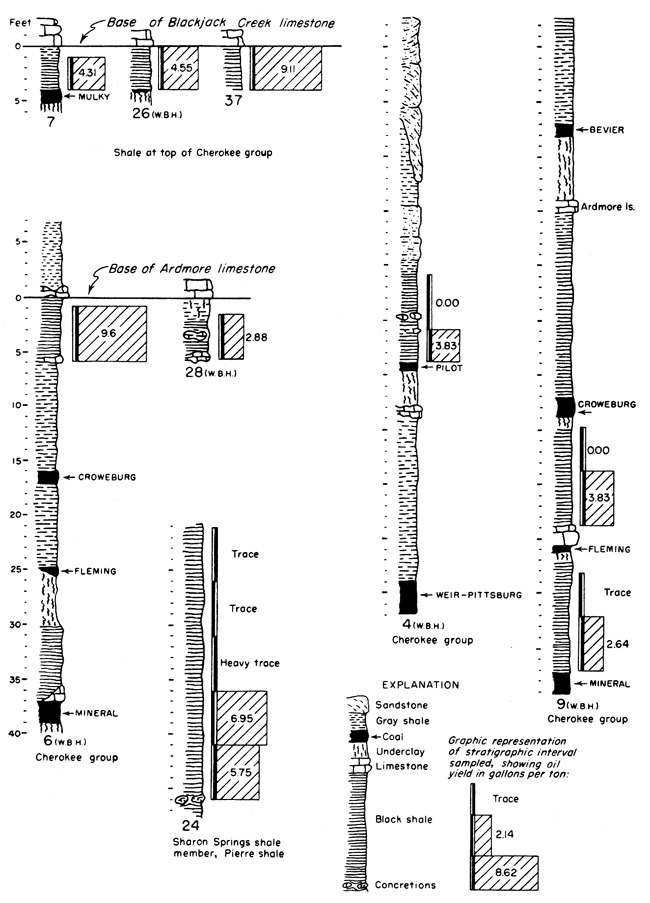
There were relatively few sampling locations in each bed tested; consequently measured yields had to be extended over comparatively long distances. Areas between outcrop and overburden limits were determined from several sources. For the area of extensive coal mining, published reports containing geological and structural maps (Pierce and Courtier, 1938) and a map of the strip-mined areas (Abernathy, 1946) are available. These maps, combined with available topographic maps of the same areas, were used. Beyond the limits of these maps, geologic maps and drillers logs on file at the State Geological Survey were used. Calculations were made only for those shale beds for which general information of this type was available. Table 3 shows locations of samples and other pertinent data for all shales tested.
Within these several limits it is calculated that about 3 billion barrels of shale oil and 3,000 billion cubic feet of gas occur in the Kansas black shales.
Table 3--Description of and shale oil yield for all locations sampled.
| Loc. no. |
Description | Sample no. |
Thickness, feet |
Stratigraphic horizon | Yield, gallons per ton |
Type of exposure |
|---|---|---|---|---|---|---|
| 1 | SW NE 9-32-25E Cherokee Co. |
1 | 5 | Black shale below Riverton coal |
trace | Sink hole |
| 2 | 5 | Black shale below Riverton coal |
0 | Sink hole | ||
| 3 | 5 | Black shale below Riverton coal |
trace | Sink hole | ||
| 4 | 5 | Black shale above Little Cabin sandstone |
0 | Sink hole | ||
| 2 | NE SW 10-34-24E Cherokee Co. |
1 | 5 | Black shales between Little Cabin sandstone and Bluejacket sandstone |
trace | Creek bank |
| 2 | 5 | Black shales between Little Cabin sandstone and Bluejacket sandstone |
trace | Creek bank | ||
| 3 | 5 | Black shales between Little Cabin sandstone and Bluejacket sandstone |
trace | Creek bank | ||
| 4 | 5 | Black shales between Little Cabin sandstone and Bluejacket sandstone |
trace | Creek bank | ||
| 3 | SW NW 24-32-24E Cherokee Co. |
1 | 5 | Black shales between Little Cabin sandstone and Bluejacket sandstone |
trace | Coal pit |
| 4 | SW 18-33N-33W Barton Co., Mo. |
1 | 3 | Black shale above Pilot coal |
3.83 | Coal pit |
| Black shale above Pilot coal |
0 | Coal pit | ||||
| 5 | NE NW 24-28-25E Crawford Co. |
1 | 5 | Black shale above Scammon coal |
0 | Coal pit |
| 2 | 5 | Black shale above Scammon coal |
0 | Coal pit | ||
| 6 | SE SW 28-29-25E Crawford Co. |
1 | 5 | Black shale below Ardmore limestone |
9.6 | Coal pit |
| 7 | NW NW 34-25-25E Bourbon Co. |
1 | 3 | Black shale below Blackjack Creek limestone |
4.31 | Coal pit |
| 8 | SE SE 17-32-19E Labette Co. |
1 | 5 | Pleasanton shale | 0 | Shale pit |
| 2 | 5 | Pleasanton shale | trace | Shale pit | ||
| 3 | 5 | Pleasanton shale | 0.72 | Shale pit | ||
| 4 | 5 | Pleasanton shale | 1.68 | Shale pit | ||
| 5 | 4 | Pleasanton shale | 8.39 | Shale pit | ||
| 6 | 5 | Pleasanton shale | 7.19 | Shale pit | ||
| 9 | NW 34-32-22E Cherokee Co. |
1 | 5 | Black shale above Mineral coal |
2.64 | Coal pit |
| 2 | 2 | Black shale above Mineral coal |
trace | Coal pit | ||
| 3 | 5 | Black shale above Fleming coal |
3.83 | Coal pit | ||
| 4 | 5 | Black shale above Mineral coal |
0 | Coal pit | ||
| 10 | 16-29-25E Crawford Co. |
1 | 5 | Black shale above Bevier coal |
0.72 | Coal pit |
| 2 | 5 | Black shale above Bevier coal |
0 | Coal pit | ||
| 3 | 5 | Black shale above Bevier coal |
trace | Coal pit | ||
| 11 | SE SE 12-30-22E Crawford Co. |
1 | 3 | Little Osage shale | 12.2 | Rock quarry |
| 12 | NW NE 21-31-19E Labette Co. |
1 | 5 | Pleasanton shale | 2.16 | Shale pit |
| 2 | 5 | Pleasanton shale | 1.68 | Shale pit | ||
| 13 | NE NW 21-29-23E Crawford Co. |
1 | 5 | Labette shale | trace | Creek bank |
| 14 | NE NE 20-29-20E Neosho Co. |
1 | 3 | Pleasanton shale | 0 | Road cut |
| 2 | 5 | Pleasanton shale | 2.64 | Road cut | ||
| 3 | 5 | Pleasanton shale | 6.47 | Road cut | ||
| 15 | NW NE 23-30-21E Crawford Co. |
1 | 4.5 | Anna shale | 9.35 | Creek bank |
| 16 | SW NE 35-32-18E Labette Co. |
1 | 5 | Ladore shale | slight trace |
Shale pit |
| 17 | SW NE 19-25-25E Bourbon Co. |
1 | 4 | Little Osage shale | 11.98 | Rock quarry |
| 18 | SW 1-35-16E Montgomery Co. |
1 | 5 | Nowata shale | trace | River bank |
| 2 | 2 | Nowata shale | trace | River bank | ||
| 3 | 5 | Nowata shale | 0 | River bank | ||
| 4 | 5 | Nowata shale | 0 | River bank | ||
| 19 | SE NE 19-33-17E Montgomery Co. |
1 | 5 | Dark shale in Cherryvale formation |
trace | Road cut |
| 2 | 5 | Dark shale in Cherryvale formation |
0 | Road cut | ||
| 20 | Cen. 1-33-18E Labette Co. |
1 | 5 | Lake Neosho shale | 1.2 | Creek bank |
| 2 | 2 | Lake Neosho shale | 0 | Creek bank | ||
| 21 | NE NW 12-25-22E Bourbon Co. |
1 | 5 | Pleasanton shale | 2.64 | Shale pit |
| 22 | NW 1-2-40W Cheyenne Co. |
1 | 5 | Pierre shale | trace | Bank of gully |
| 23 | SE NW 22-1-42W Cheyenne Co. |
1 | 5 | Pierre shale | 0 | Bank of gully |
| 2 | 5 | Pierre shale | 0 | Bank of gully | ||
| 3 | 5 | Pierre shale | 0 | Bank of gully | ||
| 24 | Cen. 36-13-40W Wallace Co. |
1 | 5 | Sharon Springs shale | trace | Bank of gully |
| 2 | 5 | Sharon Springs shale | trace | Bank of gully | ||
| 3 | 5 | Sharon Springs shale | heavy trace |
Bank of gully | ||
| 4 | 5 | Sharon Springs shale | 6.95 | Bank of gully | ||
| 5 | 5 | Sharon Springs shale | 5.75 | Bank of gully | ||
| 25 | NW SE 35-11-42W Wallace Co. |
1 | 5 | Lake Creek shale | trace | Hill side |
| 2 | 5 | Lake Creek shale | trace | Hill side | ||
| 26 | SW SE 16-31-23E Crawford Co. |
1 | 4 | Black shale below Blackjack Creek limestone |
4.55 | Road cut |
| 27 | SW NE 7-32-19E Labette Co. |
1 | 5 | Pleasanton shale | 1.44 | Shale pit |
| 2 | 5 | Pleasanton shale | 9.83 | Shale pit | ||
| 3 | 5 | Pleasanton shale | 1.92 | Shale pit | ||
| 28 | SE SE 16-31-23E Crawford Co. |
1 | 5 | Black shale below Ardmore limestone |
2.88 | Creek bank |
| 29 | NW SW 9-34-20E Labette Co. |
1 | 5 | Little Osage shale | 7.43 | Road ditch |
| 30 | NW SW 3-33-20E Labette Co. |
1 | 3 | Anna shale | 3.12 | Road cut |
| 31 | NE NW 6-15-10W Ellsworth Co. |
1 | 5 | Black shale with Dakota lignite beds |
3.36 | Hill side |
| 2 | 5 | Black shale with Dakota lignite beds |
2.88 | Hill side | ||
| 32 | SW 25-30-17W Kiowa Co. |
1 | 5 | Kiowa shale | trace | Hill side |
| 2 | 5 | Kiowa shale | trace | Hill side | ||
| 33 | SW SW 6-17-20E Franklin Co. |
1 | 8 | Eudora shale | 7.43 | Rock quarry |
| 34 | NW NW 3-13-21E Douglas Co. |
1 | 2 | Eudora shale | 4.31 | Rock quarry |
| 35 | NW NE 4-15-19E Douglas Co. |
1 | 2 | Heebner shale | 19.65 | Road ditch |
| 36 | NE SE 11-25-24E Bourbon Co. |
1 | 3.7 | Anna shale | 5.03 | Road cut |
| 37 | NW 28-32-21E Linn Co. |
1 | 4 | Black shale below Blackjack Creek limestone |
9.11 | Hill side |
| *AR-1 | NW NE 25-21-24E Linn Co. |
1 | 12 | Sandy facies of Pleasanton shale |
12.64 | Asphalt rock quarry |
| *AR-2 | SW NW 20-20-24E Linn Co. |
1 | 6 | Bethany Falls limestone | 8.15 | Asphalt rock quarry |
| *AR-3 | NW NW 21-19-24E Linn Co. |
1 | 3 | Bethany Falls limestone | 2.16 | Asphalt rock quarry |
| * Asphalt rock | ||||||
Cretaceous Shales
Sharon Springs member, Pierre shale--The Pierre shale was sampled in several stratigraphic positions, but complete sampling of its entire thickness was not attempted. Samples from the Sharon Springs shale member were the only samples which yielded oil. The Sharon Springs shale was not sampled in sufficient detail to permit calculation of possible reserve of oil; however, its total thickness is 155 feet (Elias, 1931) and it has considerable areal extent. Further sampling might show considerable obtainable oil within this shale.
Dakota lignite beds--Black shale associated with the Dakota lignite coal beds was sampled at only one locality. The yields from these samples were low, but further sampling elsewhere might show deposits of somewhat higher yield.
Pennsylvanian Shales
Eudora shale member, Stanton limestone--The Eudora shale occurs stratigraphically below the Stoner limestone member of the Stanton limestone which is quarried at many places throughout the State. The Eudora shale was not sampled in enough localities to permit calculation of the amount of available oil in the bed within the specified limits.
Pleasanton shale--The Pleasanton shale varies greatly in lithologic character (Moore, 1949); however, in Labette, Neosho, and Bourbon counties variable thicknesses of black shale are exposed. Samples from this black shale show a great variance both stratigraphically and areally with respect to their properties as oil shale. Deposits of this shale have been used locally for road material. Plate 1B shows the Pleasanton shale in Labette County where it is being quarried for county road use.
The calculated content of oil in the Pleasanton shale within the limits of 5 gallons per ton of shale and under 100 feet or less of overburden is 149,350,000 barrels. Were it not for the extreme variability of the Pleasanton shale this figure would be much larger. Further sampling of the shale may lower this figure. A drill core from the vicinity of Coffeyville fired in the ceramic laboratory of the State Geological Survey was used to aid in the determination of this quantity. This firing test showed that shale containing oil extended at least to the locality of the core and that it would be within reason to use its position as a zero point. Using a calculated average figure of 1,230 cubic feet of gas per ton of shale it is determined that 1,174 billion cubic feet of gas occurs along with the oil in the Pleasanton shale.
Anna shale member, Pawnee limestone--Samples from the Anna black shale show a variable but moderately high yield. The Myrick Station limestone member lies directly above the Anna shale and would add to the difficulty in mining it. On the other hand the limestone has protected the black shale from weathering and might be a factor contributing to its moderately high yield. Because information as to the areal extent of the Anna shale was lacking, the amount of oil contained in it, under the limiting conditions, was not calculated.
Little Osage shale member, Fort Scott limestone--Samples tested from the Little Osage shale show some of the highest consistent yields of any of the Kansas black shales tested. This shale occurs between the limestone members of the Fort Scott formation. The Higginsville limestone member, above, has protected the shale from weathering, which might have contributed to its higher yield. This upper limestone would add to the difficulty of mining the shale; however, it is quarried extensively now for crushed stone. The Blackjack Creek limestone below the shale is quarried for hydraulic cement, and the overlying Little Osage shale must be handled to quarry this rock. Plate 1A shows this shale in relation to the limestone at the Fort Scott Natural Cement Company quarry near Ft. Scott, Kansas.
The content of shale oil, within the set limits, in the Little Osage shale is determined to be 719,600,000 barrels. This high figure is due to the comparatively high yields obtained from this shale and from the consistent yield from place to place. Using a calculated average of 1,230 cubic feet of gas per ton of shale it is determined that 2,813,200 million cubic feet of gas is present concurrently with the oil in this shale.
Cherokee shale, black shale above the Mulky coal and below the Blackjack Creek limestone--An unnamed black shale occurs above the stratigraphic position of the Mulky coal. The Mulky coal is not always present below this shale, but where it does occur the coal is commonly mined and this overlying shale must be handled. The Blackjack Creek limestone, directly above the black shale, is quarried for raw material used in the manufacture of hydraulic cement. This too would contribute toward the availability of this shale. Plate 2B shows the relationship of this shale to the Mulky coal and the overlying Blackjack Creek limestone.
The calculated oil content within specified limits in this shale is 217,900,000 barrels, and 1,595 billion cubic feet of gas occurs concurrently with the oil (calculated average of 1,230 cubic feet of gas per ton of shale).
Cherokee group, black shale below the Ardmore limestone--An unnamed black shale occurs below the Ardmore limestone. This shale lies above the Mineral coal and the Fleming coal, both of which are mined at one; location (Pl. 2A) where the shale was sampled; and this shale must be handled in the strip mining of these coals. The calculated quantity of shale oil, within the specified limits, in this black shale is 81 million barrels, and if the calculated average figure of 1,230 cubic feet of gas per ton of shale is used, 530 billion cubic feet of gas occurs concurrently with the oil.
Asphalt rock in Kansas was studied and described by Jewett (1940) and therefore the deposits of this material are not redescribed in this report. However, it was judged advisable to know how asphalt rock reacts if subjected to the same test as was used for the black shale. The three locations recorded in Jewett's report as having been used for asphalt rock were selected for sampling. The results in all cases were comparatively favorable and show that this material too should be considered as a possible source of oil.
To get an idea of how these potential Kansas yields fit into the world and national picture some of the facts concerning various commercial oil shales are presented for comparison. Much of the following material was obtained from the article by Prien (1951) in the Colorado Engineers' Bulletin. The black to brownish-black finely laminated Scottish shales, among the oldest and best known of the oil shales, occur in beds 4 to 14 feet thick, and have an average obtainable oil content of approximately 22 gallons per ton. The average oil yield of the French shales is 17 gallons per ton for the richer deposits and 10 gallons per ton for the leaner ones. The shale beds of Estonia average 7 feet in thickness and production ranges from 48 to 86 gallons per ton. Yields of 80 to 180 gallons per ton are reported from certain Australian shale deposits. Noteworthy are the New South Wales deposits being used in the manufacture of motor oil. Here one refinery, with a charging capacity of 42,000 gallons of crude oil per day, is engaged in refining shale oil for production of raw and polymer gasoline, gas, and coke.
Deposits in Manchuria, occurring in beds averaging 450 feet in thickness, yield from 14 to 15 gallons of shale oil per ton of shale mined. Vast oil-shale reserves are reported in the U.S.S.R., with an annual production rate of 3 million tons anticipated. Known deposits, chiefly along the Volga River, are said to contain 35 to 50 gallons per ton of recoverable oil. Japan in 1948 had an annual production of more than 5 million barrels.
On the North American continent shales are found extensively distributed in both the United States and Canada. The richest Canadian deposits, in New Brunswick, contain more than 22 gallons per ton of obtainable shale oil and closely resemble the Scottish shales. In the United States, shale deposits considered to be of importance occur in the Tertiary shales in Colorado, Nevada, Utah, and Wyoming; in black shales in Indiana, Kentucky, and Tennessee; and in cannel coal shales in Pennsylvania and West Virginia. Total United States reserves have recently been estimated at 400 billion tons, only a small fraction of which can be claimed by Kansas. About 100 billion barrels of this oil are said to be immediately recoverable.
The Tertiary shales of the Green River formation of northwestern Colorado, eastern Utah, and southwestern Wyoming are the United States reserves of the most immediate potential industrial importance. The Green River shales have the relatively high potential shale-oil yield of approximately 25 gallons per ton and they are the most extensive of the country's oil shales. Colorado alone can claim 300 billion barrels of recoverable oil, 6 times as much as has been produced and consumed in the world since the drilling of the first oil well.
Oil production--The future of the commercial production of oil from shale is linked directly to the future of the petroleum industry. This can be interpreted to mean that though there is no immediate need for the extracting of oil from oil shale and coal it is justifiable and even expedient to anticipate future needs by being prepared to meet them. The economics of the establishing of an oil-shale industry must be backed by factual investigations. One aspect concerning the Kansas shales not to be overlooked is their close association with other mineral deposits. In some cases the black shale is overburden in the way of material being mined, and in others the shale is exposed directly through the process of mining the associated material. Such is the case in the large strip coal mining areas and to a lesser degree in limestone quarrying in the State. Under present conditions, then, the shale itself could be mined at little extra cost, and at some future date the economics of oil production in the United States may change enough to permit competition from oil made from shale.
The inorganic material of oil shale also is a possible source of raw material for the manufacture of a variety of products-cement, building bricks, mineral wool, alumina, filter acid, decolorizing carbon, and various molded articles. Upon retorting oil shale, appreciable quantities of oxygen-containing organic compounds are formed in the shale oil produced--mainly tar acids--phenol and its homologs predominating (Prien, 1951).
Other uses--The principal possibilities other than oil shale for the development of the black shales of Kansas are as fertilizer (Runnels, 1949) (because of the phosphate content and because of the trace elements) and uranium. The utilization of the phosphate in these shales is feasible even under existing economic conditions. Should a national emergency restrict the use of railroad cars in shipping rock and super phosphate into Kansas, a phosphate-shale business might become a reality. Also it is not impractical to try to find a way to utilize the shales as solid fuel per se, as the high content of bitumen along with the shale oil and gas gives these shales a relatively high gross heating value.
The recent work by the Atomic Energy Commission has prompted investigation of the uranium content of the shales. The uranium is mostly contained in the phosphate nodules and according to a recent release from the Atomic Energy Commission economic extraction of uranium during the manufacture of super phosphate has been proved. The Estonian shales are said to contain about 200 grams of uranium per ton of shale and are of interest from the uranium standpoint. Kansas shales contain in the phosphate nodules about 136 grams of uranium per ton of shale.
Another possibility not discussed previously is an investigation of the properties of these shales when used as aggregate for hot bituminous matts in road construction. The organic material in these shales suggests that with proper treatment an actual bonding action could be attained.
Abernathy, G. E. (1946) Strip-mined areas in the southeastern Kansas coal field: Kansas Geol. Survey, Bull. 64, pt. 4, pp. 125-144, fig. 1, pls. 1-3.
Elias, M. K. (1931) The geology of Wallace County, Kansas: Kansas Geol. Survey, Bull. 18, pp. 1-254, figs. 1-7, pls. 1-42. [available online]
Gavin, M. J. (1922) Oil shale: an historical, technical, and economic study: U.S. Bur. Mines, Bull. 210, pp. 1-201, figs. 1-4, pls. 1-18.
Guthrie, Boyd, and Klosky, Simon (1951) The oil-shale industries of Europe: U.S. Bur. Mines, Rept. of Investi. 4776, pp. 1-73, figs. 1-65.
Hubbard, A. B., and Robinson, W. E. (1950) A thermal decomposition study of Colorado oil shale: U.S. Bur. Mines, Rept. of Investi. 4744, pp. 1-24, figs. 1-13.
Jewett, J. M. (1940) Asphalt rock in eastern Kansas: Kansas Geol. Survey, Bull. 29, pp. 1-23, figs. 1-3, pls. 1-2. [available online]
Jewett, J. M. (1945) Stratigraphy of the Marmaton group, Pennsylvanian, in Kansas: Kansas Geol. Survey, Bull. 58, pp. 1-148, pls. 1-4.
Kraemer, A. J., and Thorne, H. M. (1951) Oil-shale operations in New South Wales, Australia: U.S. Bur. Mines, Rept. of Investi. 4796, pp. 1-48, figs. 1-23.
Latta, B. F. (1948) Geology and ground-water resources of Kiowa County, Kansas: Kansas Geol. Survey, Bull. 65, pp. 1-151, figs. 1-10, pls. 1-11. [available online]
Moore, R. C. (1949) Divisions of the Pennsylvanian System in Kansas: Kansas Geol. Survey, Bull. 83, pp. 1-203, figs. 1-37. [available online]
Pierce, W. G., and Courtier, W. H. (1938) Geology and coal resources of the southeastern Kansas coal field in Crawford, Cherokee, and Labette Counties: Kansas Geol. Survey, Bull. 24, pp. 1-122, figs. 1-13, pls. 1-12.
Runnels, R. T. (1949) Preliminary report on phosphate-bearing shales in eastern Kansas: Kansas Geol. Survey, Bull. 82, pt. 2, pp. 37-48, pls. 1-2. [available online]
Prien, C. H. (1951) World Developments in oil shale: Colorado Engineers' Bull., vol. 35, no. 4, pp. 8-9, 18-22, figs. 1-3; vol. 35, no. 5, pp. 8-9, 18-21.
Stanfield, K. E., and Frost, I. C. (1946) Method of assaying oil shale by a modified Fischer retort: U.S. Bur. Mines, Rept. of Investi. 3977, pp. 1-11, figs. 1-5.
Stanfield, K. E., and others (1951) Properties of Colorado oil shale: U. S. Bur. Mines, Rept. of Investi. 4825, pp. 1-27, figs. 1-9.
Kansas Geological Survey, Oil Shale in Kansas
Placed on web Jan. 15, 2009; originally published in March 1952.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/96_3/index.html