Kansas Geological Survey, Bulletin 204, pt. 1, originally published in 1972
Originally published in 1972 as part of Kansas Geological Survey Bulletin 204, pt. 1, p. 19-25. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version of the complete bulletin (15 MB) is also available.
A comparative organic-geochemical study has identified some of the extractable hydrocarbon components of ten Upper Pennsylvanian and Lower Permian Kansas shales, and has applied criteria for biogenicity of such compounds.
The lithologies of the shale samples examined ranged from organic-rich black shales to red shales which contain very low amounts of extractable organic material.
The extractable organic material present in these shales includes normal alkane hydrocarbons, isoprenoid and sterane hydrocarbons, and more polar compounds, including aromatic hydrocarbons.
Organic-geochemical separation techniques and identification methods utilized in this study include thin-layer, liquid, and gas chromatography, ultraviolet and infrared spectroscopy, and mass spectrometry.
The purpose of this investigation is to examine the nature of extractable hydrocarbon components present in Upper Pennsylvanian and Lower Permian Midcontinent (Kansas) shales, and to relate these components to biologic precursors.
Although the use of organic-geochemical methods endeavors to detect "chemical fossils" in natural samples in order to trace the evolution of life or life-supporting compounds, the extractable organic material present in ancient rocks today represents not only the remains of plants and animals originally incorporated in the sediment, but biogenic and abiogenic modifications which occurred during diagenesis and burial of the rock. Superimposed on these modifications are possible effects of weathering and/or migration of hydrocarbons.
Criteria for biogenicity are therefore extremely important for organic-geochemical research. Eglinton (1969) has summarized these criteria and has presented structural and presumed causal relationships between biologic precursors and compounds found in sediment extracts. Certain hydrocarbons, fatty acids, and porphyrins appear to be stable throughout geologic time, and therefore serve as criteria for biogenicity. The ability of certain compounds to rotate plane polarized light because of enzymatic activity in the original organism is, for example, another test for biogenicity.
In this study, three groups of hydrocarbons (alkanes, isoprenoids, and steranes) will be used as criteria for biogenicity.
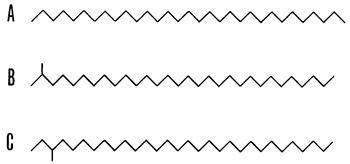
Alkanes are generally derived under geologic conditions from fatty acids produced by living organisms. Only three types of alkane hydrocarbons are found in natural samples: normal, iso-, and anteisoalkanes. For example, over one million isomers are possible for C31H64 yet only three are found in sediments (see Fig. 1); those isomers could be predicted because of biochemical pathways in present organisms.
Figure 1--Skeletal formulations of isomeric alkanes, C31H64. A, n-hentriacontane (n-alkane); B, 2-methyltriacontane (isoalkane); C, 3-methyltriacontane (anteisoalkane). The zig-zag portrayal is for convenience of representation; the molecules are flexible and are free to take up an almost unlimited number of shapes (after Eglinton, 1969).

Chlorophyll from green plants degrades to several pigments, and the phytol side chain to isoprenoid hydrocarbons (Fig. 2). Recent sediments contain phytane and some pristane, and other isoprenoids of C18, C17, C16, C15, C14, if the organic matter has been well preserved. With geologic age, the phytane degrades, and pristane often becomes the major component of the isoprenoid compounds (Robinson, et al., 1965).
Figure 2--Degradation of chlorophyll a, giving rise to two kinds of isoprenoid molecules, phytane and pristane (diagrammatic representation; after Eglinton, 1969).
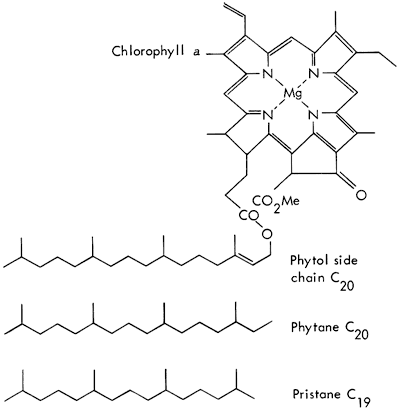
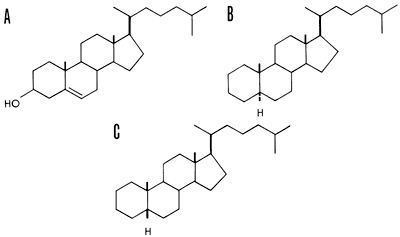
Steranes, which result from the plant and animal steroids such as cholesterol, often indicate the type of organism deposited in the sediment (Hills, et al., 1966; Speers and Whitehead, 1969). Both plants and animals contribute the steroid ring nucleus which in the sediment becomes reduced and saturated, and as a result remains very stable in sediments. Cholestane and sitostane are the most abundant triterpenes in sediments. Again, enzyme activity accounts for the specificity that only 2 out of 256 possible isomers for cholesterol have ever been detected in natural samples. Cholestane and coprostane are presumably derived from cholesterol (Fig. 3).
Figure 3--Cholesterol and its diastereomeric, C27H48 cycloalkanes. A, cholesterol; B, 5α-cholestane (cholestane); C, 5β-cholestane (coprostane). Heavy lines indicate bonds directed above the plane of the ring and dashed lines indicate bonds below the ring (after Eglinton, 1969).
The stratigraphy and lithologic characteristics of the Pennsylvanian and Permian rocks of Kansas have been described by Moore (1949) and Moore and Mudge (1956). Ten shales from the Upper Pennsylvanian and Lower Permian of Kansas were selected for this investigation; their stratigraphic positions are illustrated in Figure 4. All samples were collected from outcrops, and every effort was made to obtain fresh samples. Gloves were used to prevent hand contamination of the shales. A brief description of the location and lithology of the shales is presented in Table 1.
Figure 4--Stratigraphic position of the Upper Pennsylvanian and Lower Permian shales sampled for this study.
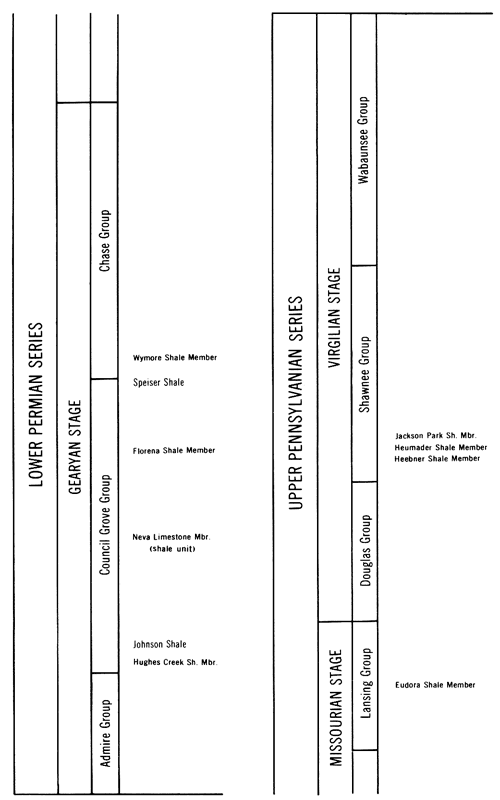
Table 1--Sample location and description.
| Shale | Location | Description |
|---|---|---|
| Wymore | Road cut, W side of K-113 cen., W2, 26-9S-7E, Riley Co. |
Mudstone, silty, gray-red (10R 4/2) |
| Speiser | Road cut, S side of 1-70 cen., 34-11S-6E, Geary Co. |
Claystone, greenish-gray (5G 6/1), calcareous |
| Florena | Quarry cut, top of Prospect Hill, W of K-177 NW 20-10S-8E, Riley Co. |
Claystone, medium light-gray (N6), calcareous |
| Neva | Road cut, base of Prospect Hill, W of K-177 NW 20-10S-8E, Riley Co. |
Clayshale, dark gray (N3), fossiliferous |
| Johnson | Road cut, base of Bluemont Hill, N of Water Works SE 7-10S-8E, Riley Co. |
Claystone, greenish-gray (5GY 6/1) |
| Hughes Creek | Road cut, base of Bluemont Hill, NW of Water Works SE 7-10S-8E, Riley Co. |
Clayshale, dark gray (N3) |
| Jackson Park | U.S. storage cave, above Gate 1 SE 7-6S-21E, Atchison Co. |
Claystone, light olive-gray (5Y 6/1), calcareous |
| Heumader | U.S. storage cave, inside cave SE 7-6S-21E, Atchison Co. |
Claystone, medium-gray (N5) |
| Heebner | Road cut, SE intersection U.S. 40 and U.S. 59 NE NE 36-12S-19E, Douglas Co. |
Clayshale, dark gray (N3), phosphatic |
| Eudora | Quarry cut SE 27-16S-20E, Franklin Co. |
Clayshale, dark gray (N3) |
The analytical scheme employed for the isolation and identification of organic matter in the Kansas shales is illustrated in Figure 5.
Figure 5--Analytical procedure.
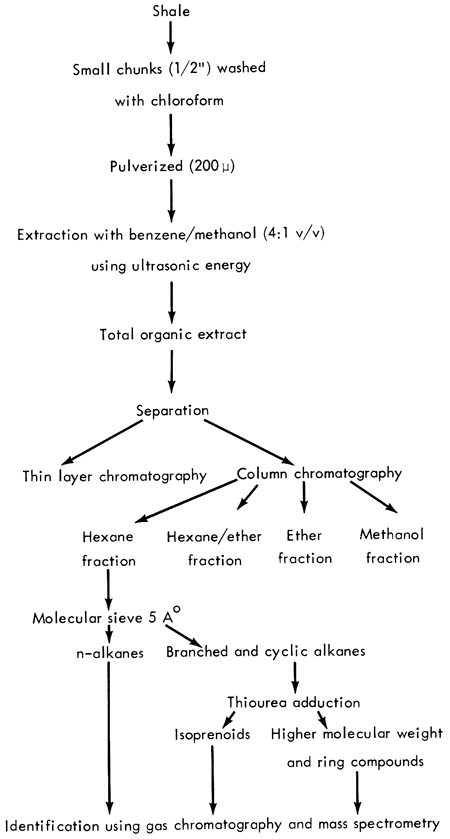
The shale samples were ground or pulverized to less than 200-μ size in a motor-driven mortar and pestle or an Angstrom grinder using a stainless steel sample holder. The powdered shales were extracted ultrasonically with benzene-methanol (4:1, v/v) for an hour; three such extractions were completed with fresh solvent for a total extraction time of three hours. After the mixture was centrifuged, the solvent was removed by flash evaporation. The last traces of solvent were evaporated under a stream of dry nitrogen. Once the weight of the extract was obtained, the general distribution of classes of components in the extract was revealed by a thin layer chromatogram on Silica Gel G developed in hexane and visualized with 0.0005% Rhodamine 6G. Information from thin layer chromatography determined the conditions for column chromatography and solvent volumes. A ratio of 1:100 sample to alumina adsorbent was always employed for optimum separation. The columns were eluted first with hexane, then hexane-ether (8:2 and 1:1), ether, and methanol.
The hexane fraction, collected in 25-ml portions, was checked for elemental sulfur using ultraviolet absorption maxima at 262 mμ and 283 mμ, as well as for aromatic hydrocarbons, using a Beckman DK-2A Ratio Spectrometer (Murphy, et al., 1965). Organic sulfur, if suspected from thin layer chromatography, was tested for with N-ethylmaleimide. The ultraviolet absorption and thin layer migration values determined which classes of hydrocarbons were in the individual fractions.
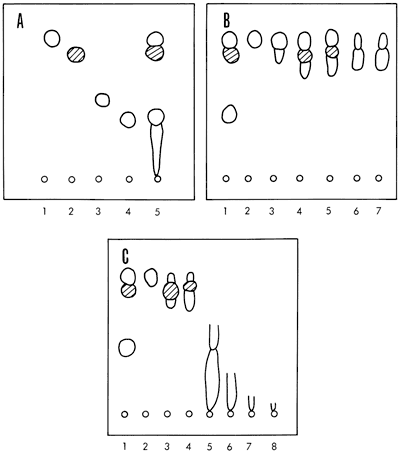
The other solvents used for elution were collected as single fractions from the column. Thin layer chromatograms confirmed the effectiveness of the column separations. The separation of classes of compounds present in the Heebner shale hexane fractions and other column eluates is illustrated in Figure 6.
Figure 6--Thin layer chromatograms of Heebner shale extract. Conditions: Silica Gel-G; n-hexane developer, 0.0005% Rhodamine 6-G visualizer; observed under ultraviolet light 254 and 365 mμ. A, standards and total extract: (1) n-octadecane, (2) elemental sulfur, (3) 2,7-dimethyl naphthalene, (4) phenanthrene, (5) total extract; B, hexane fractions: (1) standards-octadecane, 2-methylnaphthalene, phenanthrene, and sulfur, (2) fractions 1 and 2, (3) fractions 3 and 4, (4) fractions 5 and 6, (5) fraction 7, (6) fractions 8, 9, and 10, (7) fraction 11. Fractions 2-11 are the successive 25-ml portions eluted from the column; C, other column fractions: (1) standards-octadecane, 2-methylnaphthalene, phenanthrene, and sulfur, (2) hexane fractions 1 and 2, (3) hexane fraction 7, (4) hexane fractions 8, 9, and 10, (5) hexane/benzene fraction, (6) benzene fraction, (7) ether fraction, (8) methanol fraction.
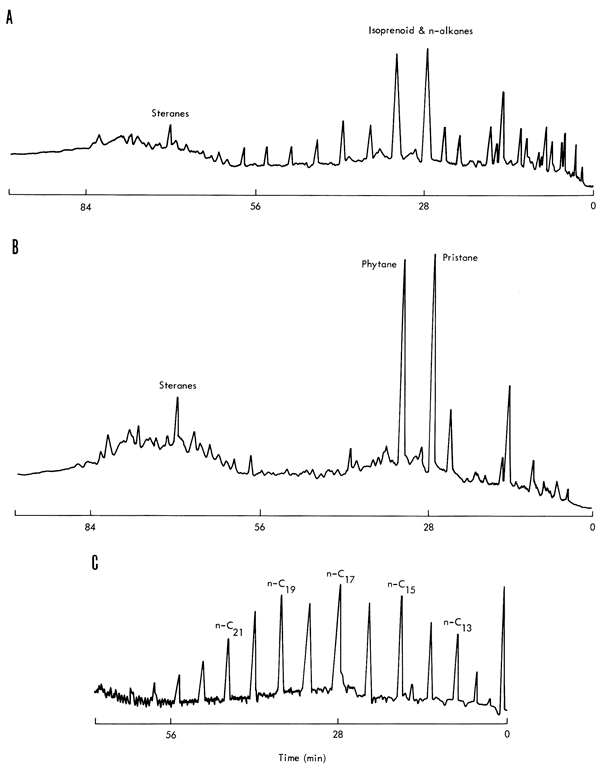
The chain and sterane ring fractions eluted prior to sulfur were separated into normal and branched and cyclic fractions with 5A molecular sieves (Thomas and Mays, 1961). These fractions were separated into individual components by gas chromatography (Perkin-Elmer 226 with an SE-30 S.C.O.T. column, 50' x 0.02", and equipped with a flame ionization detector) Many of the peaks were identified from retention time data on standard hydrocarbons with an average deviation of less than 0.25 min. A comparison of the gas chromatographic traces for the total extract, branched-cyclic fraction, and the normal alkanes for the Neva shale is illustrated in Figure 7.
Figure 7--Gas chromatograms of hexane fractions of Neva shale. Conditions: P-E 226, gas chromatograph; Column SE-30, S.C.O.T. 50' x 0.02"; temperature program, 80-240°, 2°/minute; final time, 20 minutes; chart speed 15"/hour; block temperature, 280° C; detector temperature, 180° C; carrier gas, helium. A, total hexane fraction; B, non-adduct of 5A molecular sieve; C, adduct of 5A molecular sieve.
Further identification of several compounds was obtained from mass spectra derived from combination GC/MS analyses (Hitachi RMU-6 Mass Spectrometer).
The more polar fractions obtained by elution column chromatography require further separation and identification. The aromatic hydrocarbons often have characteristic fluorescence spectra, thin layer migration values, and gas chromatographic retention times; however, mass spectrometry is required for confirmation of individual compounds.
The total lipid content of the ten Upper Pennsylvanian and Lower Permian shale samples is listed in Table 2. The results of the hydrocarbon analyses of each of these extracts are summarized in Table 3.
Table 2--Lipid content of the shales.
| Shale | Lipid-mg/1000 g shale |
|---|---|
| Wymore | 27 |
| Speiser | 16 |
| Florena | 700 |
| Neva | 3,930 |
| Johnson | 27 |
| Hughes Creek | 35 |
| Jackson Park | 180 |
| Heumader | 645 |
| Heebner | 5,000 |
| Eudora | 1,059 |
Table 3--Comparison of hydrocarbons in shale extracts.
| Shale | n-Hydrocarbons | Isoprenoid, diterpenes |
Steranes, triterpenes |
Carotane, tetraterpene |
Aromatic hydrocarbons |
Sulfur |
|---|---|---|---|---|---|---|
| Wymore | + | ? | ? | ? | ? | -- |
| Speiser | + | ? | ? | ? | -- | -- |
| Florena | + | + | ? | ? | + | + |
| Neva | + | + | + | ? | + | - |
| Johnson | + | ? | ? | ? | ? | -- |
| Hughes Creek |
+ | + | ? | ? | + | + |
| Jackson Park |
+ | + | + | ? | + | + |
| Heumader | + | + | + | -- | + | + |
| Heebner | + | + | + | -- | + | + |
| Eudora | + | + | + | ? | + | + |
The reddish Wymore shale of the Chase Group, as expected, contains very little extractable organic material; however, some normal alkane hydrocarbons were identified by gas chromatography.
Of the five shales examined from the Council Grove Group, only the gray-black Neva and Florena shales contain appreciable amounts of extractable organic material. The reddish-green Speiser shale contains very low amounts of extractable organic material. The greenish-gray to black Johnson and Hughes Creek shales contain surprisingly low amounts of extractable material; the extent to which the lipid content of these shales may have been modified by weathering processes, as compared to the other samples, is not known.
The Florena and Hughes Creek shales contain elemental sulfur; the extract of the latter contained nearly 50 percent sulfur. No other shales studied in this investigation released such large amounts of sulfur.
Hydrocarbons were present in all examined members of the Council Grove Group. The extracted normal alkanes generally exhibit a single smooth distribution from C12 to C26 with a maximum around C17; the Neva shale has a slight, but definite, odd- over even-numbered carbon atom preference (Fig. 7,C). The original preference of odd-numbered hydrocarbons, characteristic of recent sediments, disappears with geologic age, resulting in a more Gaussian distribution (Meinschein, 1969).
Phytane (C20H42) and pristane (C19H40) are the two most abundant isoprenoid hydrocarbons present in these shales (See Fig. 7,B). Pristane is present in somewhat greater concentrations than phytane, which is characteristic of older sediments. Farnasane (C15H32) frequently appears in these shales in relatively high concentrations. Steranes, such as cholestane (C27H48), are definitely present in moderate concentrations, but identification of individual components requires mass spectrometric analyses, as do the aromatic hydrocarbons and more polar compounds present in the ether and methanol column eluates.
The three gray-black shales from the Shawnee Group have been investigated previously by Gould (1969), Noonan (1971), and Prentice (1971). The Heebner shale contains a large amount of extractable organic material, while the Jackson Park and the Heumader shales contain only modest amounts of lipid material. Both the Heumader and Jackson Park extracts form a "pitch-like," black, semi-solid material which does not redissolve in methanol. All three shales contain elemental sulfur.
These shales also exhibit a single distribution of normal alkane hydrocarbons with a maximum at C17, although the Jackson Park sample has a predominant odd-over-even carbon preference. Isoprenoids and steranes present in the extracts are characteristic of sediments deposited under reducing conditions. The less common C18 isoprenoid is present in the Heumader shale, while in the Heebner shale phytane is somewhat unexpectedly more abundant than pristane. Retention times of peaks obtained by capillary gas chromatography indicate the presence of cholestane (C27H48), and possibly ergostane (C28H50) and sitostane (C29H52), in these samples. These compounds result from saturation of steroid ring compounds. Perhydrocarotene (C40H78), which is present in the Green River (Colorado) oil shale (Murphy, et al., 1967), is not detectable in the Heebner and Heumader extracts, and was not determined in the other extracts.
The black Eudora shale, the only member of the Lansing Group studied, is relatively rich in lipids. The analysis of the organic extract reveals the presence of sulfur, normal alkanes from C15 to C27, isoprenoid hydrocarbons, steranes, and aromatic hydrocarbons.
In general, the lipid compositions of the shales examined are not unexpected, and represent compounds formed by biologic synthesis and modified over geologic time. The contribution of green plants in the original organic source material is well established by the prominent isoprenoid and sterane components. However, some differences do exist among the extractable organic materials present in these shales. The distributions of the normal alkane hydrocarbons and the concentrations of the isoprenoid hydrocarbons may vary somewhat. Further work on the nature of the steranes, the aromatic fraction, and the fatty acids will provide information that may help determine differences in environment during deposition and temperature conditions after incorporation of the organic matter into the sediment.
The authors gratefully acknowledge support for this investigation from the Kansas Geological Survey, NASA-07-011-001, NASA-NGR-05-007-215, NSF-GA-1190. The mass spectra were obtained at the University of Arizona with the assistance of Drs. B. Nagy, W. Scott, and V. Modzeleski. The authors wish to thank Ann Fernandes and B. Visinski for their assistance with the analyses.
The authors also wish to thank Dr. E. E. Angino and T. C. Waugh, of the Kansas Geological Survey, for their aid in collecting the samples, and Dr. Gerard W. James, Kansas Geological Survey, for his helpful comments and suggestions.
Calvin, M., 1969, The fossil record; in, Chemical Evolution: Oxford University Press, New York, p. 9-34.
Eglinton, G., 1969, Organic geochemistry, The organic chemist's approach; in, Organic Geochemistry, Methods and Results, G. Eglinton and M. T. J. Murphy, eds.: Springer-Verlag, New York, p. 20-73.
Gould, Fr. G., 1969, Hydrocarbons in the Heebner Shale: American Chemical Society Meeting, Wichita, Kansas.
Hills, I. R., Whitehead, E. V., Cummins, J. J., and Robinson, W. E., 1966, An optically active triterpane, gammacerane, in Green River, Colorado, oil shale bitumen: Chem. Commun., v. 20, p. 752-754.
Lawlor, Sr. S., 1967, Organic matter in the red, gray, and black shale from the East Berlin Formation: Unpub. M.A. thesis, Saint Joseph College.
Meinschein, W. G., 1969, Hydrocarbons--saturated, unsaturated and aromatic; in, Organic Geochemistry, Methods and Results, G. Eglinton and M. T. J. Murphy, eds.: Springer-Verlag, New York, p. 330-356.
Moore, R. C., 1949, Divisions of the Pennsylvanian System in Kansas: Kansas Geol. Survey, Bull. 83, p. 1-203. [available online]
Moore, R. C., and Mudge, M. R., 1956, Reclassification of some Lower Permian and Upper Pennsylvanian strata in northern Mid-continent: Am. Assoc. Petroleum Geologists, Bull., v. 40, no. 9, p. 2271-2278.
Murphy, Sr. M., 1969, Analytical methods; in, Organic Geochemistry, Methods and Results, G. Eglinton and M. T. J. Murphy, eds.: Springer-Verlag, New York, p. 74-88.
Murphy, Sr. M., McCormick, A., and Eglinton, G., 1967, Perhydro-β-carotene in the Green River shale: Science, v. 157, p. 1040-1042.
Murphy, Sr. M., Nagy, B., Rouser, G., and Kritchevsky, G., 1965, Identification of elementary sulfur and sulfur compounds in lipid extracts by thin-layer chromatography: Jour. Am. Oil Chem. Soc., v. 42, p. 475-480.
Noonan, Sr. J., 1971, The organic geochemistry of the Heumader Shale of Kansas: Hydrocarbons: Unpub. M.A. thesis, Saint Joseph College, 96 p.
Prentice, D. L., 1971, A study of the hydrocarbons found in Jackson Park (Kansas) Shale: Unpub. M.A. thesis, Saint Joseph College, 105 p.
Robinson, W. E., Cummins, J. J., and Dinneen, G. U., 1965, Changes in Green River oil-shale paraffins with depth: Geochim. Cosmochim. Acta, v. 29, p. 249-258.
Speers, G. C., and Whitehead, E. V., 1969, Crude petroleum; in, Organic Geochemistry, Methods and Results, G. Eglinton and M. T. J. Murphy, eds.: Springer-Verlag, New York, p. 640-647.
Thomas, T. L., and Mays, R. L., 1961, Separation with molecular sieves; in, Physical Methods in Chemical Analysis, Vol. IV, W. G. Berl, ed.: Academic Press, New York, p. 45-98.
Kansas Geological Survey, Upper Pennsylvanian and Lower Permian Kansas Shales: Hydrocarbons
Placed on web May 8, 2009; originally published in March 1972.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/204_1D/index.html