
Kansas Geological Survey, Bulletin 202, part 2, originally published in 1971

Originally published in 1971 as Kansas Geological Survey Bulletin 202, part 2. This is, in general, the original text as published. The information has not been updated.
In samples of shaly chalk from the Greenhorn Limestone, coccolith abundance can be determined by means of a newly devised technique. A known volume of shaly chalk is disaggregated ultrasonically and the resulting sludge suspended in 2000 ml of water. A 0.001-ml aliquot of the suspension is then placed on a slide, dried, and covered. Using a polarizing microscope fitted with a mechanical stage, coccoliths are counted and the number on the slide is used to calculate total number in the suspension. Correction is made for portions of the original sample that did not disaggregate. Chief difficulty is in counting specimens that are clustered together on the slide. Duplicate counts were made for most samples, with results suggesting that the technique is valid.
Shaly chalk samples from a Mitchell County locality contain from 5 x 108 to 6 x 109 coccoliths per cubic centimeter. The lowest values are for samples adjacent to bentonite seams. Coccolith abundance in the Greenhorn samples compares favorably with estimates that have been made for Jurassic limestone of Dorset (England) and Franciscan limestone of California, and with some modern shelf deposits.
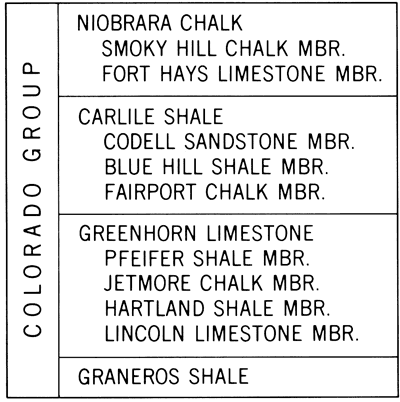
For several years the senior author has conducted studies of Upper Cretaceous strata in central and western Kansas, One of the major goals of these investigations is genetic interpretation of sedimentary rocks comprising the Greenhorn cyclothem (Hattin, 1964), a sequence of marine strata embracing (ascending) the upper part of the Dakota Formation, Graneros Shale, Greenhorn Limestone, and Carlile Shale (Fig. 1). In this cyclic bundle of strata the initial stages of transgression are marked by shallow-water, nearshore sandstones and associated sandy and/or carbonaceous shales. These are followed by sandy and silty shales of the Graneros which represent offshore, probably deeper water, marine deposition. Where the two formations are conformable locally, noncalcareous shales of the Graneros grade upward through a transitional sequence of calcareous shales and chalky shales into the chalky strata of the Greenhorn Limestone. Above the middle of the Graneros Shale gradual upward increase in carbonate content of the section reflects progressively greater distance from eastwardly migrating shoreline during deposition and consequently smaller influence of terrigenous detrital deposition. The lower half of the Greenhorn is composed mostly of impure shaly chalk. The upper half (Jetmore and Pfeifer members) contains, in addition to shaly chalk, a large concentration of medium to very thin beds of relatively pure chalky limestone and represents the maximum stage of marine transgression. Above the Greenhorn the stratigraphic section is regressive, including (ascending) impure shaly chalk of the Fairport Member, Carlile Shale, noncalcareous silty and upwardly sandy shales of the Blue Hill Member, and finally the relatively shallow-water sandstones and clayey sands of the Codell Member which terminates the cyclothem.
Figure 1--Stratigraphic nomenclature of Colorado Group in west-central Kansas.
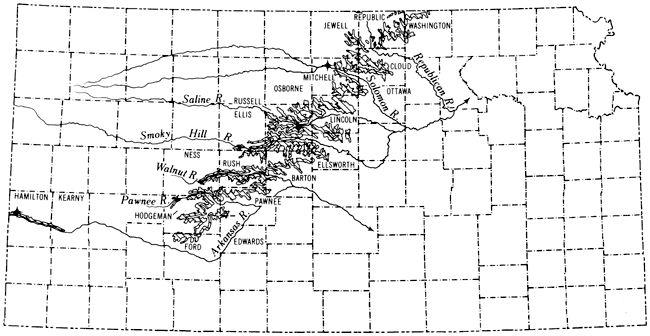
Within this cyclothem the carbonate rocks represent offshore deposition, outside the area of terrigenous-detritus-dominated sedimentation. Insoluble residue analysis of samples from three Kansas localities (Fig. 2), augmented by X-ray and thin section study of specimens from throughout the stratigraphic section, shows that the vast majority of Greenhorn shaly chalk and chalky limestone beds are composed predominantly of calcium carbonate. Exceptions are found mainly in Graneros-Greenhorn transition beds, where the formational contact is arbitrary, and in parts of the section where carbonate accumulation was diluted by volcanic ash fall, i.e., adjacent to bentonite seams.
Figure 2--Map showing Kansas outcrop of Greenhorn Limestone. Starred localities are those for which insoluble-residue data have been gathered.
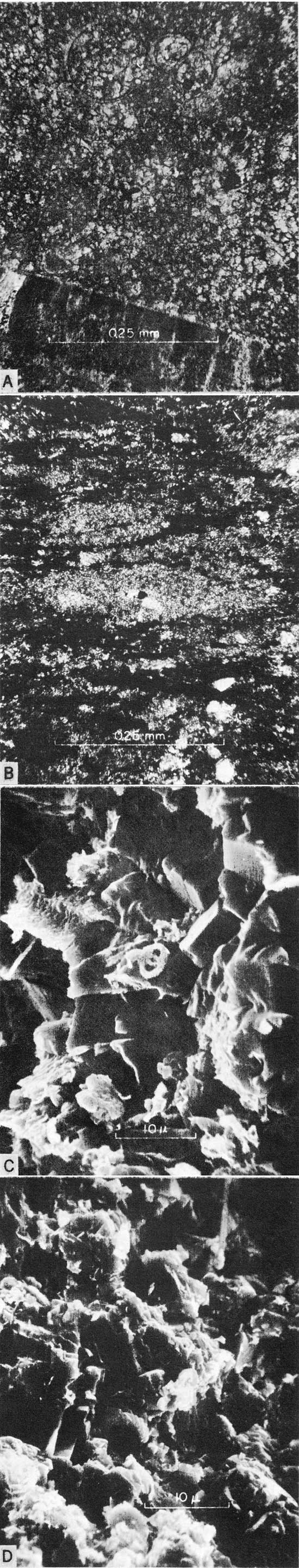
The more obvious carbonate components of Greenhorn chalks and limestones are valves, valve fragments, and isolated prisms of Inoceramus; tests of planktonic foraminifers; and, less commonly, skeletal remains of oysters, anomiids, and cirripeds. In the Lincoln Member these types of remains are concentrated in beds and lenses of biosparite and biosparrudite that characterize the member. Such concentrations are less common elsewhere in the formation. The principal kinds of carbonate rocks, including laminated shaly chalk and mostly nonlaminated chalky limestone, contain skeletal grains of coarse silt, sand and gravel size, that are nearly everywhere common and locally abundant; however, with few exceptions, such grains are collectively of less importance than the micrograined calcium carbonate matrix that characterizes these chalky strata. In thin section the matrix of Greenhorn chalky limestones appears to be largely a micrograined mosaic of interlocking calcite crystals (Fig. 3,A), the microspar of Folk (1959, p. 32). In contrast, the matrix of shaly chalk is composed of discrete grains, including huge numbers of coccoliths that are discernible only with difficulty in thin sections (Fig. 3,B). Although not well lithified, this matrix is equivalent to the micrite of Folk (1959) and the rock as a whole is comparable to the nannoagorite of Honjo (1969, p. 357).
Figure 3--Micrographs of Greenhorn carbonate rocks. [Note images enlarged for web display and magnifications recalculated.] A, Photomicrograph of chalky limestone from base of Jetmore Member, sec. 5, T. 3 S., R. 1 E., Washington County. x300; plane-polarized light. Note matrix of interlocking crystals of microsparite. B, Photomicrograph of shaly chalk from upper part of Lincoln Member, sec. 18, T. 13 S., R. 12 W., Russell County. x300; crossed nicols. C, Scanning electron micrograph of chalky limestone from lower part of Hartland Member, sec. 18, T. 13 S., R. 12 W., Russell County. x3675. Note interlocking structure of calcite microcrystals and paucity of coccoliths. D, Scanning electron micrograph of chalky limestone from top of Pfeifer Member, sec. 18, T. 13 S., R. 12 W., Russell County. x3850. Note imperfect preservation and paticity of coccoliths.
Coccoliths are minute, mostly wheel-like skeletal elements produced by more or less spherical organisms known as coccolithophores which are members of the algal class Coccolithophyceae. Cretaceous coccoliths fall generally within the size range 1 to 20 microns, but most have a maximum diameter that is between 4 and 12 microns. The individual coccolith is itself composed of discrete cubical, tabular or lath-like calcite crystals. For the purpose of this study coccoliths are considered only as a major component of chalk matrix but these fossils have proved useful in Cretaceous biostratigraphic studies (e.g., Hay and others, 1967; Čepek and Hay, 1969; Bukry, 1969).
Ehrenberg (1836) was first to recognize coccoliths as an important constituent of chalk; however, he regarded them as inorganic structures. The organic nature of coccoliths in the English Chalk was determined by Sorby (1861) who compared them with similar structures in recent deep-Atlantic mud and with entire skeletons (coccospheres) of the coccolith-producing organism. Williston (1890a, p. 249; 1890b, p. 100) reported coccoliths as one of the most important constituents of the Niobrara Chalk of Kansas. After studying the Niobrara Chalk in Iowa and adjacent states, and also some pieces of the English Chalk, Calvin (1895, p. 215) stated that the bulk of all chalk is made up of coccoliths, that foraminifera comprise one-fourth to one-third of the Iowa Niobrara, and that chalk matrix is composed chiefly of coccoliths. Kansas coccoliths were first illustrated in a paper by McClung (1898). Following these early reports by American workers, the study of coccoliths in Western Interior chalks was essentially neglected for more than half a century. In 1958, Rezak and Burkholder (p. 1742) stated that coccoliths compose at least 80 percent of some chalk samples from the Western Interior of the United States, including the Niobrara and chalky parts of the overlying Pierre Shale. Although chalky strata of the Greenhorn Limestone and Fairport Chalk member, Carlile Shale, apparently were ignored in earlier studies, coccoliths were reported as an important constituent of chalky rocks of the Fairport by Hattin (1962, p. 106), and Trexler (1962, 1967) reported coccoliths in the Greenhorn, Carlile, and several other formations in South Dakota, Wyoming, and Colorado. The first study of coccoliths in the Kansas Greenhorn was by Čepek and Hay (1969) who described the zonation of calcareous nannofossils in a complete section of the formation in Russell County, Kansas, a section first described by Hattin 1965, p. 33, 50).
In these recent studies, the abundance of coccoliths has been stated only in general terms if mentioned at all. Indeed, Bramlette (1958, p. 123) deemed impracticable the accurate determination of percentages of these microfossils in rock samples owing to the difficulty of separating the constituent particles. One aspect of the Greenhorn Limestone about which little is known is the numerical abundance of coccoliths in the chalky rocks of this formation. From such data better estimates of the relative importance of these rock components in different parts of the formation and a comparison with abundance of coccoliths in modern marine sediments can be made. To meet this need the senior author devised a laboratory procedure for counting coccoliths in chalk samples of known volume. The junior author carried out the actual task of counting coccoliths.
In order for any count of coccoliths to be meaningful one must express numerical abundance in terms of a known volume of sediment or rock. We chose to use cubes of rock measuring 1 cm on an edge for several reasons: (1) smaller sized cubes could not be cut on available equipment with sufficient accuracy to guarantee constant volume, (2) larger cubes would have necessitated more cumbersome processing techniques, and (3) many previous workers have expressed microfossil abundance in terms of number per cubic centimeter. We found that 1-cm cubes of both chalky limestone and relatively soft shaly chalk could be prepared easily and with sufficient accuracy on an ordinary rock saw. A nearly perfect 1-cm cube can be obtained by the following method:
Each of the resulting cubes was dried overnight at 80° C, cooled, and then weighed on an analytical balance.
Cubes of chalky limestone apparently are too well indurated to be broken down by common methods of disaggregation. Neither boiling slowly in a 20 percent stock solution of Quaternary O nor soaking in photographic hypo, freezing, and then thawing the specimen had discernible effect on the cubes. Prolonged ultrasonic treatment in beakers of water did little more than blunt the edges of the cubes. Later examination of the chalky limestones by scanning electron microscopy (Fig. 3,C,D) showed that such rocks consist largely of tightly interlocking masses of calcite microcrystals, a fabric that is highly resistant to mechanical disaggregation. Furthermore it was revealed that the chalky limestones contain relatively few coccoliths and that some of these have been partially obliterated during the recrystallization process that produced the limestones (Hattin, 1971).
Mechanical disaggregation of the softer, more friable, shaly chalk was effected by the following procedure:
This procedure resulted in a satisfactory disaggregation of the cubes into a muddy sediment, although usually a few small pieces of the sample remained intact. These pieces were taken into account in final calculations of coccolith abundance.
Each disaggregated sample was processed for coccolith counting according to the following procedure:
Mounted subsamples of disaggregated chalk were examined with a binocular petrographic microscope having a lens combination giving magnification of 860x. The microscope stage was fitted with a mechanical stage and two minute pieces of spider web were placed in the distal end of one ocular for use as reference lines. Coccoliths were then counted in the following manner:
In all calculations the number of coccoliths per slide was multiplied by 2 x 106 because the counted subsample represented only 0.001 ml of the original 2000-ml suspension. Because the suspended material in the flask did not include nondisaggregated portions of the 1-cm cube, the number of coccoliths in the suspension was multiplied by a correction factor to give number of coccoliths per cubic centimeter. The correction factor was determined by the following equation:
Correction factor = (weight of cube / weight of disaggregated fraction)
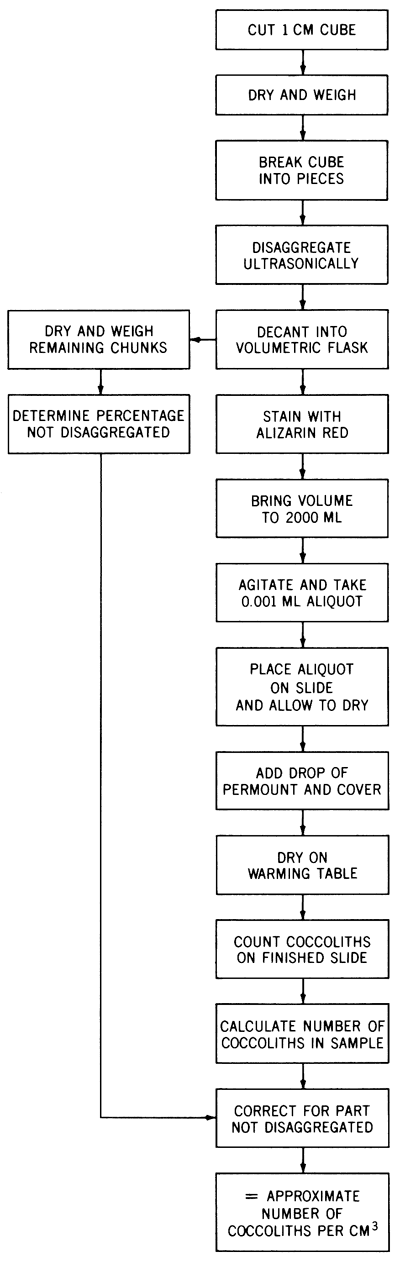
A flow diagram summarizing the laboratory procedure is depicted in Figure 4.
Figure 4--Flow diagram outlining operational steps for counting coccoliths in shaly chalk.
Coccoliths were counted in slides representing 19 samples taken from shaly chalk beds distributed through the entire Greenhorn section in SW sec. 27, T. 6 S., R. 9 W. at Glen Elder Dam, Mitchell County, Kansas. The results of these counts, after correction, are listed in Table 1.
Table 1--Numerical abundance of coccoliths in shaly chalk samples from Greenhorn Limestone at Glen Elder Dam. Samples listed in ascending order. Numbers rounded to nearest hundred thousand.
| Sample | Unit | Slide | No. of coccoliths (x 106) per cubic cm |
|---|---|---|---|
| KGH-1-4:10' to 15' | upper part Graneros Shale |
1 | 1,937.9 |
| 2 | 1,967.4 | ||
| KGH-1-4:15' to 16.7' | 1 | 2,303.0 | |
| 2 | 2,045.8 | ||
| KGH-1-6 | Lincoln Limestone Member |
1 | 534.6 |
| 2 | 669.1 | ||
| KGH-1-13 | 1 | 848.0 | |
| 2 | 1,187.8 | ||
| KGH-1-21 | Hartland Shale Member |
1 | 3,888.2 |
| 2 | 4,013.1 | ||
| KGH-1-23 | 1 | 1,867.8 | |
| 2 | 1,799.9 | ||
| KGH-1-25 | 1 | 655.2 | |
| 2 | 741.7 | ||
| KGH-1-29 | 1 | 2,129.9 | |
| 2 | 2,330.5 | ||
| KGH-1-32 | 1 | 2,531.4 | |
| 2 | 2,894.9 | ||
| KGH-1-36 | Jetmore Chalk Member |
1 | 1,266.0 |
| 2 | 1,837.8 | ||
| KGH-1-40: #1 | 1 | 4,336.2 | |
| 2 | 4,234.6 | ||
| KGH-1-40: #2 | 1 | 2,811.2 | |
| 2 | 3,283.2 | ||
| KGH-1-42 | 1 | 1,142.9 | |
| 2 | 1,426.8 | ||
| KGH-1-47 | 1 | 3,398.4 | |
| 2 | 2,990.3 | ||
| KGH-1-48 | 1 | 2,306.8 | |
| 2 | 2,325.8 | ||
| KGH-1-50 | Pfeifer Shale Member |
1 | 1,182.2 |
| 2 | 937.2 | ||
| KGH-1-52 | 1 | 1,908.3 | |
| 2 | 1,544.6 | ||
| KGH-1-56: # 1 | 1 | 5,498.6 | |
| 2 | 5,867.0 | ||
| 3 | 5,401.4 | ||
| 4 | 6,090.7 | ||
| KGH-1-56: #2 | 1 | 3,798.9 |
Throughout laboratory preparation special care must be taken to prevent contamination of samples from external sources such as airborne dust, tap water, and blackboard chalk. These precautions, necessary in our laboratory because of the quantity of Cretaceous rock samples located there, included use only of distilled water for preparing samples and cleaning slides, and keeping slides covered during disaggregation and drying processes.
During initial phases of the investigation we took a 0.05-ml aliquot from a 1000-ml suspension of disaggregated rock. The first slide contained 22,418 coccoliths which were counted at great expense of time. By increasing the volume of suspension to 2000 ml and reducing the pipetted aliquot to 0.001 ml, the number of coccoliths to be counted was reduced by 99 percent.
In order to test the variation in coccolith abundance related to the position in flask from which the 0.001-ml aliquot was taken, one suspension was sampled from near the top of the volumetric flask, two from near midheight of the flask, and one from near the bottom of the flask. The stoppered flask was shaken vigorously before each sample was withdrawn. The corrected coccolith counts resulting from this test are as follows:
| Top | 5,401,400,000/cm3 |
| Middle #1 | 5,498,600,000/cm3 |
| Middle #2 | 5,867,000,000/cm3 |
| Bottom | 6,090,700,000/cm3 |
These test data suggest that the midheight position is probably best.
Counting the individual coccoliths is difficult where several are grouped on the slide, are mixed with other particles, or are exceptionally small. The optical microscope has relatively low resolution at magnification required for counting coccoliths. The counting process often can be aided by use of cross-polarized light in which many coccoliths exhibit a swastika-shaped pseudouniaxial cross. Staining with alizarin red-S aids in distinguishing the coccoliths in plane polarized light.
In the procedure outlined in this paper, possibility exists for wide margins of error. For this reason two slides were prepared and counted for all but one sample reported in Table 1. The data in Table 1 show that with few exceptions the two counts for a given sample are not only of the same order of magnitude, but that for several pairs of counts the larger number is only 1 or 2 percent greater than the smaller. Examples are the two counts from KGH-1-4:10' to 15' and those from KGH-1-48. Of 20 pairs of counts compared, only 4 involved a difference greater than 25 percent, but 15 had a difference less than 15 percent.
As recorded from respective counts of both pairs of slides, only two samples contained less than a billion coccoliths. Both are from shaly chalk units that contain more-than-usual amounts of clay because of proximity to former volcanic ash beds. The senior author often has observed that shaly chalk units lying adjacent to thicker bentonite seams are somewhat bentonitic, probably owing to dilution of the original carbonate mud by volcanic ash.
Coccolith abundance estimates commonly are stated in terms of percentage of the rock in which they occur (e.g., Downie, 1956; Bramlette, 1958; Rezak and Burkholder, 1958). However, Gümbel (1873) reported that Eocene marls from the Alpine region contain 8 x 108 coccoliths per cubic centimeter, and Black (1965, p. 136) stated that coccolith percentage reported from certain limestone beds in the Kimeridge Shale of Dorset, England (Downie, 1956, p. 418) is equivalent to approximately 4 x 109 coccoliths per cubic centimeter. Garrison (1968, p. 76) reported that red pelagic limestones from the Franciscan Group of California are composed almost wholly of coccoliths, containing approximately 4 to 6 million per cubic millimeter. These estimates compare very well with abundance calculated for samples of shaly chalk in the Greenhorn Limestone. In comparison, Hay and others (1967, p. 431) state that modern deep sea sediments contain approximately 1 x 1012 calcareous nannofossils per cubic centimeter whereas shallower shelf deposits contain approximately 1 x 109 calcareous nannofossils per cubic centimeter. The latter figure is of the same order of magnitude as most Greenhorn samples and prompts one to speculate that some of the recent coccolith-rich shelf sediments may be present-day counterparts of Cretaceous chalk-forming ooze. Perhaps admixture of terrigenous detritus masks the carbonate content of most shelf sediments, whereas in Cretaceous chalk-forming seas the sediment was dominantly calcium carbonate.
With regard to coccolith abundance one must be reminded that a cubic centimeter of Greenhorn shaly chalk represents a once-larger volume of soft, noncompacted pelagic ooze. Fecal pellets in Greenhorn chalky limestones are usually elliptical in cross section whereas in shaly chalk such pellets are mostly fusiform, testifying to the compaction suffered by the shaly-chalk-forming sediment. Furthermore, shaly chalk laminae bend around such objects as Inoceramus shell fragments. Comparison of measurements of fecal pellets in thin sections and measurement of laminae that wrap around skeletal grains suggest that an average of a little more than 50 percent compaction has taken place in shaly chalk. Precompactional abundance of coccoliths per cubic centimeter was apparently less by approximately half the numbers given in Table 1.
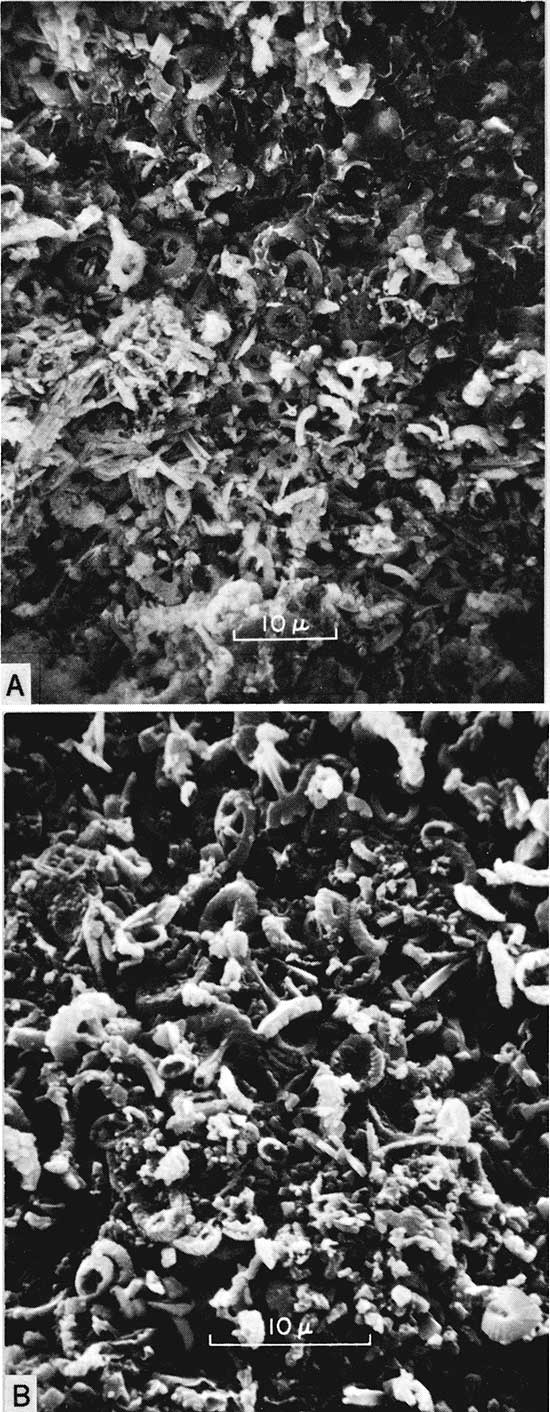
Like other chalks the Greenhorn shaly chalks contain enormous numbers of essentially unaltered coccoliths (Fig. 5,A,B). However, these structures apparently do not make up the bulk of the shaly chalk. Tests of planktonic foraminifera, valves and valve fragments of inoceramids and oysters, fish scales and bones, organic matter, fecal pellets, and terrigenous detritus are common. constituents. The last comprises anywhere from 5.8 to 50 percent of shaly chalk residues studied by the senior author (Hattin, 1971). Furthermore, much of the fine-grained matrix consists of calcite grains representing disarticulated coccoliths. And, of course, part of the volume of these rocks is pore space. To determine the approximate percentage of rock composed of recognizable coccoliths the senior author made measurements of coccoliths in scanning electron micrographs. In each of three micrographs measurements were made of length, breadth, and thickness of equal-sized conspecific specimens representing a large species. The volume of one coccolith was calculated as if the structure were a solid disc. That volume was multiplied by 6 x 10", the largest number of coccoliths counted in any sample. By this method it was calculated that the space occupied by coccoliths is on the order of 20 percent or less of the total volume of rock. This conclusion is based on a small number of micrographs suitable for measurement, however, and must be regarded as tentative.
Figure 5--Scanning electron micrographs of shaly chalk from Greenhorn Limestone. [Note images enlarged for web display and magnifications recalculated.] A, Sample from upper part of Lincoln Member, sec. 18, T. 13 S., R. 12 W., Russell County. x2600. B, Sample from lower part of Jetmore Member, sec. 18, T. 13 S., R. 12 W., Russell County. x4120. In both pictures note abundance of coccolith debris.
Black, Maurice, 1965, Coccoliths: Endeavour, v. 24, p. 131-137.
Bramlette, M. N., 1958, Significance of coccolithophorids in calcium-carbonate deposition: Geol. Soc. America, Bull., v. 69, p. 121-126.
Bukry, David, 1969, Upper Cretaceous coccoliths from Texas and Europe: Univ. Kansas, Paleont. Contr., Article 51 (Protista 2), 79 p. [available online]
Calvin, Samuel, 1895, Composition and origin of Iowa chalk: Iowa Geol. Survey, Reports, v. 3, p. 213-236.
Čepek, Pavel, and Hay, W. W., 1969, Calcareous nannoplankton and biostratigraphic subdivision of the Upper Cretaceous: Trans. Gulf Coast Assoc. Geol. Societies, v. 19, p. 323-336.
Downie, Charles, 1956, Microplankton from the Kimeridge Clay: Quart. Jour. Geol. Soc. London, v. 112, p. 413433.
Ehrenberg, C. G., 1836, Über mikroskopische neue Charaktere der erdigen und derben Mineralien: Ann. Phys. u. Chem. (Poggendorff), v. 39, p. 101-106.
Folk, R. L., 1959, Practical petrographic classification of limestones: Am. Assoc. Petroleum Geologists, Bull., v. 43, p. 1-38.
Garrison, R. E., 1968, Electron microscopy of Franciscan pelagic limestones, California (abs.): Geol. Soc. America, Spec. Paper 101, p. 75-76.
Gümbel, C. W., 1873, Coccolithen im Eocänmergel: Neues Jahrb. Min. Geol. u. Paleont., p. 299-304.
Hattin, D. E., 1962, Stratigraphy of the Carlile Shale (Upper Cretaceous) in Kansas: Kansas Geol. Survey, Bull. 156, 155 p. [available online]
Hattin, D. E., 1964, Cyclic sedimentation in the Colorado Group of west-central Kansas: Kansas Geol. Survey, Bull. 169, v. 1, p. 265-217. [available online]
Hattin, D. E., 1965, Upper Cretaceous stratigraphy, paleontology, and paleoecology of western Kansas: Field Conf. Guidebook, Ann. Meetings, Geol. Soc. America, Kansas City, Missouri, 1965, 69 p.
Hattin, D. E., 1971, Widespread, synchronously deposited, burrow-mottled limestone beds in Greenhorn Limestone (Upper Cretaceous) of Kansas and southeastern Colorado: Am. Assoc. Petroleum Geologists, Bull., v. 55, p. 412431.
Hay, W. W., Mohler, H. P., Roth, P. H., Schmidt, R. R., and Boudreaux, J. E., 1967, Calcareous nannoplankton zonation of the Cenozoic of the Gulf Coast and Caribbean-Antillean area, and transoceanic correlation: Trans., Gulf Coast Assoc. Geol. Societies, v. 17, p. 428480
Honjo, Susumo, 1969, An electron microscopic study of fine-grained carbonate matrix--Nannoagorite, orthomicrite, and an aspect of carbonate sedimentation and diagenesis (Japanese with English summary): Geol. Soc. Japan Jour., v. 75, p. 349-364.
McClung, C. E., 1898, Microscopic organisms of Upper Cretaceous: Geol. Survey Kansas, v. 4, p. 415-427. [PDF available online]
Rezak, Richard, and Burkholder, R. E., 1958, Cretaceous coccoliths from the western interior of the United States (abs.): Geol. Soc. America, Bull., v. 69, p. 1742.
Sorby, H. C., 1861, On the organic origin of the so-called 'Crystalloids' of the Chalk: Ann. Mag. Nat. History, s. 3, v. 8, p. 193-200.
Trexler, D. W., 1962, Coccolithophorid assemblages from the Benton and Niobrara groups, Canon City area, Colorado (abs.): Geol. Soc. America, Spec. Paper 68, p. 107- 108.
Trexler, D. W., 1967, Stratigraphic distribution of Upper Cretaceous nannoplankton (coccoliths) in central and northern Colorado and the Black Hills region: Jour. Paleontology, v. 41, p. 1355-1364.
Williston, S. W., 1890a, Chalk from the Niobrara Cretaceous of Kansas: Science, v. 16, p. 249.
Williston, S. W., 1890b, On the structure of the Kansas chalk: Trans., Kansas Acad. Science, v. 12, p. 100.
Kansas Geological Survey, Coccolith Abundance in Shaly Chalk of Greenhorn Limestone, Kansas
Placed on web Feb. 18, 2009; originally published in July 1971.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/202_2/index.html