
Kansas Geological Survey, Bulletin 200, originally published in 1970
A PDF version is available

Originally published in 1970 as Kansas Geological Survey Bulletin 200. This is, in general, the original text as published. The information has not been updated.
Six kimberlites are known from northern Riley County, Kansas. Five (Bala, Leonardville, Randolph No. 1, Randolph No. 2, Stockdale) crop out, and a sixth (Winkler) has been discovered by drilling. Magnetometer studies reveal that the kimberlites are pipelike bodies that dip steeply to the southeast and are oriented approximately normal to the Abilene anticline, the main structural feature in northern Riley County.
Primary kimberlite minerals and their alteration products are serpentinized forsteritic olivine, serpentinized clino- and ortho-pyroxenes, ilmenite, magnetite, carbonates, perovskite, apatite, ± chloritized phlogopite, ± partially kelyphitized pyrope, and ± carbonated melilite (?). More than 90 percent of the kimberlites consist of serpentine (predominantly lizardite) and calcite , the latter being derived chiefly from the intruded lower Permian calcareous country rocks. Leonardville, Stockdale, and probably the Winkler, are classified as micaceous kimberlites, whereas the Bala, Randolph No. 1, and Randolph No. 2 are classified as lamprophyric kimberlites. Despite this division, no two kimberlites are exactly alike in their mineralogy, bulk chemistry, or texture. Randolph No. 2 contains only a few serpentinized xenoliths whereas the remaining five (with the possible exception of the Winkler) contain abundant accidental xenoliths of locally derived calcareous country rocks with a paucity of xenoliths of the Precambrian basement rocks (arkose, basalt, granite). Cognate xenoliths of eclogite, pyroxenite, and gabbro-metagabbro are abundant in the Stockdale.
A 745 ± 100 m.y. age for pyrope from the Stockdale may indicate time of original kimberlitic crystallization in the upper mantle. The kimberlites may have come close to the surface during final emplacement in a post-Dakota sandstone during the Late Cretaceous. The lack of pyrometamorphic contact effects coupled with preservation of pre-emplacement K-Ar dates on chloritized phlogopite indicates a temperature range of 75° to 150°C accompanying emplacement. Carbonatitic material helped fluidize the kimberlite and kept temperatures low, but the gas-rich phase was probably dissipated at depth.
Six bodies of ultramafic rock found in northern Riley County, Kansas, are now recognized as kimberlites. Five of these (Bala, Leonardville, Randolph No. 1, Randolph No. 2, Stockdale) crop out, and a sixth (Winkler) has been discovered by drilling (Fig. 1, Table 1).
Figure 1--Map of Riley County, Kansas, showing locations of kimberlites. 1, Bala; 2, Leonardville; 3, Stockdale; 4, Randolph No. 1; 5, Randolph No. 2; 6, Winkler.
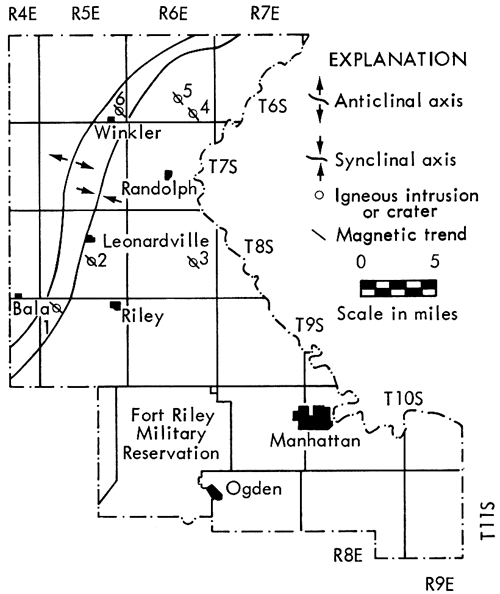
Table 1--Locations and dates of discovery of Riley County kimberlites.
| Kimberlite | Location | Discoverer | Date | Reference |
|---|---|---|---|---|
| Bala | NW NW Sec. 6, T. 9 S., R. 5 E. | T. S. Harrison | before 1920 | Moore and Haynes (1920) |
| Stockdale | NW SE Sec. 23, T. 8 S., R. 6 E. | G. H. Failyur | Jewett (1941) | |
| Leonardville | SE NW Sec. 22, T. 8 S., R. 5 E. | A. B. Sperry | 1935(?) | Tolman and Landes (1939) |
| Randolph No. 1 | NW NW Sec. 35, T. 6 S., R. 6 E. | K. L. Parish | 1950 | Byrne, et al. (1956) |
| Randolph No. 2 | SW SW Sec. 27, T. 6 S., R. 6 E. | K. L. Parish | 1950 | Byrne, et al. (1956) |
| Winkler | NE NW Sec. 36, T. 6 S., R. 5 E. | D. G. Brookins | 1969 | Brookins (1970) |
Either the Stockdale or the Bala was the first to be discovered, although not recognized as being igneous at the time of discovery. Mudge (1879-80) reported the occurrence of ruby spinel (subsequently disproved by Bagrowski, 1941; Brookins, 1967a) from the Stockdale location, although the rock was then referred to as clay shale. Jewett (1941, p. 97) credits G. H. Failyur of Manhattan with the discovery of the Stockdale, and Moore and Haynes (1920, p. 183) credit T. S. Harrison of Denver with the discovery of the Bala. A. B. Sperry discovered the Leonardville in, or shortly prior to, 1935 (Tolman and Landes, 1939, p. 100). It is not known exactly when the two Randolph kimberlites were discovered, but Byrne, Parish, and Crumpton (1956) credit K. L. Parish, of the U.S. Geological Survey, for recognizing them as igneous in origin in 1950. The Winkler was discovered by drilling in May, 1969, in what previously had been described as a possible meteorite crater (Barringer, 1964; Freeberg, 1966), and it has been little studied to date (Brookins, 1970). There may well be more kimberlites in the area that are unrecognized or that do not crop out, since kimberlites commonly occur in swarms. Unfortunately, glacial and fluvial action has obscured much of the Riley County terrane so that stream prospecting (for garnets, etc.) has not been successful. Surface magnetometer surveys (Dowell, 1964) have also proven unsuccessful, as the magnetic anomalies associated with the kimberlites are restricted to an extremely small area, and gravimetric studies also have been unsuccessful because the density of the serpentinized, carbonated kimberlite is not appreciably different from the surrounding calcareous country rocks.
Prior to 1964 investigations of the Riley County kimberlites have been largely restricted to a brief description of hand specimens, qualitative microscopic work, tectonic-emplacement studies, and magnetometer surveys. Jewett (1941) briefly described Bala, Stockdale, and Leonardville and thought them to be clearly intrusive into the surrounding rocks, suggesting the possibility that the Stockdale exposure might be a surface flow (p. 97). Dreyer (1947) demonstrated that the Bala is a vertical dike-like body surmounted by a cylindrical mass. Cook (1955) subsequently conducted magnetometer surveys over Leonardville, Randolph No. 1, Randolph No. 2, and Stockdale and found them to be pipelike bodies also with a nearly vertical dip to the southeast. Byrne, et al. (1956) summarized the existing information on the kimberlites to that date and their possible effect on the occurrence of oil. Merriam (1963, p. 151-157) presented a brief summary of the Riley County rocks and their possible relationship to the ultramafic igneous rocks of Woodson County, Kansas.
Unpublished masters' theses at Kansas State University (formerly Kansas State College) that have dealt with the Riley County kimberlites include the works of Neff (1949), Taylor (1950), Nelson (1952), Bridge (1953), Dowell (1964), Eastwood (1965), Rosa (1966), McDermott (in preparation), and Dyer (in preparation). Published reports and papers in press on the petrography and geochemistry of the Riley County kimberlites include Eastwood and Brookins (1965), Rosa and Brookins (1966), Brookins (1966a, 1966b, 1967a, 1967b, 1967c, 1969a, 1969b, 1969c, 1969d, 1970, and in press), Brookins and McDermott (1967), Dyer and Brookins (1969), and McDermott and Brookins (in press).
Three other recent reports have mentioned the Riley County kimberlites. Wheeler (1965) has referred to them as klippen of an ancient overthrust, but this theory has been severely criticized by Franks (1966). O'Connor (1968) mentioned very briefly the Riley County kimberlites but included little of the more recently accumulated information.
Acknowledgment is due H. O. A. Meyer, Geophysical Laboratory, Carnegie Institution, Washington, D.C., for helpful discussion of some of the problems involved as well as for analytical and petrographic determinations. Several faculty and student personnel of Kansas State University have been most helpful: I wish to thank P. C. Twiss, R. W. Vian, A. B. Sperry, R. L. Eastwood, the late F. Rosa, P. L. Dowling, R. G. Dyer, and R. L. Methot. J. R. O'Neil, of the U.S. Geological Survey, made the carbon and oxygen isotopic determinations. Financial support was provided by Kansas State University, the National Science Foundation (Grants GA-317 and GA-893), the Research Corporation (New York), and the State Geological Survey of Kansas. Special thanks are due E. E. Angino, of the State Geological Survey of Kansas, who encouraged the writer to compile the information for this report.
The Riley County kimberlites intrude rocks of the Chase Group, Gearyan Stage, Lower Permian Series (nomenclature in Zeller, 1968). According to Merriam (1963) there may have been a cover of only a few hundred feet of Cretaceous rocks overlying the lower Permian host rocks into which the kimberlites were intruded, and it is possible that the kimberlites may have come close to or even reached the surface. Glacial and/or fluvial action has masked field evidence, however. Kimberlite-wall-rock contacts have been exposed at the Randolph kimberlites by trenching (at Randolph No. 1 by the U.S. Geological Survey; at Randolph No. 2 by V. J. McDermott), and the contact at the northwestern edge of the Stockdale kimberlite has been infrequently exposed after very dry summers. The contacts of the Bala and Leonardville kimberlites are not exposed, and the Winkler kimberlite does not crop out. Some characteristics of the individual kimberlites are noted below.
The Bala kimberlite (Dreyer, 1947) forms a distinct topographic high in the form of a rounded knoll approximately 15 feet in height and 200 feet in diameter. The uppermost country rock is the Cresswell Limestone Member of the Winfield Limestone, and it is folded 0.1 mile west of the pipe, and the Stovall Limestone Member of the Winfield Limestone, which lies 11 feet stratigraphically below the Cresswell Limestone Member, is faulted near the kimberlite. However, the Stovall Limestone Member, a thin unit (1 to 3 feet thick) overlain and underlain by much thicker shales, is subject to faulting in areas removed from the kimberlite. Core to a depth of 144 feet from the Bala kimberlite is stored at Kansas State University.
The Stockdale kimberlite (Rosa and Brookins, 1966) crops out in an irregular fashion in a stream bed. This is the only one of the five kimberlites which crops out to form a topographic low. The wall rock is reported to be the Holmesville Shale Member of the Doyle Shale by Jewett (1941, p. 97), but in actuality the uppermost part of the Fort Riley Limestone Member of the Barneston Limestone is exposed, though infrequently, at the contact. The kimberlite-Fort Riley Limestone Member contact is thought to be a fault contact, as an opening partially filled with debris to a maximum width of 1 inch separates the two rocks. The dip of the limestone is nearly horizontal. Many samples of subsurface kimberlite and xenoliths were recovered from a 2.5-foot diameter drill core to a depth of 15 feet. The stone was to have been used for ornamental purposes, but due to the high content of calcite this was not feasible.
The Leonardville kimberlite crops out in two very small mounds on the west side of a gently sloping hill. The unexposed country rock is the Winfield Limestone. Magnetometer surveys indicate the Leonardville kimberlite to be the largest of the five exposed, but its outcrop area is the smallest, and it is very poorly exposed.
The Randolph No. 1 kimberlite is a nearly circular 10-foot-high knoll 200 feet in diameter. The country rock is the Cresswell Limestone Member, which is bent upward at the contact with the kimberlite with dips of 45° and 36° away from the contact found on the west and northeast sides respectively (McDermott and Brookins, in press). Within a few feet of the contact, the dips in the limestone are nearly horizontal. No pyrometamorphic contact effects are noted, but the upwarping of the limestone indicates that the kimberlite was intruded into the country rock.
The Randolph No. 2 kimberlite forms a topographic high 50 feet in diameter on an eastward-sloping hill of the Holmesville Shale Member. The country rock has been exposed by trenching, and part of the geometry of the kimberlite has been outlined by drilling (McDermott and Brookins, in press). A mushroom-like cap suggests that the kimberlite may have come very close to the surface, a view proposed by Merriam (1963). In addition, weathered Fort Riley Limestone was recovered beneath the cap from one of the drill holes.
The Winkler kimberlite does not crop out but forms the most striking topographic feature in northern Riley County--Winkler Crater, a 950-foot-diameter topographic low which appears as an almost perfect circle on an aerial photograph but possesses crater-like morphology only in its west-southwestern portions (Brookins, 1970). The entire northern half is dissected by small southward-trending streams that join a more pronounced eastward-trending stream, which divides the crater into two nearly equal parts. The entire eastern "rim" has been eroded away, and the maximum relief (50 feet) within the feature is found at that point. Drilling over magnetic anomaly has revealed the presence of a kimberlite-poor brecciated and partially recemented pipelike body. The highest rimrock of the feature is composed of the cherty, resistant Florence Limestone Member of the Barneston Limestone. Two other cherty, resistant limestones, the Schrover and Threemile Limestone members of the Wreford Limestone, were not encountered in the drilling, and the feature is therefore presumed to be due at least in part to solution promoted by the arching associated with the kimberlitic emplacement. The exact structure of the circular feature and the kimberlite geometry remains to be investigated in detail.
The geometry of the Riley County kimberlites and their relationship to the regional structure has been determined by magnetometer surveys reported by Dreyer (1947), Cook (1955), Dowell (1964), and Brookins (1970). The five exposed kimberlites are characterized by strong (2,000 to 5,000-γ) positive anomalies with a less intensive negative anomaly (800 to 2,000 γ) on the north edge due to the 70° N declination of the earth's magnetic field in the area. The trends of the magnetic fields surrounding each kimberlite are oriented more or less normal to the Abilene anticline (Fig. 1).
The magnetic anomaly over the Winkler kimberlite differs from that of the other kimberlites, due, in part, to the f act that the main body is not exposed at the surface. Baysinger first studied this anomaly and reported a +650-γ value (Baysinger, B. H., 1962, Pfaff Crater: Unpub. report and map submitted to C. W. Shenkel, Dept. Geol., Kansas State Univ., Manhattan, Kansas.). Dowell (1964) reported a +350-γ anomaly, but it is not certain that the surveys were conducted over the same area within the circular feature. The magnetic survey was rerun in replicate by Dyer and Methot (Brookins, 1970), and a +550-γ anomaly was found. The reason for the discrepancy between the various investigations is not clear, but presumably it is due to instrumental error. The +550-γ value is probably the most reliable. No negative anomaly was found on the north side of the Winkler kimberlite, although the gradient is extremely steep on that side (Dowell, 1964; Brookins, 1970). The importance of these magnetometer studies is that a similar geometry, that of a steeply dipping pipelike body, is common to all the Riley County kimberlites (Table 2). Further, a similar mechanism of emplacement for all of the kimberlites is suggested even though local variations exist. According to Dawson (1967a), the pipes of Riley County should be classified as intrusive kimberlite breccias, probably originating from diatremes as opposed to dikes.
Table 2--Magnetic characteristics of Riley County kimberlites.*
| Kimberlite | Positive anomaly |
Negative anomaly |
Magnetic trend |
|---|---|---|---|
| Bala | > 3,900 γ | 2,000 γ | N 77° W |
| Leonardville | a) > 2,900 γ | a) 700 γ | N 55° W |
| b) 2,000 γ | b) 700 γ | N 55° W | |
| Randolph No. 1 | 3,800 γ | 2,800 y | N 26° W |
| Randolph No. 2 | 2,045 γ | 885 γ | N 28° W |
| Stockdale | > 4,000 γ | 1,125 γ | N 41° W |
| Winkler | 550 γ | none (?) | N 42° W |
| *Data from Dowell(1964) and Brookins (1970). †Negative anomalies are located on the north flank of the magnetic highs and are due to the 70° N declination of the earth's magnetic field in northern Riley County. |
|||
Although the age of the kimberlites has been definitely placed as post-Chase Group (early Permian) from field relationships, most previous investigators have suggested a Cretaceous age for the emplacement. Jewett (1941, p. 97) stated that the age,of the rock was probably Cretaceous, and Byrne, et al. (1956) also argue for emplacement in late Cretaceous time because the rocks are thought to be somewhat similar to the ultrabasic rocks of the Murfreesboro, Arkansas, area which are Cretaceous in age. Merriam (1963, p. 154) states that because of Cretaceous vulcanism in Arkansas and Texas, it would seem logical that the igneous rocks in Riley County are Cretaceous, and further (p. 157) that the Riley County rocks were emplaced under the same conditions and at about the same time as the ultrabasic rocks of Woodson County, Kansas, which have been dated by Zartman, Brock, Heyl, and Thomas (1967) as 88-91 m.y. (late Cretaceous). I do not agree that these rocks were emplaced under the same conditions as those in Woodson County, because there is abundant evidence for high-temperature contact metamorphism in the country rocks surrounding the Woodson County ultrabasic rocks, whereas such effects are absent in Riley County. Joint patterns in the rocks of northern Riley County contain two pronounced sets of joints, one set striking N 79° 23' E and another, less intense, set striking N 15° 26' W. Both of these sets are readily discernible in the lower Permian rocks and can be traced into the Dakota sandstone of late Cretaceous age in northwestern Riley County, but are not found in the outcrops of the kimberlitic rocks. The kimberlites possess a randomly oriented set of joints which can also be found in the surrounding country rocks where they often cut and/or offset the pronounced joint sets. On this evidence I believe the rocks to be post-Dakota in age, a view in agreement with Jewett, Byrne, et al., and Merriam, but my interpretation is based on a study of the joints. The pipes are thought to be pre-Kansan, as glacial debris is found in Winkler Crater. A much younger age than late Cretaceous possibly can be inferred from the presence of the weathered Fort Riley Limestone recovered from beneath the cap rock at the Randolph No. 2 kimberlite (McDermott, in preparation).
The following description is directly applicable only to the five exposed kimberlites, and the texture of the Winkler kimberlite can only be inferred. The kimberlites have a light- to dark-green, brecciated and porphyritic appearance and are cut by numerous veins of calcite filling joints and other fractures (Brookins, 1967c). Some color zoning is apparent (Rosa and Brookins, 1966), and flow banding is evident in a few places. The kimberlites can be divided into three groups based on their texture, number and nature of xenoliths, and phenocryst mineralogy.
The Randolph No. 2 kimberlite is unique in that it contains very few xenoliths and only a few megascopic pseudomorphs of serpentine, usually after olivine. Ilmenite and magnetite phenocrysts are present in extremely small amounts. Pyrope and phlogopite are absent. The few xenoliths present are completely serpentinized. The only pronounced similarity between the Randolph kimberlite and the other kimberlites is its light-green color and abundance of calcite veins.
The Stockdale and Leonardville kimberlites are highly porphyritic and brecciated. They contain megascopic phenocrysts of dark-red pyrope, ilmenite, chloritized phlogopite, magnetite, and serpentinized olivine, as well as many cognate and accidental xenoliths. The xenoliths vary in size from barely megascopic to 40 cm in maximum dimension. Some are highly altered, others scarcely altered at all. The degree of roundness varies also. In general, the smaller and more highly altered xenoliths are more rounded than larger, less altered xenoliths. Most of the xenoliths, including the nearly unaltered ones, contain some type of apparent reaction rim. The Winkler kimberlite contains abundant red pyrope, ilmenite, cholritized phlogopite, and serpentine pseudomorphs after olivine and is on this basis tentatively grouped with the Leonardville and Stockdale kimberlites pending further investigation
The Bala and Randolph No. 1 kimberlites are grouped together because both are less brecciated than the Stockdale and Leonardville kimberlites and do not contain significant amounts of red pyrope or chloritized phlogopite, although they do contain abundant ilmenite, magnetite, and serpentinized olivine phenocrysts. Moore and Haynes (1920) have reported biotite that occurs in large books in the Bala kimberlite, but I have observed none. The Bala kimberlite also contains large (0.5 to 1.5 cm maximum dimension) phenocrysts or xenocrysts of calcite. Due to local accumulation of accidental xenoliths of the surrounding country rocks, the Bala and Randolph No. 1 kimberlites appear to be highly brecciated. Cognate xenoliths are much less abundant than in the Leonardville and Stockdale kimberlites. Finally, the "chromite" from the Bala (Moore and Haynes, 1920) is almost certainly ilmenite, as chromite is known from the kimberlites as a minor accessory mineral only (Brookins, 1969d).
The kimberlites have an approximate serpentine-carbonate mineral ratio of 8:2. Features common to all include (1) a porphyritic and microporphyritic texture with olivine the dominant phenocryst followed by (2) ilmenite, magnetite, calcite, pyroxenes, ± pyrope, ± phlogopite, and ± melilite in a panidiomorphic matrix of serpentine and carbonates containing numerous grains of perovskite, magnetite, ilmenite, secondary mica, apatite, chromite, and other minerals (Brookins, 1969d). The kimberlites show variations in mineralogical composition and texture. Flow banding and/or preferred orientation of phenocrysts and microphenocrysts does not appear to be a function of distance from wall rocks or large inclusions, fractured zones, or geometry of the body.
Olivine (0.3 to 10 mm maximum dimension) occurs as anhedral to euhedral crystals altered to serpentine and/or replaced by carbonates (usually calcite) and often contains crystals of secondary magnetite as well as minor amounts of bowlingite, iddingsite, talc, brucite(?), and other hydrous (± ferro-)magnesian silicates and oxides. The phenocrysts usually contain a reaction rim of some sort, often fine-grained serpentine with a columnar structure, and chlorite with occasional secondary amphibole (in part tremolite). Clino- and ortho-pyroxenes (0.1 to 0.5 mm maximum dimension) are much less abundant than olivine and have been altered in the same manner. Rounded ilmenite varies from 2 to 20 mm maximum dimension and is occasionally coated by leucoxene (± small crystals of enclosed brookite). It may also contain exsolved magnetite. Magnetite occurs in several generations. It occurs as primary phenocrysts and microphenocrysts (0.2 to 5 mm maximum dimension) which are occasionally altered to hematite along grain edges and/or altered in part to geothite and/or limonite. In addition, it is a common secondary product of serpentinization of ferromagnesian minerals and is commonly found oriented parallel to cleavage or parting planes and in reaction rims. Another generation of magnetite of uncertain origin occurs in primary carbonate veins. Phenocrysts (or xenocrysts?) of primary calcite (15 mm maximum dimension) are noted in several thin sections. The phenocrysts have an oikilitic texture and surround euhedral crystals of serpentinized-carbonated olivine and/or chloritized phlogopite. Other carbonates (usually calcite and/or dolomite) of possible primary origin occur in veins and networks of :optically continuous, magnetite-bearing material located between closely spaced euhedral to subhedral kimberlite minerals. In these latter carbonates, serpentine replaces calcite, and later fractures contain calcite with a random optical orientation. Pyrope varies from 0.2 to 12 mm maximum dimension and occurs in several varieties. It possesses a subhedral to anhedral outline and is commonly rimmed by kelyphite, which may be partially or completely removed during emplacement. Fractures within the grains are commonly filled with calcite. Some grains contain minute inclusions of rutile; other grains contain naturally decorated dislocations in otherwise unaltered portions. Phlogopite (0.2 to 15 mm maximum dimension) occurs in euhedral to subhedral crystals that have been largely altered to chlorite and/or vermiculite. It exhibits undulatory extinction, and some grains are bent or broken while others possess "kink bands." Many grains show two or three sets of kink bands that formed after initial alteration as indicated by secondary magnetite grains oriented parallel to cleavage traces, which were formed during initial chloritization and which are offset by the kinking. Calcite pseudomorphs (<0.5 mm maximum dimension) after a lathlike mineral, probably melilite(?), are noted in the Randolph kimberlites and are molded around larger crystals of olivine and/or pyroxenes.
Secondary calcite replaces primary minerals and serpentine in the phenocrysts and groundmass and apparently formed later than the serpentine. Calcite fills most of the fractures in the kimberlites and probably is derived from the surrounding calcareous country rocks. However, calcite veins between the Randolph No. 1 kimberlite and the Cresswell Limestone Member originate within the kimberlite and cut across the contact into the limestone. Calcite is also abundant in the accidental limestone and calcareous shale xenoliths.
Serpentine minerals include lizardite, antigorite, chrysotile, and serpophite (serpentine so fine-grained as to appear isotropic). Lizardite is far more abundant than antigorite, and chrysotile is rare and restricted to reaction rims.
Apatite, perovskite, and chromite are ubiquitous accessories found in all the kimberlites, although their abundance may vary greatly even within a single pipe. Some of the salient characteristics of the kimberlites are shown in Figures 2 to 6, and the minerals are listed in Brookins 1969d).
Figure 2--Photomicrographs of Riley County kimberlites. A, Highly altered pyrope grain, Leonardville kimberlite. Pyrope is almost completely replaced by serpentine (antigorite) and iddingsite (dark patches). Calcite fills fractures in phenocryst and has replaced kelyphite rim. Altered olivine oriented around pyrope in magnetite-perovskite-ilmenite-choked serpentine and carbonate groundmass. B, Leonardville kimberlite, highly altered olivine microphenocrysts showing random orientation and extensive serpentinization followed by carbonation of groundmass. C, Typical pyrope phenocryst, Stockdale kimberlite. The pyrope is rimmed by kelyphite and cut by fractures filled with calcite. Note the calcite filling a separation between the pyrope and kelyphite. Surrounding groundmass consists of magnetite-perovskite-ilmenite-choked serpentine and carbonates. Microphenocrysts of serpentinized and carbonated olivines are oriented in semi-circular arrangement around pyrope. D, Highly altered olivine phenocryst, Leonardville kimberlite, showing anhedral nature of olivine (altered to antigorite). Phenocryst is cut and rimmed by magnetite-choked dolomite vein of probable carbonatitic origin which in turn (lower left-hand corner) has been partially replaced by younger serpentine (lizardite) and calcite. E, Leonardville kimberlite. Photomicrograph shows (upper left-hand corner) chloritized phlogopite crystal with magnetite formed along cleavage traces broken after initial alteration. Strong fracture system is evident by alignment in groundmass and in altered pyrope (lower right-hand corner) and kelyphite, despite random orientation of other altered olivine phenocrysts. F, Chloritized phlogopite phenocryst, Stockdale kimberlite. Note evidence of two periods of kinking after initial chloritization and formation of magnetite along cleavage traces. Evidence for flow around phenocryst is indicated by altered carbonate laths (originally melilite?) in Ti-Fe ore-choked serpentine-carbonate groundmass.
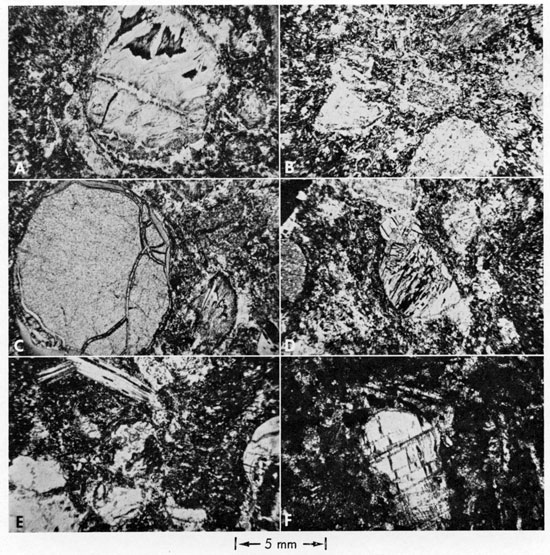
Figure 3--Photomicrographs of Riley County kimberlites. A, Chloritized phlogopite phenocryst, Stockdale kimberlite. Note development of magnetite along cleavage traces in phlogopite, followed by kinking normal to cleavage, followed by fracture parallel to cleavage (filled with light-colored serpentine). B, Subhedral altered olivine phenocryst, Randolph No. 1 kimberlite. Three periods of deformation are indicated by the fractures within the olivine phenocryst. Interior of grain is filled with serpentine and younger carbonate. Magnetite crystal in upper right-hand corner of phenocryst is altered; magnetite of a younger generation chokes the dolomitic vein which rims the grain. Orientation of microphenocrysts around olivine is random. C, Randolph No. 1 kimberlite showing some orientation of subhedral altered olivine microphenocrysts around large oikicryst of calcite (upper left-hand corner) partially replaced by serpentine. Microphenocrysts near oikicryst show random orientation as do perovskite and ilmenlte (± some magnetite) grains in serpentine-carbonate groundmass. D, Randolph No. 2 kimberlite showing flow banding of subhedral clinopyroxene (upper half) and olivine (lower half) microphenocrysts followed by younger deformation indicated by subcurvalinear hands of magnetite.
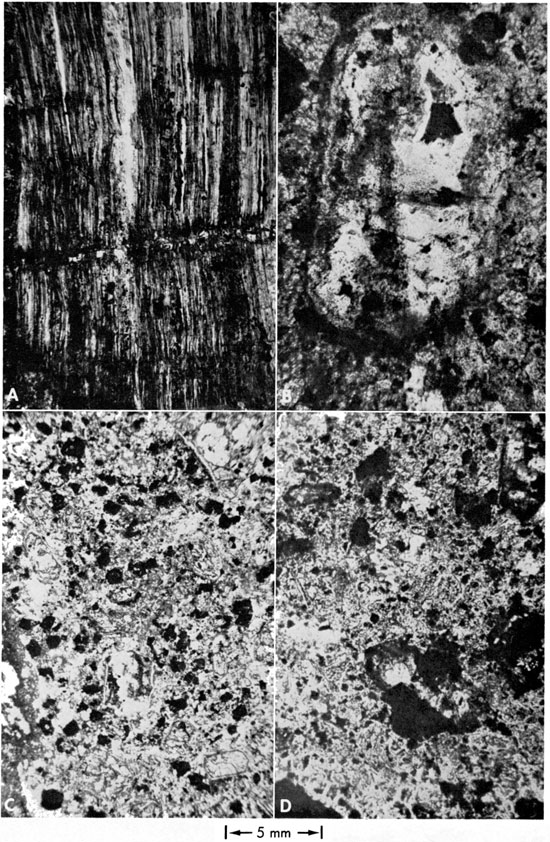
Figure 4--Photomicrograph of Randolph No. 1 kimberlite. Note extensive replacement of euhedral to subhedral altered olivine by magnetite (black) and bowlingite (dark gray) plus serpentine (light gray) and calcite (white). An older generation of almost completely altered (without magnetite and bowlingite) olivine is noted in the perovskite-rich serpentine-carbonate groundmass.
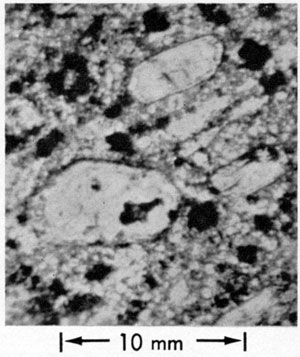
Figure 5--Photomicrographs of kimberlite contacts and carbonate veins. A, Complex vein, Bala kimberlite, showing older magnetite-choked carbonate (calcite ± dolomite ± ankerite) vein with pronounced flow banding. Younger fracturing along the vein accounts for the presence of the younger, magnetite-free calcite. B, Higher power view of 5, A. The carbonates in the older (magnetite-rich) vein material are optically continuous, the younger (clear) calcite grains are not, indicating their deposition in the fracture without significant post-formational deformation. C, Complex vein, Randolph No. 1 kimberlite, showing older, magnetite-choked calcite-dolomite (upper left-hand corner) in contact with younger, relatively magnetite-free calcite. Some magnetite and serpentine (light-gray patches and stringers) are noted in the younger vein, indicating some reaction between the two types of veins. D, Higher power view of 5, C showing the pronounced continuity of the older vein material and textural evidence for deformation normal to it, accounting for its reopening which allowed the deposition of the younger calcite.
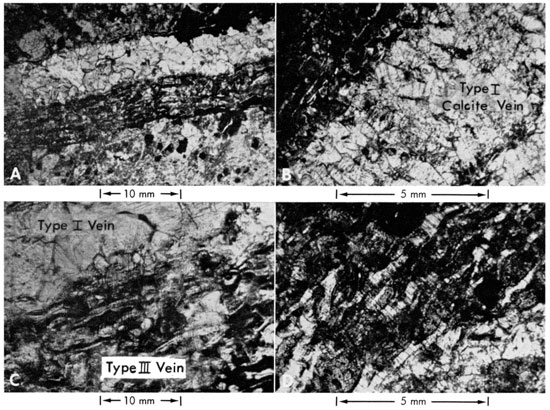
Figure 6--Photomicrographs of kimberlite contacts, carbonate veins, and limestone. A, Post-emplacement calcite vein in contact with Randolph No. 2 kimberlite. Crack in kimberlite parallel to contact is filled with clear hydrous mineral, possibly brucite. Note carbonates replacing possible melilite (lower left-hand corner) and lack of orientation of kimberlite minerals near calcite vein. B, Contact zone, Randolph No. 2 kimberlite. Sample taken from drill hole shows slight alignment of microphenocrysts parallel to contact with Fort Riley Limestone. The contact appears locally gradational due to serpentinization of limestone which is slightly weathered (McDermott and Brookins, in press). C, Contact zone, Randolph No. 1 kimberlite. Crystals in kimberlite show crude alignment parallel to contact (upper right-hand corner); carbonates in complex vein (remainder of photomicrograph) show optically continuous calcite (exhibiting strong NW-SE cleavage) and younger calcite veins in Cresswell Limestone (non-vein carbonates). The older calcite veins originate from within the kimberlite (Bridge, 1953; Brookins, 1967b). D, Towanda Limestone Member 30 feet from contact with Randolph No. 2 kimberlite. Interlocking mosaic of microspar calcite with intergranular limonite-stained clay and some dolomite. No flow or fracture texture present.
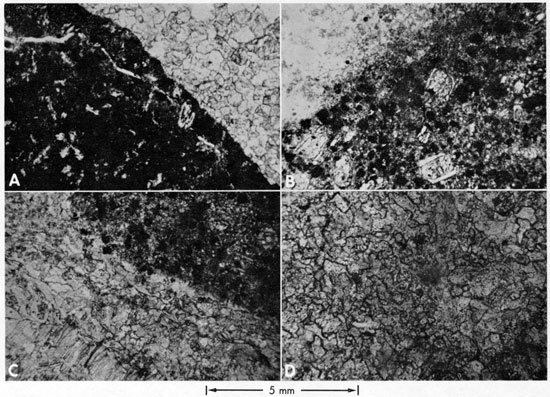
The kimberlites may be divided into two groups, micaceous and lamprophyric. The Stockdale and Leonardville kimberlites can be classified (Dawson, 1967a) as the micaceous variety because of the abundance of original phlogopite. Some of the smaller hand specimens contain up to 15 percent by volume of this mineral. These two kimberlites also contain abundant pyrope and rounded grains of ilmenite. The altered olivine (originally near Fo92) in both kimberlites is largely anhedral to subhedral in crystal outline, and contains very small amounts of pyroxene (also anhedral to subhedral). Due to the very highly brecciated nature of the kimberlites, it is uncertain whether any of the calcite is primary. Though most of the calcite appears to be secondary in origin, the possibility of remobilized carbonatitic calcite cannot be ruled out.
The Bala and Randolph No. 1 kimberlites are very much alike, and the Randolph No. 1 and Randolph No. 2 kimberlites also are similar in some respects. All three can be classified as lamprophyric because they contain virtually no phlogopite, and much of that which is present is presumed to be either secondary or xenocrystalline in origin. An exception may be the biotite from the Bala reported by Moore and Haynes (1920), which has not been observed since. Pyrope is extremely rare in these kimberlites, and occurs in small crystals (0.1 mm maximum dimension) in the groundmass. These three kimberlites are characterized by euhedral to subhedral olivine and pyroxene and contain more of the latter than do the Leonardville and Stockdale. In addition, ilmenite in the lamprophyric kimberlites is usually smaller and less frequently coated by leucoxene than in the micaceous varieties. The lamprophyric kimberlites contain phenocrysts (or xenocrysts?) of primary calcite and pre-emplacement veins. The Randolph No. 2 contains more euhedral olivine and pyroxene than either the Randolph No. 1 or Bala, possesses a higher apatite content, and contains abundant carbonated laths of original melilite(?). The Randolph No. 1 contains some apatite and possibly some original melilite, but the Bala contains very little apatite and no original melilite. Randolph No. 1 appears to be intermediate in composition between Randolph No. 2 and Bala, although these three are distinctly different from the Stockdale and Leonardville. The Winkler kimberlite contains abundant chloritized phlogopite and partially kelyphitized pyrope and probably belongs in the micaeous group with Leonardville and Stockdale.
The xenoliths from the Riley County kimberlites are classified as either cognate or accidental. Cognate xenoliths contain material characteristic of kimberlites regardless of their geographic location, and accidental xenoliths contain material characteristic ;of rocks that occur in a particular area. Angular accidental xenoliths of shale and limestone, some of which are entirely unaltered, make up the bulk of included material, approximately 60 to 70 percent of the total. These xenoliths have been derived from local calcareous country rocks and, in addition to their relative lack of alteration, usually show no preferred orientation within any particular kimberlite, indicating late entrapment. Other recognizable sedimentary or metasedimentary xenoliths are extremely rare. One large (12 cm maximum dimension) arkosic xenolith and two small pelitic slaty-schist xenoliths are the only others identified to date. Franks (1966) cites the presence of a fossil in a limestone xenolith from the Bala kimberlite.
Most of the xenoliths studied have been found in the Stockdale and Randolph No. 1 kimberlites. At least 13 different rock types totaling 36 different specimens have been identified (Table 3). Most of these are cognate and indicate a deep-seated origin. The eclogites, pyroxenites, lherzolites, and serpentine (probably original pyroxenite) are almost certainly derived from the upper mantle (Brookins, 1969a). The gabbros and metagabbros, which contain garnet and some spinel, indicate a high-pressure reaction of the type plagioclase + pyroxene = garnet and are derived either from the deep crust or upper mantle (H. O. A. Meyer, written communication, 1969; Brookins, 1969a). These deep-seated cognate xenoliths constitute more than 80 percent of the total (Table 3). Xenoliths from the Stockdale and Randolph No. 1 kimberlites are 1 to 3 inches in diameter, rounded, and composed of: limestone (32), shale (10), pyroxenite (30), gneiss (8), gabbro (15), schist (5) (Bridge, 1953). Not all of the specimens are available for restudy, however, and several misidentifications have been noted in the descriptions of thin sections. Alteration within the deep-seated xenoliths is variable. All are rimmed by at least a thin layer of serpentine or kelyphite, but alteration within the xenoliths varies from slight sausseritization of plagioclase in the gabbroic rocks to almost complete serpentization in some pyroxenites(?). Although the cognate xenoliths vary in size and degree of roundness, they are smaller and more rounded than the accidental xenoliths. Where pronounced angularity is noted in the former, it is usually due to fragmentation of the xenolith caused by excessive churning during emplacement. The largest of the cognate xenoliths examined to date is a 10-cm (maximum dimension), well-rounded eclogite. Preliminary examination of the eclogites indicates that they belong to the A-group (those characteristic of kimberlites in general; terminology in Coleman, Lee, Beatty, and Brannock, 1965), although some similarities to the eclogites from the kimberlites of northeastern Arizona (Watson, 1967a; Watson and Morton, 1968) are noted.
Table 3--Nonsedimentary xenoliths from Riley County kimberlites.*
| Rock type | Xenolith classification | Abundance |
|---|---|---|
| Basalt | Accidental | 1 |
| Diorite | Accidental | 1 |
| Eclogite | Cognate | 6 |
| Gabbro | Cognate | 7 |
| Granite | Accidental | 4 |
| Granulite | Cognate | 1 |
| Magnetite pyroxenite | Cognate | 2 |
| Garnet pyroxenite | Cognate | 1 |
| Spinel pyroxenite | Cognate | 3 |
| Metagabbro | Cognate | 3 |
| Pyroxenite | Cognate | 5 |
| Serpentine | Cognate | 5 |
| Spinel lherzolite | Cognate | 1 |
| Schist | Accidental | 3 |
| * Most cognate xenoliths from the Stockdale kimberlite. | ||
The remaining xenoliths (Table 3) consist of: granite (4), basalt (1), diorite (1), and schist (3). These are accidental xenoliths and presumably were derived from the basement rocks of Precambrian age in northeastern Kansas (Muehlburger, Hedge, Denison, and Marvin, 1966). If this is the case, then their paucity relative to the cognate xenoliths is probably genetically significant because northern Riley County is underlain by basic sedimentary and metasedimentary rocks of the Rice Formation and by basalt (Woollard, 1959; Lidiak, 1969a,b), which in turn overlie granitic and granodioritic rocks (Farquhar, 1957; Lidiak, 1969a,b). The granite xenoliths are highly altered and contain chloritized biotite and some hornblende plus sodic (Ab78-88) plagioclase. The plagioclase has been altered to sericite, talc, and clay minerals. Original K-feldspars have been almost completely altered and/or replaced by sericite, clay minerals, and calcite respectively. This type of alteration is to be expected for granitic rocks exposed to high partial pressure of CO2 (such as in the Riley County kimberlites) under moderate to high hydrostatic pressure. The basalt, diorite, and schist xenoliths are moderately altered and may have been derived from uppermost Precambrian rocks.
Analytical methods employed in this and previous studies have been described in detail elsewhere and references are given where appropriate rather than repeating the entire descriptions.
Mineral identifications were made by conventional X-ray diffraction, density, hardness, and flame tests, in addition to standard optical microscopic techniques. ASTM standard file cards were used where applicable and, where not available, well-documented, naturally occurring minerals were used for reference standards. Unit-cell and approximate chemical compositions for some primary kimberlite minerals were determined using curves, data, and methods outlined in Klug and Alexander (1954), Sriramadas (1957), Troger (1959), Smirnov (1959), Walker (1961), Brindley (1961), Brown (1961), Deer, Howie, and Zussman (1962), Nixon, Knorring, and Rooke (1963), Page (1966; 1968), and Page and Coleman (1967), as well as unpublished information on file at the Department of Geology, Kansas State University, Manhattan, Kansas.
Chemical analyses for ma)or elements in three kimberlite whole-rock and one pyrope sample were done by the Japan Analytical Chemistry Research Institute and by Kansas State University using standard gravimetric, colorimetric, and flame photometric techniques. One olivine sample was analyzed by the electron microprobe (Meyer and Boyd, 1968).
Trace elements were analyzed by emission spectrography, X-ray spectrography, flame photometry' atomic absorption, and isotope dilution. Some neutron activation analyses have been carried out in the Department of Nuclear Engineering, Kansas State University, using a TRIGA Mark 11 reactor with all supporting facilities (Eckhoff, Hill, and Kimel, 1968).
Minerals and whole rocks from the Bala and Stockdale kimberlites were analyzed for Zr, Cr, Ti, Co, Ni, and Cu, using a Bausch and Lomb emission spectrograph with Littrow-mount (Eastwood and Brookins, 1965).
Standard flame photometric procedures were used for the determination of K in conjunction with the K-Ar age studies (Brookins, 1969b), and K tests were made by atomic absorption techniques to test for alkali contamination of pyrope used in Rb-Sr age study (Brookins, in press). Isotope dilution was employed for the very accurate determination of Rb and Sr at extremely low levels (sub-ppm) of concentration in the same study.
Standard procedures were followed for the dissolution of samples for isotopic ratio determinations. Carbonate rocks were dissolved in 15 ml vycor-distilled 2N HCl with constant stirring for 15 min, filtered, and Sr separated by ion exchange using radioactive Sr85 as a tracer and vycor-distilled 2N HCl as eluant. One g samples of the silicates and oxides were dissolved in a mixture of reagent-grade HF and vycor-distilled HClO4 (20 ml:3 ml) in a teflon dish. The contents were evaporated to dryness without boiling and then redissolved in 80 ml of a 1:1 mixture of vycor-distilled 2N HCl and deionized water. This mixture was evaporated to about 10 ml, cooled overnight, filtered, and Sr separated by ion exchange. Samples for isotope dilution determination of Rb and Sr were dissolved in the same way after the addition of known amounts of Rb87-enriched and Sr86-enriched spike solutions and Rb and Sr separated by ion exchange. A flame test was used to monitor the position of Rb on the column. The Rb and Sr concentrates were evaporated to dryness, redissolved in a few ml 2N HCl, transferred to a 4-ml beaker, evaporated to dryness, and fused to burn off resin. The samples were then stored for mass spectrometric analysis.
The mass spectrometer used for the isotopic ratio (including isotope dilution) analyses is a 6-inch, 60-degree surface ionization direction-focusing instrument with Faraday cup collection and amplification by a vibrating reed electrometer. Peaks were recorded on a strip recorder with expanded scale. Some of the measurements were made at the Massachusetts Institute of Technology, Cambridge, Mass., using an identical type of instrument. Replicate analysis of the Eimer and Amend interlaboratory standard SrCO3 (lot no. 492327) were made during the course of the investigation with an average value of 0.7082 ± 0.0002 (σ). An error about a single determination is ± 0.0003. The error for Rb and Sr determined by isotope dilution is ± 1 percent. The Sr content of carbonates and carbonate-rich kimberlites was determined by X-ray spectrography using a Norelco instrument with a molybdenum tube and either a topaz or LiF detector crystal (Dowling, 1968). Commercially analyzed samples or samples in which Sr had been determined by isotope dilution were used as standards. The error about a single Sr determination is ± 2 percent.
Analyses of whole rocks from four of the Riley County kimberlites are compared with analyses of kimberlites from -other locations (Table 4). Kimberlites are characterized by low SiO2 and Al2O3, high MgO and H2O, fairly high TiO2, Fe2O3 > FeO, K2O > Na2O, and fairly high CO2 and P2O5 (Heinrich, 1966). The micaceous variety usually contains more CaO, CO2, and K2O and less H2O than the lamprophyric variety. These differences are not always present (Dawson, 1967b), and there appears to be a complete chemical gradation between the two types. Further, the analyses for the Riley County kimberlites differ from the average values for micaceous and lamprophyric kimberlites (Table 4, col. E, F) due to the large amount of CaO and CO2 derived from the surrounding calcareous country rocks, and both K2O and Na2O are extremely low due to the excessive alteration. Phlogopite, the principal K-bearing phase in most kimberlites, is so highly altered that K contents range from 0.09 to 0.50 percent (Table 5). Consequently it is not surprising that the K2O/Na2O ratios for the kimberlites vary from approximately 0.3 to 1.0. Depth does not appear to have an appreciable effect on the degree of alteration of the kimberlites. A whole-rock analysis of the Bala kimberlite from 119 feet below the surface (Table 4, col. D) is nearly identical to analyses of other Riley County kimberlite samples taken from the surface. Petrographically, the Bala kimberlite is as highly weathered at the 144-foot depth as at the surface. The criteria that MgO/Fe2O3 + FeO be lower than for other types of ultramafic rocks, and that Fe2O3 > FeO are met, however. The latter condition is due to the large amount of Fe3+-rich lizardite relative to other Fe-bearing species.
Table 4--Chemical analyses of Riley County and other kimberlites.
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 21.13 | 22.91 | 24.15 | 22.23 | 35.2 | 31.1 | 22.86 | 27.30 |
| TiO2 | 1.80 | 1.72 | 1.50 | 2.20 | 2.32 | 2.03 | 2.98 | 1.31 |
| Al2O3 | 4.28 | 4.63 | 2.03 | 3.59 | 4.4 | 4.9 | 3.78 | 3.44 |
| Cr2O3 | 0.12 | 0.12 | 0.18 | 0.06 | nd | |||
| Fe2O3 | 7.44 | 6.80 | 6.51 | 7.19 | 4.79 | 3.92 | ||
| Fe2O3+FeO | 9.8 | 10.5 | ||||||
| FeO | 1.79 | 2.94 | 1.85 | 2.89 | 5.32 | 5.10 | ||
| CaO | 18.62 | 16.68 | 15.87 | 13.07 | 7.6 | 10.6 | 22.24 | 12.70 |
| MgO | 20.04 | 20.36 | 24.45 | 25.47 | 27.9 | 23.9 | 14.58 | 30.30 |
| K2O | 0.16 | 0.15 | 0.30 | 0.02 | 0.98 | 2.1 | 1.52 | 0.26 |
| Na2O | 0.27 | 0.51 | 0.30 | 0.04 | 0.32 | 0.31 | 0.33 | 0.20 |
| H2O (+) | 8.65 | 8.76 | 8.50 | 9.81 | 7.4 | 5.9 | 3.42 | 4.63 |
| H2O (-) | 1.31 | 1.79 | 0.84 | 1.71 | 1.65 | 0.48 | ||
| CO2 | 11.72 | 10.69 | 12.04 | 9.38 | 3.3 | 7.1 | 14.84 | 9.23 |
| MnO | 0.22 | 0.14 | 0.21 | 0.11 | 0.10 | 0.17 | nd | |
| P2O5 | 1.39 | 1.19 | 0.62 | 1.56 | 0.72 | 0.66 | 1.32 | 0.65 |
| SO3 | 0.34 | 0.29 | 0.20 | 0.24 | ||||
| Totals | 99.28 | 99.68 | 99.16 | 99.79 | 100.05 | 99.20 | 99.86 | 99.52 |
|
(A) Randolph No. 2 kimberlite, Riley County, Kansas (McDermott and Brookins, in press). (B) Randolph No. 1 kimberlite, Riley County, Kansas (McDermott and Brookins, in press). (C) Leonardville kimberlite, Riley County, Kansas. Erroneously reported in previous investigations as an analysis for the Stockdale kimberlite by Bridge (1953) and Eastwood and Brookins (1965) due to incorrect reference to Sperry (1929). Analysis in 1938 by A. T. Perkins, Department of Chemistry, Kansas State University. (D) Bala kimberlite, Riley County, Kansas; 119 foot depth; new analysis. (E) Average basaltic kimberlite (Dawson, 1967b, p. 271). (F) Average micaceous kimberlite (Dawson, 1967b, p. 271). (G) Bachelor Lake kimberlite, Quebec (Watson, 1955, p. 573). (H) Elliott County kimberlite, Kentucky (Koenig, 1956, p. 49). |
||||||||
Table 5--Potassium-argon age determinations. Data for S-1, S-2, S-3, L-1 from Brookins (1969b). S = Stockdale, L = Leonardville, W = Winkler, ST = Stockdale xenolith. Sample ST-1 is chloritized biotite from a gabbroic xenolith; all other samples are chloritized (and/or vermiculitized) phlogopites. Discrepancies between duplicate analyses for *Ar40 and K are due Primarily to sample inhomogeneity (see text for discussion).
| Sample* | *Ar40, ppm |
*Ar40 / total Ar40 |
Average *Ar40, ppm |
K, % |
Average K, % |
K40, ppm |
Age, m.y. |
|---|---|---|---|---|---|---|---|
| S-1 | 0.00504 | 0.081 | 0.00508 | 0.180 | 0.197 | 0.240 | 331 ± 50 |
| 0.00511 | 0.091 | 0.215 | |||||
| S-2 | 0.00515 | 0.314 | 0.00489 | 0.200 | 0.200 | 0.244 | 315 ± 20 |
| 0.00463 | 0.274 | ||||||
| S-3 | 0.00266 | 0.502 | 0.00282 | 0.088 | 0.093 | 0.114 | 380 ± 40 |
| 0.00299 | 0.476 | 0.098 | |||||
| L-1 | 0.00138 | 0.181 | 0.00150 | 0.096 | 0.097 | 0.119 | 204 ± 20 |
| 0.00163 | 0.247 | 0.099 | |||||
| W-1 | 0.00157 | 0.123 | 0.00180 | 0.142 | 0.123 | 0.150 | 195 ± 10 |
| 0.00204 | 0.101 | 0.104 | |||||
| SI-1 | 0.01095 | 0.823 | 0.01138 | 1.392 | 1.382 | 1.686 | 112 ± 6 |
| 0.01182 | 0.588 | 1.373 |
Analyses of olivine, pyrope, and ilmenite from Riley County kimberlites are compared with analyses of the same minerals from other locations (Table 6). The unaltered olivine from the Stockdale kimberlite (Table 6, col. A) is highly magnesian (Fo92). Presumably most of the olivine from the other Riley County kimberlites is close to this in composition, but the analysis reported is on the only unaltered grain yet found in any of the kimberlites. The pyrope analysis (ibid, col. D) should be considered as an average of red pyropes from the Stockdale kimberlites as 20 g of material were hand-picked using a binocular microscope from a + 100 / -40-mesh ground sample. Kelyphite and obviously altered grains were avoided. Carbonate contamination was checked by X-ray analysis for calcite peaks after first washing the sample in dilute glacial acetic acid. The resultant pyrope formula is Mg2.22 Fe0.46 Ca0.29 Al1.76 Fe0.08 Cr0.14 Si2.98 O12 (Brookins, 1967a) with n = 1.747 ± 0.003 and a0 = 11.535. The Cr2O3 content (2.77 percent) is higher than the 1.0 percent value reported by Rosa and Brookins (1966) for another red pyrope sample from the Stockdale, but significantly lower than the 7.90-percent value reported by Bagrowski (1941) and shown to be in error by Brookins (1967a). Green pyrope noted from the Stockdale kimberlite by Eastwood and Brookins (1965) and Rosa and Brookins (1966) is more Cr-rich and Ca-poor than the red pyrope (unpublished emission spectrographic data) with n = 1.753 ± 0.003 and a0 = 11.558. This may suggest the presence of significant amounts of the knorringite garnet end member (Mg3Cr2Si3O12) in solid solution with pyrope. Dyer and Brookins (1969) have reported variation in composition of pyropes from the Leonardville kimberlite as well. A yellowish-orange variety yields n = 1.748 ± 0.005 with a0 = 11.556 and a purplish-red variety with n = 1.752 ± 0.006 with a0 = 11.567. The error about the average unit-cell determination is ± 0.005. It should be reemphasized that (1) these values are averages for pyropes of variable composition, and (2) attempts to obtain a chemical formula based on the unit-cell and refractive index values are not warranted. Only microprobe analysis of individual grains will give meaningful results.
Table 6--Chemical analyses of Riley County and other kimberlite minerals.
| Oxide | (A) | (B) | (C) | (D) | (E) | (F) | (G) |
|---|---|---|---|---|---|---|---|
| SiO2 | 40.8 | 40.7 | 40.94 | 41.11 | 41.71 | ||
| TiO2 | 0.02 | 0.004 | 0.00 | 0.27 | 0.61 | 47.90 | 49.35 |
| Al2O3 | 0.02 | 0.05 | 0.06 | 21.50 | 21.55 | 6.46 | 0.33 |
| Fe2O3 | 0.00 | 1.37 | 0.02 | 13.71 | |||
| Cr2O3 | 0.00 | 0.10 | 0.00 | 2.77 | 2.97 | ||
| FeO | 7.88 | 7.02 | 7.30 | 7.55 | 7.36 | 34.76 | 27.57 |
| CaO | 0.01 | 0.08 | t | 4.64 | 5.22 | 0.08 | 0.20 |
| MgO | 51.7 | 51.1 | 50.80 | 20.57 | 20.79 | 9.71 | 8.65 |
| MnO | 0.10 | 0.12 | 0.01 | 0.37 | 0.31 | 1.90 | 0.05 |
| Totals | 100.53 | 99.174 | 99.11 | 100.15 | 100.54 | 100.81 | 99.86 |
| (A) Forsterite, Stockdale kimberlite (Meyer and Boyd, 1968). (B) Average of five forsterite inclusions in diamonds (Meyer and Boyd, 1968). (C) Forsterite, saxonite nodule, Basutoland (Nixon, et al., 1963, p. 1118, sample E8). (D) Pyrope, Stockdale kimberlite (Brookins, 1967a). (E) Chrome pyrope, lherzolite nodule, Basutoland (Nixon, et al., 1963, p. 1104, sample E1). (F) Ilmenite, Riley County (exact location unknown; Willard, 1883-84). (G) Ilmenite, Kao pipe, Basutoland (Nixon, et al., 1963, p. 1122). t = Trace. |
|||||||
Ilmenite (Table 6, col. F) from Riley County (exact location not given by Willard, 1883-84) contains appreciable Mg, a characteristic of kimberlitic ilmenite. The presence of Mg is also indicated by low unit-cell values for ilmenite from the Stockdale and the Randolph kimberlites; a0 = 5.05, c0 = 14.14 (Rosa and Brookins, 1966; McDermott and Brookins, in press), although the presence of dissolved Fe2O3 would cause the same effect.
Magnetite from the Bala, Stockdale, and the Randolph kimberlites associated with probable primary carbonatitic calcite yields a low unit-cell value of a0 = 8.379 (average) which may indicate the presence of Al3+ substituting for Fe3+ within the structure, but a magnetite sample from the Leonardville kimberlite yields a0 = 8.397, very close to "ideal" FeFe2O4. Considerable Ti occurs in the Riley County magnetites, however, and solid solution with ulvospinel (Fe2TiO4) may be offset by solid solution with hercynite (FeAl2O4). There is also no guarantee that the magnetite samples represent the same generation of crystallization. Again, microprobe techniques must be employed in future investigations.
Eastwood and Brookins (1965) have reported on the distribution of Co, Cu, Cr, Ni, Ti, and Zr in some kimberlite minerals and whole rocks from the Bala and Stockdale kimberlites. The data are highly variable and difficult to compare with trace element studies on samples from other locations due to many factors, including (1) impurities in the mineral separates, (2) varying composition within mineral separates from grain to grain, (3) inclusion of some minerals (i.e., magnetite) formed under different T, P conditions, and (4) analytical errors. The analyses were made by emission spectrography and are therefore subject to greater error than by other methods; still certain trends are apparent. Magnetite shows a pronounced enrichment in Zr, which is probably due to its close affinity for Ti in ultrabasic rocks. Exsolved ilmenite and the possibility of dissolved ulvospinel in the magnetite may then account for the Zr concentrations noted. The 767 ppm Zr noted in serpentine from the Bala kimberlite may be due to either minute amounts of Ti-rich magnetite or the presence of small amounts of zircon. Grains in some crushed fragments exhibit orange fluorescence, a useful way of identifying zircon in kimberlites (Nixon, et al., 1963).
Dyer and Brookins (1969) have reported concentrations of 370 ppm La and 13 ppm Sc in a whole-rock sample from the Leonardville kimberlite. The analyses were made by neutron activation. These values are consistent with data for other recognized kimberlites (Dawson, 1967b).
Brookins (1966b, 1967b, 1967c) has discussed the strontium geochemistry of carbonates in kimberlitic veins and groundmass material and in some limestones in Riley and nearby counties.
Carbonatitic magmas may provide the source for fluidization of kimberlites (Franz and Wyllie, 1967), and intimate carbonate: kimberlite relationships have been noted in the field by numerous investigators (Heinrich, 1966, p. 93106; Wyllie, 1967, p. 279-323). In the Riley County kimberlites some of the phenocrysts and optically continuous veins and networks of calcite surrounding euhedra of primary kimberlite minerals are thought to be at least partly carbonatitic in origin, although it is equally certain that much of the carbonate material present replacing kimberlite minerals and serpentine and in post-emplacement veins must have been derived from the surrounding calcareous country rocks. Petrographic examination of carbonates usually does not provide a clear distinction between carbonatitic and sedimentary material, especially when remobilization has occurred. In the case of remobilized material, even accessory trace elements characteristic of carbonatites (i.e., Ti, REE, etc.) may be depleted or absent due to their highly different solubilities.
Usually the Sr isotopic composition of carbonatitic and sedimentary carbonates are quite different (Powell, 1967). Carbonatites are characterized by Sr87/Sr86 ratios in the range of 0.702 to 0.705 whereas, with the exception of rare early to mid-Precambrian limestones, sedimentary carbonates yield ratios in the range of 0.706 to 0.7093 (the last figure being that for modern sea water). Total Sr is usually higher in carbonatites as well; Gold (1963) reports an average value of 3500 ppm. Graf (1960) reports an average value of 475 ± 50 ppm Sr for limestones. Limestones in which much of the original calcareous shelly material was aragonitic will usually contain fairly high Sr values as well, a reflection of the ease of substituting Sr2+ in the aragonite structure relative to the calcite structure under low T, P aqueous conditions. High Sr contents in some limestones are due to the presence of small amounts of celestite. When aragonite is changed to calcite, some Sr escapes and will readily combine with SO4= ion if present. Runnels and Schleicher (1956) report an average value of 490 ppm Sr for eastern Kansas limestones, but there is a great deal of scatter in the data. They do note, however, that many of these limestones appear to have been recrystallized. Very low Sr contents are often found in calcite veins formed in limestones by redeposition of locally dissolved carbonates because Sr2+ will preferentially remain in solution during calcite precipitation (Brookins, 1967b).
Calcite from post-emplacement veins yields Sr87/Sr86 ratios between 0.7079 and 0.7093 and Sr contents between 171 ppm and 442 ppm; both ranges are experimentally identical to the values for limestones: Sr87/Sr86 = 0.7075 to 0.7091; total Sr = 119 to 473 ppm. At least one sample contained some celestite, and this accounts for its high Sr content of 1100 ppm. Pre-emplacement vein material yields Sr87/Sr86 ratios between 0.7028 and 0.7044, with Sr contents between 1480 ppm and 9169 ppm. Interstitial and replacement carbonates yield intermediate results. Kimberlite whole rocks were leached with 2N HCl to remove the carbonate fraction which on analysis, yields Sr87/Sr86 ratios between 0.7051 and 0.7075. The Sr contents of the whole rocks before leaching range from 660 ppm to 1481 ppm; these are minima for the carbonate fractions, however. The data do indicate that the groundmass and interstitial carbonate material is a mixture of carbonatitic and sedimentary material. In one case (Fig. 5,A) a pre-emplacement vein which consists of magnetite-choked, optically continuous calcite and/or dolomite (with minor ankerite) has re-opened and has been filled with post-emplacement calcite. The Sr isotopic composition of the preemplacement vein material is 0.7045 and that of the post-emplacement vein material 0.7080. The δO18 and δC13 values for the carbonatitic material are +22.9 and -6.4 respectively (J. R. O'Neil, written communication, 1969). The δC13 value is consistent with a carbonatitic origin, but the δO18 value is suggestive of low temperature material deposited by ground water according to the suggested ranges (Taylor, Frechen, and Degens, 1967) for fresh carbonatitic material (δC13: -5.0 to -8.0; δO18: +6.0 to +8.5). Since the Riley County kimberlites have passed through zones where metamorphism could have occurred, it is also conceivable that the isotopic information is misleading. Metamorphism may yield apparent carbonatitic values for Sr87/Sr86 and O18/O16 and C13/C12 respectively from non-carbonatitic sources (Hayatsu, et al., 1965; Deines and Gold, 1969). However, the data (Brookins, 1967a) do support the petrographic evidence for the presence of both primary (carbonatitic) and secondary (sedimentary) carbonates. That the δO18 value for the pre-emplacement vein material in one instance is high also supports the idea of remobilization of much of the carbonatitic material, and mixing of the two types of carbonates is also indicated by the Sr87/Sr86 ratios for interstitial and replacement carbonates. Since oxygen is present in equal amounts in both carbonatitic and sedimentary calcite and Sr is greatly enriched in the former, the variations noted are not surprising.
An attempt has been made to date the time of formation of the Riley County kimberlites by applying the Rb-Sr geochronological method to pyrope from the Stockdale kimberlite (Brookins, in press). The age is given by the approximate formula:
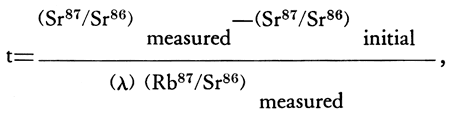
where λ is the decay constant for Rb87 . A value of 1.39 X 10-11y-1 has been assigned to λ by Aldrich and Wetherill (1958) based on geological evidence in conjunction with other geochronological work by different methods and is the value used at Kansas State University. Flynn and Glendenin (1959) have proposed the decay constant to be 1.47 X 10-11y-1, however, and hence a 6 percent uncertainty is present in Rb-Sr age determinations until this problem is resolved.
Since Rb and/or radiogenic Sr87 (*Sr87) may be enriched in secondary sites in kimberlitic minerals (Erlank, 1969; Allsopp, Nicolaysen, and Hahn-Weinheimer, 1969), great care must be taken to avoid secondary material. The pyrope separated for study was hand-picked from a +100/-40-mesh crushed sample using a binocular microscope. Kelyphite and other obvious contaminants were easily avoided. The sample was then crushed to -325 mesh and subjected to a variety of tests for contaminants. X-ray diffraction analysis showed the sample to be free of significant amounts of calcite and/or other carbonates, but did not rule out their possible presence in small amounts. Even small amounts of carbonate material, especially if carbonatitic, can markedly contaminate a Sr-deficient sample. The same is true for possible alkali contamination by small amounts of kelyphite or post-formational inclusions near the grain surface. Two 0.5-g aliquots were leached with vycor-distilled 0.05 N HCl for 24 hr with occasional stirring, filtered, washed, and the filtrate and washes combined and evaporated to dryness. The leach residue was then dissolved in 5 ml 0.05 N HCl and analyzed for K and Ca by atomic absorption techniques. Both aliquots showed less than 0.01µ g/ml Ca and less than 0.005µ g/ml K. These data, presuming Ca > Sr and K > Rb, indicate that at least the surfaces of the crushed pyrope grains do not contain enough Sr or Rb to pose a contamination problem (Brookins, in press).
The corrected values for Rb and Sr are 0.2315 ± 0.010 and 0.420 ± 0.020 ppm respectively, and the corrected measured Sr87/Sr86 ratio is 0.7186 ± 0.0007. Using an assumed initial Sr87/Sr86 ratio of 0.702 ± 0.001 for the upper mantle (Hurley, 1967) an age of 745 ± 100 m.y. is calculated as the age of formation for the Stockdale pyrope. Using λ = 1.47 x 10-11y-1 this age is lowered to 705 ± 100 m.y. Both ages are well in excess of the proposed maximum age of final kimberlitic emplacement of approximately 100 m.y. It is possible that the Rb is present in the pyrope as an impurity, but it is even more likely that the Rb was trapped during original crystallization. Furthermore, the age is probably a minimum for the true age of crystallization, since some *Sr87 may have been lost by thermal diffusion prior to emplacement.
In order to study the temperature of kimberlitic emplacement, several samples of altered kimberlitic phlogopite and one sample of altered biotite from a diorite xenolith were studied by the K-Ar geochronological method. The age is given by the formula:
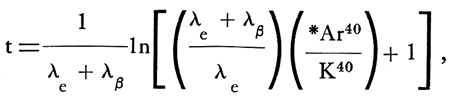
where λb = 4.72 X 10-10y-1, λe = 0.585 X 10-10y-1, and K40/K = 1.22 X 10-4 g/g.
In all but one case, K and *Ar40 were determined by commercial analysis (Geochron Laboratories, Inc.). K was determined by flame photometry, and *Ar40 by isotope dilution techniques (Schaeffer and Zahringer, 1966) (Table 5). The errors reported for the individual age determinations are due primarily to sampling. Since the micas were separated by hand picking, they were essentially free of contamination or dilution by other minerals, but petrographic examination indicates that no two phlogopite grains are altered to the same degree and are therefore chemically different. Since K and Ar determinations were made on separate aliquots, a sampling error is usually introduced. The hand-picked mica cannot be crushed fine enough to assure sample homogeneity because some *Ar40 may be lost during the crushing. Still the reproducibility of both K and *Ar40 content in separate aliquots is good, even when air corrections vary, and it is assumed that the reported dates are analytically reliable.
The dates reported in Table 5 vary from 95 ± 6 m.y. for chloritized phlogopite from the Winkler kimberlite to 380 40 m.y. for a chloritized phlogopite from the Stockdale kimberlite. Other chloritized phlogopites yield dates of 204 ± 20 m.y., 315 ± 20 m.y., and 331 ± 50 m.y., and a chloritized biotite from a diorite xenolith yields a date of 112 ± 6 m.y. All of the samples possess extremely low K contents (0.088 to 1.392 percent), and dates equal to or younger than the age of emplacement would be expected if the temperature accompanying final intrusion were elevated. This is not the case, and ages in excess of even lower Permian (280 m.y. according to Smith, 1964) are obtained. Zartman, et al., (1967) have demonstrated that certain peridotitic micas contain excess *Ar40, presumably due to a relatively high PAr in the rocks at the time of crystallization. This cannot be tested for the Riley County kimberlites because of their highly altered nature. The cases cited by Zartman, et al., (1967) apply to micas with a nearly stoichlometrlc K content. Conversely, one can argue that K has been removed preferentially to *Ar40. Chlorites yielding "ages" in excess of the age of the earth (Gerling, et al., 1967) may indicate this to be the case, and Kirsten (1968) cites the experimental evidence of Seifert and Schreyer (1966) in which it is demonstrated that at relatively high temperatures (500° to 900°C) small amounts (up to 2 percent) of K may be incorporated into a fluid phase which allows movement of the entire mass as a crystal mush, and at still lower temperatures the K is incorporated into a gas phase which can escape from the system. It is thus possible that the reactive K will escape from the system, whereas the inert *Ar40 will remain trapped.
Using existing diffusion data obtained by laboratory studies and inferred from some field evidence, Brookins (1969b) has used the data in Table 5 to estimate the maximum temperature to which the chloritized phlogopites could have been emplaced and still preserve their *Ar40. Careful consideration of some of the many factors involved leads to an estimate of 75° to 150°C maximum; at higher temperatures ages equal to or lower than 100(?) m.y. would result. Low temperature processes removing K but not *Ar40 are unlikely, and the abundance of sedimentary carbonates in the kimberlites argues against a high PAr near the time of emplacement. The low temperatures indicated by the K-Ar dates are consistent with the lack of pyrometamorphic contact effects observed in the field.
The ultramafic rocks of Riley County, Kansas, may be classified as kimberlites based on their mineralogy, chemistry, geometry, and other features. The original kimberlitic mineralogy consisted of forsteritic olivine, magnesian clino- and ortho-pyroxenes, Mg-bearing ilmenite, titaniferous magnetite, carbonate minerals (usually calcite), perovskite, apatite, ± phlogopite, ± pyrope-rich garnet, and possible ± melilite. These minerals occur both as megascopic and microscopic phenocrysts and in the groundmass of the kimberlite, suggesting more than one period of formation. The kimberlites are largely altered to serpentine (usually lizardite) and carbonated to a high degree. Only in the Stockdale is there an unaltered forsteritic (Fo92) olivine; the remaining olivine and pyroxenes have been almost completely altered to serpentine and other hydrous silicates and oxides of Mg, replaced by carbonate minerals, and contain abundant secondary magnetite. Primary ilmenite and magnetite are relatively unaltered, although the former is rounded and sometimes coated by leucoxene and the latter partially eroded along grain edges and altered to hematite. Perovskite and apatite are ubiquitous accessory minerals found only in the kimberlite groundmass. Phlogopite is altered to chlorite and/or vermiculite and contains secondary magnetite oriented along cleavage planes. Phlogopite in the groundmass is less altered and is closely associated with probable carbonatitic calcite (± other carbonates) veins and networks. Garnet phenocrysts are pyrope-rich but exhibit a variable chemistry. They are often rimmed completely or partially by kelyphite, part or all of which has commonly been stripped away during the kimberlitic emplacement. Probable original melilite laths in the Randolph No. 2 kimberlite have been completely replaced by calcite.
Accidental xenoliths of unaltered to partially altered calcareous shale and argillaceous limestone are very common in the Stockdale and Leonardville kimberlites but less abundant in the others. These xenoliths have been derived from the surrounding country rocks and have not been appreciably affected by their kimberlite host rocks. They are much more angular and usually larger than the cognate xenoliths. There is a surprising paucity of accidental xenoliths of Precambrian basement rocks. The basement rocks of northern Riley County consist of arkosic sedimentary rocks (Rice Formation) and basalt overlying granitic to granodioritic rocks (Lidiak, 1969a). These accidental igneous xenoliths constitute less than 20 percent of the total (Table 3), and only one accidental xenolith of arkose has been found. These xenoliths are fairly small, well rounded, and highly altered. That the acidic xenoliths are highly altered is not surprising since the presence of carbonatitic material implies a high partial pressure of CO2 within the kimberlite, and "granitic" minerals are usually very reactive in such an environment (Bailey, 1964), and K-feldspar is probably not stable under such conditions below 20 km. It is conceivable that some deep-seated acidic rocks may have been included in the kimberlite, but have been completely destroyed by alteration, and lack of evidence of such alteration may be masked by incorporation of K into a supercritical fluid dissipated before final emplacement (Seifert and Schreyer, 1966). The accidental basaltic, dioritic, and schistose xenoliths are highly altered as well, probably under highly aqueous conditions at a depth of 1 to 5 km (Brookins, 1969a).
Several types of cognate xenoliths (Table 3) have been found in the Stockdale kimberlite. These xenoliths are usually small, well rounded, and possess a reaction rim of magnetite-bearing hydrous silicates. Where this rim has been fractured, extensive serpentinization and/or carbonation has occurred within the xenolith. Where the rim is preserved, it has effectively acted as armor against alteration of the interior minerals. This group of xenoliths includes eclogites, gabbros, metagabbros, pyroxenites (some spinel-, garnet-spinel-, and magnetite-bearing), lherzolite(?), and granulite. Some of the eclogites may be similar to those found in Buell Park and Garnet Ridge, Arizona (Watson, 1967b; Watson and Morton, 1968) in which the composition of the garnet and pyroxene resemble C-group eclogites (characteristic of apline-type metamorphic rocks) rather than A-group eclogites (characteristic of kimberlites) according to the terminology proposed by Coleman, et al. (1965). Most of the eclogites appear to belong to the A-group, however.
The Riley County kimberlites can be subdivided into two or three groups based on their mineralogy. The Stockdale, Leonardvllle, and Winkler kimberlites are classified as the micaceous variety because of their abundance of original phlogopite. This group is also characterized by the presence of abundant phenocrysts of pyrope-rich garnet, anhedral to subhedral olivine, very few pyroxenes, abundant accidental and cognate xenoliths, and abundant calcite veins. The Bala, Randolph No. 1, and Randolph No. 2 kimberlites contain only minute amounts of groundmass phlogopite and pyroperich garnet and are classified as lamprophyric. This group is characterized by the presence of more abundant euhedral olivine and pyroxene, fewer xenoliths and veins, and more apatite. Randolph No. 2 may be even further distinguished by a paucity of xenoliths and the presence of possible original melilite, now altered to calcite. Randolph No. 2 may be intermediate between lamprophyric kimberlite and allnöite (Eckerman, 1967). It should be emphasized that even in the micaceous group pronounced differences in garnet chemistry exist within each and between the Stockdale and Leonardville kimberlites (Dyer and Brookins, 1969).
The chemical analyses of the Bala, Randolph No. 1, Randolph No. 2, and Leonardville kimberlites (Table 4) appear to be intermediate between analyses of the average micaceous and lamprophyric kimberlites (Dawson, 1967a). This may be due to accidental sampling error, or, more likely, to the nearly complete serpentinization of most of the primary minerals coupled with the presence of high amounts of locally derived carbonate material. It is interesting to note that only the Bala (Table 4, col. D), shows virtually no K, and that the Leonardville (Table 4, col. C), contains more K than a chloritized phlogopite separate (Table 5, sample L-1).
Randolph kimberlites also contain significant amounts of K, which is probably concentrated in minute inclusions, along grain boundaries, or in some other secondary site. Erlank (1969) has found this to be true for K in some South African kimberlite eclogites and, considering the highly altered state of the Riley County kimberlites coupled with lack of evidence for Kenrichment (such as feldspathization) of the contact country rocks, this is probably true for the latter as well.
Other similarities between the Riley County and other kimberlites include: (a) more Al2O3, TiO2, CaO, CO2, P2O5, SO3, and H2O than other ultrabasic rocks, (b) a high Fe2O3/FeO ratio, (c) relatively high concentrations of Sr, Cr, and Zr, and (d) a low Mg/Fe ratio. On the other hand, the Riley County kimberlites are different from many other, but by no means all, kimberlites in possessing a low K content and a low K/Na ratio. They are also extremely silica deficient, a fact that cannot be explained by dilution of the kimberlite by calcite and water. Table 7 shows analyses of Riley County and other kimberlites, as well as a garnet lherzolite and an average of the metal-free portion of stony meteorites recalculated after subtracting all CO2 as calcite and water. The Riley County kimberlites are clearly more silica deficient than the average micaceous and lamprophyric kimberlites. Of the similarities between Riley County and other kimberlites cited above, the high Fe2O3/FeO ratio is due to the large proportion of lizardite among the serpentine minerals and abundant magnetite. The relatively high concentrations of TiO2, Sr, and Zr may well be due to the presence of carbonatitic material; but the concentrations of the other enriched elements (Al, P, S, Cr) and water appear to be a unique characteristic of kimberlites independent of carbonatitic contributions.
Table 7--Recalculated chemical analyses of some ultramafic rocks, minus CO2 (as calcite) and water.
| Oxide | (1) | (2) | (3) | (4) | (5) |
|---|---|---|---|---|---|
| SiO2 | 40.84 | 35.25 | 40.53 | 45.85 | 47.85 |
| TiO2 | 2.69 | 2.81 | 1.84 | 0.41 | 0.13 |
| Al2O3 | 5.73 | 5.65 | 4.00 | 2.05 | 3.34 |
| Fe2O3 | 10.89 | 7.86 | 0.71 | ||
| 12.52 | |||||
| FeO | 3.67 | 4.18 | 6.45 | 14.69 | |
| MnO | 0.14 | 0.29 | 0.15 | 0.32 | |
| MgO | 31.91 | 35.16 | 34.76 | 41.63 | 29.41 |
| CaO | 3.06 | 3.30 | 3.96 | 1.76 | 2.33 |
| K2O | 1.90 | 0.25 | 1.19 | 0.08 | 0.12 |
| Na2O | 0.39 | 0.46 | 0.43 | 0.15 | 1.11 |
| P2O5 | 0.81 | 1.85 | 1.25 | 0.03 | 0.24 |
| Cr2O3 | 0.21 | 0.48 | 0.37 | ||
| Totals | 99.99 | 99.79 | 100.00 | 100.01 | 99.91 |
| (1) Average of micaceous and lamprophyric kimberlites (Dawson, 1967a). (2) Average of Riley County kimberlites (this report). (3) Average of 13 South African kimberlites (Kennedy and Nordlie, 1968). (4) Water-free garnet lherzolite (Ito and Kennedy, 1967). (5) Metal-free portion of stony meteorites (Urey and Craig, 1953). |
|||||
The ultramafic rocks of Riley County and the mica peridotite in Woodson County, Kansas, may well be similar and might have been simultaneously emplaced under essentially identical conditions (Tolman and Landes, 1939; Jewett, 1941; Merriam, 1963). The mica peridotite of Woodson County crops out in the vicinity of the Silver City Dome (Hills Pond Peridotite) and has been discovered by drilling in the vicinity of Rose Dome. The mica peridotite forms sill-like bodies roughly conformable to the late Pennsylvanian rocks they intrude and probably originating from a dike (Wagner, 1954; Franks, 1959, 1966). The mica peridotite contains phlogopite, olivine, augite, amphibole, with minor amounts of magnetite, perovskite, and apatite (Franks, 1959). The country rock in both the Silver City and Rose domes has been contact metamorphosed by the peridotite intrusion. These locations lie on the western end of a 400-mile-long east-west-trending explosive axis anchored on its eastern end by the Hicks dome structure in southern Illinois (Snyder and Gerdemann, 1965). Ultrabasic igneous rocks occur in several places along this structure and crystallization from a magma accompanying intrusion is evident in many. The phlogopite from the Woodson County peridotites crystallized approximately 90 m.y. ago (Zartman, et al., 1967). It is significant that the three phlogopites dated contain between 7.85 and 8.52 percent K2O. The Rose Dome location is famous for the presence of large granitic boulders of Precambrian age over which controversy has raged for 50 years (Merriam, 1963). Bickford, Mose, and Wetherill (1969), attribute the presence of the granitic boulders to the peridotite emplacement, but more work is needed in order to understand fully the granite-peridotite relationship at Rose Dome.
Thus, there are significant differences between the Woodson County peridotites and the Riley County kimberlites. The former were intruded explosively and phlogopite crystallized from a melt at that time; contact metamorphism of the country rocks is fairly extensive and high temperatures accompanying intrusion are indicated by the presence of high sanidine in entrapped granite boulders (Bickford, et al., 1969). Furthermore, the phlogopite contains a near stoichlometric amount of K and yields an average K-Ar date of 90 m.y., thus dating the time of intrusion as late Cretaceous (Zartman, et al., 1967). Somewhat tenuous field evidence indicates that the Riley County kimberlites are post-Dakota (late early Cretaceous) in age (Brookins and McDermott, 1967; Brookins, 1969b; McDermott and Brookins, in press)--or they may be Tertiary in age. In addition, a possible age of crystallization of pyrope from the Stockdale kimberlite of 745 ± 100 m.y. (Brookins, in press) indicates that crystallization occurred long before emplacement. The phlogopite in the Riley County kimberlites has been highly altered to chlorite and/or vermiculite and contains only 0.088 to 0.22 percent K and yields pre-emplacement dates ranging from 95 ± 6 m.y. to 380 ± 40 m.y. (Table 5). Brookins (1969b) has used some of the K-Ar data to estimate maximum temperature of injection of the kimberlites with a resultant range of 75° to 150°C. These low temperatures are consistent with very little pyrometamorphic contact effects. Structural deformation is noted at the Randolph No. 1 and Randolph No. 2 kimberlites and probably at the Winkler kimberlite, but the contact rocks are unaltered except at the Randolph No. 1 kimberlite where the Cresswell Limestone Member is cut and infiltrated on a very local scale by carbonatitic calcite stringers. The contact rocks at Stockdale have been altered, though the cause of the alteration is unknown (D. G. Brookins, cited in Franks, 1966). The Fort Riley Limestone is apparently in contact with the Stockdale kimberlite along its northwestern edge and appears gray to gray-brown in color. Farther from the contact the normal buff to yellowish-brown Fort Riley Limestone is observed. Examination of thin sections of the altered and unaltered Fort Riley reveals that at the contact it is slightly more weathered and contains very small amounts of serpentine-like material. The actual contact is obscured by a joint or small fault, and mud and rubble up to a width of 1 inch separate the kimberlite from the limestone. All of the evidence supports the hypothesis that a cold, plastic mush of kimberlitic material was intruded along tectonic-induced zones of weakness (probably joint systems overlying fracture zones in the basement complex). The Riley County kimberlites may have been emplaced at about the same time as the Woodson County peridotites, and both are broadly classified as ultrabasic rocks, but those are the only significant similarities between the two.
The 745 ± 100 m.y. age for the pyrope from the Stockdale kimberlite requires more discussion. Many of the alkali and alkaline earth elements are commonly concentrated in secondary sites in kimberlitic eclogites (Erlank, 1969, Allsopp, et al., 1969; Gurney, Berg, and Ahrens, 1966; and Berg, 1968). This in turn makes primary kimberlite minerals suspect as well, particularly a mineral such as pyrope which is rimmed by kelyphite, veined by secondary minerals (calcite in the Stockdale kimberlite), and which contains both inclusions and naturally decorated dislocations. Assuming that contamination from these and analytical sources are negligible (see discussion earlier in this report) the 745 ± 100 m.y. age is significant. It is probable that at least some, if not all, the Rb and possibly the *Sr87 are located in minute inclusions or along dislocations, but it is equally as probable that entrapment occurred at the time of pyrope crystallization. Obviously more data are needed, but the present age does indicate ancient formation for the pyrope and, considering the possibility of *Sr87 loss by thermal diffusion during the long pre-emplacement history, the 745 ± 100 m.y. age is probably a minimum. Work is now in progress on Rb-Sr dating of eclogite and spinel-pyroxenite xenoliths from the Stockdale kimberlite to help answer the questions of original crystallization, age of the various cognate xenoliths, and the distribution of Rb, Sr, and *Sr87 within the xenoliths.
As mentioned above, the field relationships are best explained by an injection of a cold, plastic mush of kimberlitic material due to tectonic disturbances in the basement complex. However, none of the kimberlite emplacement theories is entirely satisfactory for the Riley County kimberlites. One theory calls for an explosive volcano (or cryptovolcano) with rapid emplacement of a hot kimberlite magma, but this seems unlikely due to the lack of pyrometamorphic contact effects, the presence of unaltered xenoliths, the lack of radially oriented structures around the kimberlites, and the lack of explosion craters and associated agglomerate. Winkler "crater" is circular, but its shape is due at least in part to solution of the calcareous country rocks over the kimberlite. Another theory suggests that kimberlites are bodies of magmatic rock that never reached the surface; however, this theory is inconsistent with the field relationships. Very rapid, jet-like piercements of the earth's crust by kimberlitic magma also have been postulated but this theory is refuted not only by field relationships but also by the high velocities necessarily implied in such a process. Mikheyenko and Nenashev (1960) postulate that essentially plastic kimberlite flows are produced by the extreme pressures that occur during subsidence of the earth's crust and are forced upward into zones of weakness. The flows are circular in part, as indicated by megascopic evidence of flow banding around xenoliths and phenocrysts, especially near the wall rocks where accidental xenoliths are sporadic and where the viscosity of the flow is subjected to the greatest change. This theory does not require powerful gaseous explosions or high velocity magmatic jetlike streams. The process is somewhat similar to that proposed for salt dome formation, except that the patterns of flow are quite different for the two. Crystallization of all the primary kimberlite minerals prior to injection in a diapir-like form into the earth's crust is also postulated in such a model. The minerals thus may yield ages older than the "geologic" or emplacement age, as is the case for the Riley County kimberlites. It is possible that the K-Ar ages on chloritized phlogopite are not truly pre-emplacement and are due to an excess *Ar40 or loss of K relative to *Ar40. However, the pre-emplacement age for pyrope from the Stockdale kimberlite obtained by the Rb-Sr method substantiates to some degree the ideas of Mikheyenko and Nenashev (1960).
Mikheyenko (1968) has discussed the importance of flow banding in Russian kimberlites and his interpretations are applicable to the Riley County kimberlites. Flow banding is noted on at least a local scale in the five kimberlites which crop out, and it is especially noticeable in the Stockdale, Bala, and Randolph No. 1 kimberlites. Since the temperature of intrusion is thought to be very low, this flow banding is due to cold plastic flow. The most pronounced flow is noted near wall-rock contacts where the velocity of the flow is less than in the interior of the body, and the type of flow is dependent upon the geometric dimension of the flow, viscosity, density, and velocity (Mikheyenko, 1968). The cold, plastic kimberlites can be thought of as non-Newtonian liquids in which viscosity is a function of shear rate and which will move as pressurized flows in the direction of easiest access. Since the kinetic energy of cold, plastic flows is low, the viscosity is high and the velocity low. In the kimberlites the hydrodynamic pressure will be greater in a stream with a lower velocity relative to a neighboring slow-moving stream; hence, most xenoliths, large minerals, and gas bubbles will be squeezed out due to the hydrodynamic pressure gradient and will be transferred to the fast-moving stream where they will accumulate as a layer (in a flat flow) or band parallel to the pipe geometry. The xenoliths, minerals, and gas bubbles thus accumulated will decrease the velocity and increase the viscosity of that part of the entire plastic flow relative to the surrounding areas and, since the total energy of the flow remains essentially constant, two symmetrically situated neighboring streams will increase in velocity. These in turn will allow more zones of inclusions to arise. These processes will continue until the width of such zones will be nearly equal to the dimensions of the xenoliths transported by them. The xenoliths will be constantly rotated in the zones of accumulation because of the velocity gradient in the plane of its kinetic section. Hence, in zones where the width exceeds the dimension of the largest xenoliths, they are arranged chaotically, whereas a linear arrangement will result when the zone width is less than the dimension of some of the xenoliths, and the linearity is due to the action of the neighboring, faster-moving stream. The Riley County kimberlites are thought to have been intruded at low temperatures. However, they are altered and we do not know the carbonatite: sedimentary carbonate ratio. Thus, an accurate estimation of the viscosity and velocity within the flows is not possible.
Kimberlite may be the end product of reaction between granitic crustal rocks and a carbonatitic magma, and emplacement may have occurred as diatremes above parent dike systems (Dawson, 1967a). This theory assumes that a fluid mush of highly gas-charged kimberlite has been injected as dikes along deep-seated tension fractures, part of the material escaping explosively by the easiest access to the surface. As breakthrough to the surface or near-surface occurs, the pressure drops drastically and much of the serpentinization presumably occurs at this time. Dawson (1967a) cites examples of extensively serpentinized forsteritic olivine from surface or near-surface kimberlites as opposed to less altered olivines in kimberlites encountered at depth by drilling or mining. The explosion vent is enlarged and infilled by kimberlite, and it "sand-blasts" its way to the surface along zones of weakness created by jointing. Often the surface expression of a diatreme formed in such a fashion will consist of a crater with an olivine-rich ring or cone of tuff. Dawson's (1967a) model is based largely on South African kimberlites and is different in many respects from the model postulated by Mikheyenko and Nenashev (1960), although both emphasize the role of fluidization in kimberlitic emplacement. However, the nature of the fluidizing agents and the initial site of formation of a kimberlitic "magma" are critical. Dawson's model calls for formation of kimberlite within the crust, the presence of granitic rocks, a carbonatitic magma, and fluidization promoted by high partial pressure of C02- Mikheyenko's model calls for formation of the kimberlite in the mantle and subsequent upward flow by diapir-like movements which may be facilitated by, but do not depend upon, the presence of a gas-rich phase.
As to the formation of the kimberlite itself, I believe that the assemblage of high-pressure minerals found in most kimberlites is indicative of an upper mantle origin, probably in the depth range of 120 to 150 km. This speculation is based on the following facts:
Figure 7--Pressure-temperature diagram showing approximate fields of diamond crystallization. Upper hatched area from Kennedy and Nordlie (1968), lower hatched area from Meyer and Boyd (1968). Curve b from Brookins (1969a); all other data from MacGregor and Ringwood (1964) and text.
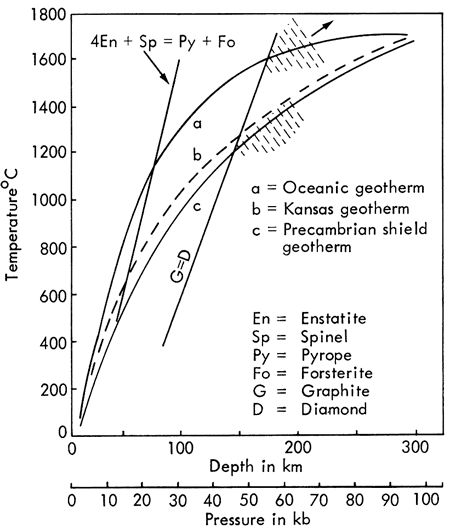
The role of carbonatitic fluids and the behavior of the H2O:CO2 ratio and K in a possible supercritical gas phase associated with (and in part responsible for?) kimberlite genesis is unknown, but the carbonatite:kimberlite association in many parts of the world cannot be considered as accidental. No calcite or other carbonate inclusions have been found in diamonds, and most cognate xenoliths of probable upper mantle origin do not contain primary (carbonatitic) calcite. Yet petrographic evidence in some cases (Watson, 1967b) indicates that calcite crystallized at, or close to, the time of crystallization of the other primary kimberlite minerals. At moderate to low pressures, artificial carbonatitic magmas are in equilibrium with most kimberlite minerals (Franz and Wyllie, 1967), but the relationships at depths of 120 to 150 km are unknown. It is possible that a CO2-rich gas phase associated with early formed Mg-rich kimberlite minerals could react with the somewhat Ca-enriched fluid to form calcite. In any event there is substantial evidence that the large amounts of carbonate associated with kimberlite are not due to hydrothermal alteration, weathering, or some other secondary process (Franz and Wyllie, 1967; Brookins, 1967b; Brookins, 1967c). However, in the case of the Riley County kimberlites, although much of the carbonate material has been derived from the calcareous country rocks, the presence of some carbonatitic material has been demonstrated by Sr isotopic studies (Brookins, 1967b). The suggestion of kimberlitic, or any alkaline-rich, magma being generated on a large scale by granitic magma assimilation of limestone seems unlikely in view of recent low pressure experimental work (Watkinson and Wyllie, 1969).
The presence of probable melilite laths, now altered to calcite in the Randolph No. 2 kimberlite, further complicates the problem of the genesis of the kimberlite. Yoder (1967, 1968), Yoder and Schairer (1968), and Schairer and Yoder (1968) do not support the idea that the magmatic portion of kimberlites may be hydrothermally altered basaltic magma due to probable melilite-plagioclase incompatibility. Plagioclase in the Riley County kimberlites is noted only in xenoliths, however. It is unaltered in cognate gabbroic and metagabbroic xenoliths except where the "armoring" rim has been penetrated, in which case the entire xenolith is usually highly serpentinized. The only xenoliths present in the Randolph No. 2 kimberlite are small, serpentinized nodules of uncertain origin. Very high pressure (upper mantle equivalent) studies of melilite with other primary kimberlite minerals in the presence of calcite are needed to resolve the melilite:kimberlite relationship.
The relationship between kimberlites and cognate eclogite and garnet peridotite nodules is still uncertain, although considerable evidence points to formation of the nodules at upper mantle depths of 100 to 140 km (O'Hara, 1967; Kushiro and Aoki, 1968). Yet the relationship between eclogite and garnet peridotite is also uncertain, although the two can be clearly distinguished by the composition of their constituent garnets. It has been suggested that eclogites have been derived from parent garnet peridotites (Rickwood, Mathias, and Siebert, 1968), yet phase equilibria studies (O'Hara and Yoder, 1967) show that eclogites melt at higher temperatures than do garnet peridotites. Rickwood, et al., (1968) agree with O'Hara and Yoder (1967) that (1) the derivation of eclogite is a late-stage crystal accumulate formed by partial melting with reaction and migration of successive liquids; (2) there is a lack of xenoliths intermediate in composition between eclogites and garnet peridotites; (3) there is a variability in composition of eclogite xenoliths and their constituent garnets, yet with an apparently constant almandine:uvarovite ratio in the garnets of the garnet peridotites; and (4) there is a higher temperature of melting of eclogites relative to garnet peridotites. However, Rickwood, et al., (1968) do not mention the possible presence of knorringite-rich garnet which may play an important part in the chemical reactions in the upper mantle (Meyer, 1968). Cr-rich kimberlitic pyropes have been noted by Nixon, et al., (1963) and Brookins (1967a). As Meyer (1968, p. 1447) points out the composition of garnet based on refractive index and unit-cell determinations alone usually do not take knorringite into consideration, and this omission may lead to serious errors in estimates of composition. Furthermore, since diamonds have been recovered from A-group eclogites as well as from kimberlites, and Cr-rich pyrope is found in diamonds, it is unlikely that these eclogites are xenoliths of crustal material trapped in mantle-derived kimberlite.
Rickwood, et al., (1968) have proposed that reactions of the type: garnet-peridotite1 = garnet-periodotiteII + liquid occur where the liquid will have a higher Fe/Mg ratio, and as the process is continued, eclogite residua and kimberlitic fluid (both of a continually changing composition) result. This is inconsistent, as eclogite has a higher melting temperature than garnet peridotite, although the constancy of garnet compositions in garnet peridotites is explained. This last fact is not readily explainable if garnet peridotites are formed by the partial melting of eclogites. A further complication results from the fact that both eclogites and garnet peridotites are extremely K-deficient, and where high K values are reported, secondary sites (grain boundaries, veins, etc.) are extremely K-rich (Erlank, 1969; Allsopp, et al., 1969; Berg, 1968). Hence the genesis of kimberlitic fluids from garnet peridotite or eclogite is unlikely. Partial melting of parental pyrolite, especially if a hydrous phase is present (even in small amounts), would probably account for the generation of a gas- and K-rich kimberlitic fluid, but this fails to explain adequately the formation of eclogite during such a process. However, the enrichment of K in secondary sites in eclogites is then explained by contamination from kimberlite. In eclogites armored by a protective reaction rim, the K-enrichment in the interior of the eclogites may be due to local partial melting within the nodule. This last possibility remains to be investigated in detail.
Kyanite- and corundum-bearing eclogites from South Africa and Russia are also known (Sobolev, Kuznetsova, and Zyuzin, 1968; Godovikov and Kennedy, 1968), but none has yet been found in the Riley County kimberlites.
It appears likely that after crystallization in the upper mantle, the kimberlitic fluid was injected into the crust as a diapir-like piercement in a manner similar to that proposed by Mikheyenko and Nenashev (1960), but with the presence of a gas-rich phase, which facilitated the process. The cognate eclogite and pyroxenite xenoliths were probably entrapped either just before or during piercement of the upper part of the crust, but the cognate gabbro and metagabbro xenoliths may have been entrapped in the deepest part of the crust. Possible near-adiabatic insulation of the kimberlites coupled with rapid movement in the deeper crust is indicated by preservation of minerals such as plagioclase, which under normal high-temperature conditions at elevated pressures would be expected to be highly altered. It is noteworthy that olivine and pyroxene in the cognate xenoliths are often unaltered, but the same minerals in the kimberlite are highly altered. The unaltered xenoliths possess a rim which evidently prevented alteration by infiltration and may have acted as a partial thermal insulator as well.
The history of the kimberlites after this initial phase is even more speculative. At least two or three periods of deformation occurred after chloritization of phlogopite, as indicated by kink bands and fractures cutting and offsetting preformed alteration products. Since kink banding is commonly attributed to shock processes (Short, 1966), its presence probably indicates periods of abrupt movement within the crust. In addition, much of the serpentinization and chloritization took place prior to the abrupt movements that caused the kinking. Secondary magnetite trains oriented along cleavage planes in phlogopite and in reaction rims around olivines are often offset and broken. In some cases fractures in serpentine are filled by calcite (carbonatitic?), which in turn are cut and filled by still younger serpentine, all of which are then cut by kink bands. It is unlikely that the serpentinization and chloritization occurred near the time of final emplacement as proposed by Dawson (1967a), because no K-enrichment of contact rocks is noted, and the Bala kimberlite, at least, is as highly altered at a depth of 144 feet as at the surface. Finally, field evidence for final emplacement by explosive intrusion is lacking. Presumably the kimberlites were forced upward along joints or faults as a well-lubricated, cold, plastic mush and much of the gas-rich (and K-rich?) phase dissipated at depth. Final emplacement (near the surface?) must have been relatively rapid and in abrupt movements as indicated by sporadic distribution in size, degree of angularity, lack of alteration, and lack of orientation of accidental xenoliths of the calcareous country rocks. Only infrequently are these xenoliths preferentially oriented with respect to wall-rock contacts, fracture zones, or pre-existing flow bands.
The following conclusions concerning the ultrabasic rocks of Riley County, Kansas, have been reached:
Aldrich, L. T., and Wetherill, G. W., 1958, Geochronology by radioactive decay: Ann. Rev. Nuclear Sci., v. 8, P. 257-298.
Allsopp, H. L., Nicolaysen, L. O., and Hahn-Weinheimer, P., 1969, Rb/K ratios and Sr-isotopic compositions of minerals in eclogitic and peridotitic rocks: Earth Plan. Sci. Letters, v. 5, p. 231-244.
Bagrowski, B. P., 1941, Pyrope garnet vs. spinel in Kansas: Am. Mineralogist, v. 26, p. 675-676.
Bailey, D. K., 1964, Temperature and vapor composition in carbonatite and kimberlite: Carnegie Inst. Wash., D.C., Yearbook 63, p. 79-81.
Barringer, R. W., 1964, World's meteorite craters, "Astroblemes": Meteoritics, v. 2, p. 169-174.
Berg, G. W., 1968, Secondary alteration in eclogites from kimberlite pipes: Am. Mineralogist, v. 53, p. 1336-1346.
Bickford, M. E., Mose, D. G., and Wetherill, G. W., 1969, Age of the Rose Dome granite, Woodson County, Kansas (abs.): Geol. Soc. America, Abstracts for 1969, Pt. 2, South-Central Section, p. 2.
Boyd, F. R., 1966, Electron probe study of dioosidic pyroxenes from kimberlites: Carnegie Inst. Wash., D.C., Yearbook 65, p. 252-260.
Boyd, F. R., and England, J. L., 1964, The system enstatite-pyrope: Carnegie Inst. Wash., D.C., Yearbook 63, p. 157-161.
Boyd, F. R., and MacGregor, I. D., 1964, Ultramafic rocks: Carnegie Inst. Wash., D.C., Yearbook 63, p. 152-156.
Bridge, T. E., 1953, The petrology and petrography of the igneous rocks of Riley County, Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 57 p.
Brindley, G. W., 1961, Chap. 6, Chlorite Minerals; in, The X-ray Identification and Crystal Structures of Clay Minerals, G. Brown, ed.: Mineralogical Society, London, p. 242-296.
Brookins, D. G., 1966a, Kimberlites from Riley County, Kansas (abs.): Geol. Soc. America, Spec. Paper 101, p. 27.
Brookins, D. G., 1966b, The strontium geochemistry of carbonates associated with kimberlites from Riley County, Kansas (abs.): Geol. Soc. America, Spec. Paper 101, p. 26-27.
Brookins, D. G., 1967a, Re-examination of pyrope from the Stockdale kimberlite, Riley County, Kansas: Min. Mag., v. 36, p. 450-452.
Brookins, D. G., 1967b,The strontium geochemistry of carbonates in kimberlites and limestones from Riley County, Kansas: Earth Plan. Sci. Letters, v. 2, p. 235-240.
Brookins, D. G., 1967c, Kansas kimberlites: Earth Sci. v. 20, p. 109-114.
Brookins, D. G., 1969a, Riley County, Kansas, kimberlites and their inclusions (abs.): Geol. Soc. America, Abstracts for 1969, Pt. 2, South-Central Section, p. 4.
Brookins, D. G., 1969b, The significance of K-Ar dates on altered kimberlitic phlogopite from Riley County, Kansas: Jour. Geol., v. 77, p. 102-107.
Brookins, D. G., 1969c, Spinel from the Stockdale kimberlite, Riley County, Kansas: Trans. Kansas Acad. Sci., v. 72, p. 262-263.
Brookins, D. G., 1969d, A list of minerals found in Riley County kimberlites: Trans. Kansas Acad. Sci., v. 72, p. 365-373.
Brookins, D. G., 1970, Kimberlite at Winkler Crater, Kansas: Geol. Soc. America, Bull., v. 81, p. 541-546.
Brookins, D. G., (in press), Possible age of crystallization of pyrope from the Stockdale kimberlite, Kansas: Geochem. Jour.
Brookins, D. G., and McDermott, V. J., 1967, Age and temperature of intrusion of kimberlite pipes, Riley County, Kansas (abs.): Geol. Soc. America, Spec. Paper 115, p. 366.
Brookins, D. G., and Watson, K. D., 1969, The strontium geochemistry of calcite associated with kimberlite from Bachelor Lake, Quebec: Jour. Geology, v. 77, p. 367-371.
Brown, G., ed., 1961, The X-ray identification and crystal structures of clay minerals: Mineralogical Society, London, 544 p.
Byrne, F. E., Parish, K. L., and Crumpton, C. F., 1956, Igneous intrusions in Riley County, Kansas: Am. Assoc. Petroleum Geologists, Bull., v. 40, p. 377-387.
Clark, S. P., and Ringwood, A. E., 1964, Density distribution and constitution of the mantle: Rev. Geophys., v. 2, p. 35-88.
Cole, V. B., Merriam, D. F., and Hambleton, W. W., 1965, Final report of the Kansas Geological Society Basement Rock Committee and list of Kansas wells drilled into Precambrian rocks: Kansas Geol. Survey, Spec. Distrib. Pub. 25, 48 p.
Coleman, R. G., Lee, D. E., Beatty, L. B., and Brannock, W. W., 1965, Eclogites and eclogites: their differences and similarities: Geol. Soc. America, Bull., v. 76, p. 483-508.
Cook, K. L., 1955, Magnetic surveys over serpentine masses, Riley County, Kansas: Mining Eng., v. 7, p. 481-488.
Davidson, C. F., 1964, On diamantiferous diatremes: Econ. Geol., v. 59, p. 1368-1380.
Davidson, C. F., 1967a, 8, III. The Kimberlites of the U.S.S.R.; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 251-261.
Davidson, C. F., 1967b, 10, IV. The So-called "Cognate Xenoliths" of Kimberlite; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc.. New York, p. 342-346.
Davis, B. T. C., 1964, The system diopside-forsterite-pyrope at 40 kilobars: Carnegie Inst. Wash., D.C., Yearbook 63, p. 165-171.
Dawson, J. B., 1962, Basutoland kimberlites: Geol. Soc. America, Bull., v. 73, p. 545-560.
Dawson, J. B., 1964, Carbonate tuff cones in northern Tanganyika: Geol. Mag., v. 101, p. 129-137.
Dawson, J. B., 1967a, 8, II. A Review of the Geology of Kimberlite; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 241-251.
Dawson, J. B., 1967b, 8, V. Geochemistry and origin of kimberlite; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 269-278.
Dawson, J. B., 1968, Recent researches on kimberlite and diamond geology: Econ. Geol., v. 63, p. 504-511.
Deer, W. A., Howie, R. A., and Zussman, J., 1962, Rock-forming minerals, Vol. 1, Ortho- and Ring-silicates: John Wiley and Sons, Inc., New York, 333 p.
Deines, P., and Gold, D. P., 1969, The change in carbon and oxygen isotopic composition during contact metamorphism of Trenton limestone by the Mount Royal pluton: Geochim. Cosmochim. Acta, v. 33, p. 421-424.
Dowell, A. R., 1964, A magnetic investigation of northern Riley County, Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 84 p.
Dowling, P. L., 1968, Carbonate petrography and geochemistry of the Eiss Limestone of Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 159 p.
Dyer, R. G., (in prep.) Mineralogy, geochemistry, and petrogenesis of the Leonardville intrusion, Riley County, Kansas: M.S. thesis, Dept. Geol., Kansas State University.
Dyer, R. G., and Brookins, D. G., 1969, Mineralogy and petrography of the Leonardville kimberlite, Riley County, Kansas (abs.): Geol. Soc. America, Abstracts for 1969, Pt. 2, South-Central Section, p. 10.
Dreyer, R. M., 1947, Magnetic survey of the Bala intrusive, Riley County, Kansas: Kansas Geol. Survey, Bull. 70, pt. 2, p. 21-28. [available online]
Eastwood, R. L., 1965, A spectrochemical investigation of the Bala and Stockdale intrusions, Riley County, Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 50 p.
Eastwood, R. L., and Brookins, D. G., 1965, A spectrochemical investigation of the Bala and Stockdale intrusions, Riley County, Kansas: Trans. Kansas Acad. Sci. , v. 68, P. 72-87.
Eckerman, H. von, 1967, 9, IV. A Comparison of Swedish, African, and Russian Kimberlites; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons Inc., New York, p. 302-312.
Eckhoff, N. D., Hill, T. R., and Kimel, W. R., 1968, Trace element determinations by neutron activation analysis: theory and development: Trans. Kansas Acad. Sci., v. 71, p. 101-135.
Erlank, A. J., 1969, Microprobe investigation of potassium distribution in mafic and ultramafic nodules (abs.): Trans. Am. Geophys. Union, v. 50, p. 343.
Farquhar, O. C., 1957 [19581, The Precambrian rocks of Kansas: Kansas Geol. Survey, Bull. 127, Pt. 3, p. 49-122. [available online]
Flynn, K. F., and Glendenin, L. E., 1959, Half-life and beta spectrum of Rb87: Phys. Rev., v. 116, p. 744-748.
Franks, P. C., 1959, Pectolite in mica peridotite, Woodson County, Kansas: Am. Mineralogist, v. 44, p. 1082-1086.
Franks, P. C., 1966, Ozark Precambrian-Paleozoic relations: Discussion of igneous rocks exposed in eastern Kansas: Am. Assoc. Petroleum Geologists, Bull., v. 50, p. 1035-1042.
Franz, G. W., and Wyllie, P. J., 1967, 9, VI. Experimental Studies in the System CaO-MgO-SiO2-CO2-H2O; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 323-326.
Freeberg, J. H., 1966, Terrestrial impact craters--a bibliography: U.S. Geol. Survey, Bull. 1220, 91 p. [available online]
Gerling, E. K., Morozova, I. M., Shukolyukov, Yu. A., Maslenikov, V. A., Levchenkov, O. A., Matveyeva, I. I., and Vas'kovskiy, D. P., 1967, Excess of Ar40 in chlorite: Geokhimya, no. 10, p. 1035-1043 (Eng. Transl.).
Gold, D. P., 1963, Average chemical composition of carbonatites: Econ. Geol., v. 58, p. 988-991.
Godovikov, A. A., and Kennedy, G. C., 1968, Kyanite eclogites: Contrib. Mineral. Petrol., v. 19, p. 169-176.
Graf, D. L., 1960, Geochemistry of carbonate sediments and sedimentary carbonate rocks. Pt. 3, Minor element distribution: Illinois State Geol. Survey Circ. 301, p. 5-71.
Gurney, J. J., Berg, G. W., and Ahrens, L. H., 1966, Observations on cesium enrichment and the potassium/rubidium/cesium relationship in eclogites from the Roberts Victor mine, South Africa: Nature, v. 210, p. 1025-1027.
Hayatsu, A., York, D., Farquhar, R. M., and Gittins, J., 1965, Significance of strontium isotope ratios in theories of carbonatite genesis: Nature, v. 207, p. 625.
Heinrich, E. W., 1966, The Geology of Carbonatites: Rand, McNally & Co., Chicago, 555p.
Hurley, P. M., 1967, 11, IV. Rb87-Sr87 Relationships in the Differentiation of the Mantle; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 372-375.
Ito, K., and Kennedy, G. C., 1967, Melting and phase relations in a natural peridotite to 40 kilobars: Am. Jour. Sci., v. 265, p. 519-538.
Jewett, J. M., 1941, The geology of Riley and Geary counties, Kansas: Kansas Geol. Survey, Bull. 39, 164 p. [available online]
Kennedy, G. C., and Nordlie, B. E., 1968, The genesis of diamond deposits: Econ. Geol., v. 63, p. 495-503.
Kirsten, T., 1968, Extremely high 40Ar/K ratios in xenolithic rocks: A remark: Earth Plan. Sci. Letters, v. 4, p. 219-220.
Klug, H. P., and Alexander, L. E., 1954, X-ray diffraction procedures for polycrystalline and amorphous materials: John Wiley and Sons, Inc., New York, 716 p.
Koenig, J. B., 1956, The petrography of certain igneous dikes of Kentucky: Kentucky Geol. Survey, ser. 9, Bull. 21, 57 p.
Kushiro, I., and Aoki, K. I., 1968, Origin of some ecologite inclusions in kimberlite: Am. Mineralogist, v. 53, p. 1347-1367.
Kushiro, I., Syono, Y., and Akimoto, S., 1967, Stability of phlogopite at high pressures and possible presence of phlogopite in the earth's upper mantle: Earth Plan. Sci. Letters, v. 3, p. 197-203.
Lidiak, E. G., 1969a, Buried Precambrian rocks of Nebraska (abs.): Geol. Soc. America, Abstracts for 1969, Pt. 2, South-Central Section, p. 17.
Lidiak, E. G., 1969b, Buried Precambrian rocks of eastern Kansas (abs.): Geol. Soc. America, Abstracts for 1969, Pt. 2, South-Central Section, p. 17-18.
McDermott, V. J., (in prep.), Determinative mineralogy of the Randolph intrusions, Riley County, Kansas: M.S. thesis, Dept. Geol., Kansas State University.
McDermott, V. J., and Brookins, D. G., (in press), The mineralogy of the Randolph kimberlites, Riley County, Kansas: Trans. Kansas Acad. Sci.
MacGregor, I. D., 1964, The reaction 4 enstatite + spinel = forsterite + pyrope: Carnegie Inst. Wash., D.C., Yearbook 63, p. 157.
MacGregor, I. D., 1965, Aluminous diopsides in the three-phase assemblage diopside solid solution + forsterite + spinel: Carnegie Inst. Wash., D.C., Yearbook 64, p. 134-135.
MacGregor, I. D. and Ringwood, A. E.,, 1964, The natural system enstatite-pyrope: Carnegie Inst. Wash., D.C., Yearbook 63, p. 161-163.
Markov, V. K., Petrov, V. P., Delitsin, I. S., and Ryabinin, Y. N., 1966, Phlogopite transformations at high pressures and temperatures: Akad. Nauk S.S.S.R. Izv., Ser. Geol., no. 6, p. 10-20.
Merriam, D. F., 1963, The geologic history of Kansas: Kansas Geol. Survey, Bull. 162, 317 p. [available online]
Meyer, H. O. A., 1967, Mineral inclusions in diamonds: Carnegie Inst. Wash., D.C., Yearbook 66, p. 446-450.
Meyer, H. O. A., 1968, Chrome pyrope: an inclusion in natural diamond: Science, v. 160, p. 1446-1447.
Meyer, H. O. A., and Boyd, F. R., 1968, Mineral inclusions in diamond: Carnegie Inst. Wash., D.C., Yearbook 67, p. 130-135.
Mikheyenko, V. I., 1968, Mode of origin of the banded flow texture in kimberlite: Doklady Akad. Nauk S.S.S.R. (Eng. Trans].), v. 179, p. 145-148.
Mikheyenko, V. I., and Nenashev, N. I., 1960, Absolute age of formation and relative age of intrusion of the kimberlites of Yakutia: Internatl. Geol. Rev., v. 4, p. 916-924.
Moore, R. C., and Haynes, W. P., 1920, An outcrop of basic igneous rock in Kansas: Am. Assoc. Petroleum Geologists, Bull., v. 4, p. 183-187.
Mudge, B. F., 1879-80, List of minerals found in Kansas: Trans. Kansas Acad. Sci. v. 7, p. 27-29.
Muehlberger, W. R., Hedge, C. E., Denison, R. E., and Marvin, R. F., 1966, Geochronology of the Midcontinent Region, United States, 3. Southern area: Jour. Geophys. Res., v. 71, p. 5409-5426.
Neff, A. W., 19495 A study of the fracture patterns of Riley County, Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 47 p.
Nelson, P. D., 1952, The reflection of the basement complex in the surface structure of the Marshall-Riley County area of Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 73 p.
Nixon, P. H., Knorring, O. von, and Rooke, J. M., 1963, Kimberlites and associated inclusions of Basutoland, a mineralogical and geochemical study: Am. Mineralogist, v. 48, p. 1090-1132.
O'Connor, H. G., 1968, Igneous and metamorphic rocks; in, The Stratigraphic Succession in Kansas, D. E. Zeller, ed.: Kansas Geol. Survey, Bull. 189, p. 67-68. [available online]
O'Hara, M. J., 1967, 10, V. Crystal-liquid Equilibria and the Origins of Ultramafic Nodules in Basic Igneous Rocks; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 346-349.
O'Hara, M. J., and Yoder, H. S., 1967, Formation and fractionation of basic magmas at high pressures: Scottish Jour. Geol., v. 3, p. 67-117.
Page, N. J., 1966, Mineralogy and chemistry of the serpentine group minerals and the serpentinization process: Unpub. Ph.D. dissertation, Dept. Geol., Univ. California (Berkeley), 390.
Page, N. J., 1968, Chemical differences among the serpentine "polymorphs": Am. Mineralogist, v. 53, p. 201-215.
Page, N. J., and Coleman, R. G., 1967, Serpentine-mineral analyses and physical properties: U.S. Geol. Survey, Prof. Paper 575-B, p. B103-BIO7.
Powell, J. L., 1967, isotopic composition of strontium in carbonatites and kimberlites: Indian Mineral., IMA Volume, p. 58-66.
Rickwood, P. C., Mathias, M., and Siebert, J. C., 1968, A study of garnets from eclogite and peridotite xenoliths found in kimberlite: Contrib. Mineral. Petrol., v. 19, p. 271-301.
Ringwood, A. E., MacGregor, I. D., and Boyd, F. R., 1964, Petrological constitution of the upper mantle: Carnegie Inst. Wash., D.C., Yearbook 63, p. 147-152.
Rosa, F., 1966, Mineralogy and petrogenesis of the Stockdale intrusion, Riley County, Kansas: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 77 p.
Rosa, F., and Brookins, D. G., 1966, The mineralogy of the Stockdale kimberlite pipe, Riley County, Kansas: Trans. Kansas Acad. Sci., v. 69, p. 335-344.
Runnels, R. T., and Schleicher, J. A., 1956, Chemical composition of eastern Kansas limestones: Kansas Geol. Survey, Bull. 119, pt. 3, 81 p. [available online]
Schaeffer, O. A., and Zahringer, J., 1966, Potassium-argon dating: Springer-Verlag, Berlin, 234 p.
Schairer, J. F., and Yoder, H. S., 1968, The joint albite-anorthite-akermanite: Carnegie Inst. Wash., D.C., Yearbook 67, p. 104-105.
Seifert, F., and Schreyer, W., 1966, Fluide phasen im system K2O-MgO-SiO2-H2O und ihre mögliche bedeutung für die entstehung ultrabasischer gesteine: Ber. Bunsenges. Physik. Chemie., v. 70, p. 1045-1054.
Short, N. M., 1966, Shock processes in geology: Jour. Geol. Ed., v. 14, p. 149-166.
Skinner, B. J., and Boyd, F. R., 1964, Aluminous enstatites: Carnegie Inst. Wash., D.C., Yearbook 63, p. 163-165.
Smirnov, G. I., 1959, Mineralogy of Siberian kimberlites: Internatl. Geol. Rev., v. 1, p. 21-39.
Smith, D. B., 1964, The Permian Period: Quart. Jour. Geol. Soc. London, v. 120S, p. 211-220.
Snyder, F. G., and Gerdemann, P. E., 1965, Explosive igneous activity along an Illinois-Missouri-Kansas axis: Am. Jour. Sci., v. 263, p. 465-493.
Sobolev, N. V., Kuznetsova, I. K., and Zyuzin, N. I., 1968, The petrology of grospydite xenoliths from the Zagadochnaya kimberlite pipe in Yakutia: Jour. Petrol., v. 9, p. 253-280.
Sperry, A. B., 1929, [by title only], The intrusive rocks of Riley County, Kansas: Trans. Kansas Acad. Sci., v. 32, p. ?.
Sriramadas, A., 1957, Diagrams for the correlation of unit-cell edges and refractive indices with the chemical composition of garnets: Am. Mineralogist, v. 42, p. 294-298.
Taylor, H. P., Frechen, J., and Degens, E. T., 1967, Oxygen and carbon isotope studies of carbonatites from the Laacher See district, West Germany, and the Alnö district, Sweden: Geochim. Cosmochim. Acta, v. 31, p. 407-430.
Taylor, W. K., 1950, Study of the structural relationship of the Riley County intrusions to the Abilene arch: Unpub. M.S. thesis, Dept. Geol., Kansas State University, 25 p.
Tolman, C., and Landes, K. K., 1939, Igneous rocks of the Mississippi Valley lead-zinc districts: Geol. Soc. America, Spec. Paper 24, p. 71-103.
Troger, E., 1959, Die granatgruppe: Beziehungen zwischen Mineralchemismus und Gesteinsart: Neus jahr. Mineral. v. 93, p. 1-44.
Urey, H. C., and Craig, H., 1953, The composition of the stone meteorites and the origin of meteorites: Geochim. Cosmochim. Acta, v. 4, p. 36-82.
Wagner, H. C., 1954, Geology of the Fredonia quadrangle, Kansas: U.S. Geol. Survey Geol. Quad. Map GQ49.
Walker, G. F., 1961, Vermiculite minerals; in, The X-ray Identification and Crystal Structure of Clay Minerals, G. Brown, ed.: Mineralogical Society, London, p. 297-324.
Watkinson, D. H., and Wyllie, P. J., 1969, Phase equilibrium studies bearing on the limestone-assimilation hypothesis: Geol. Soc. America, Bull., v. 80, p. 1565-1576.
Watson, K. D., 1955, Kimberlite at Bachelor Lake, Quebec: Am. Mineralogist, v. 40, p. 565-579.
Watson, K. D., 1967a, 8, IV. Kimberlite Pipes of Northeastern Arizona; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 261-269.
Watson, K. D., 1967b, 9, V. Kimberlites of Eastern North America; in, Ultramafic and related rocks, P.J. Wyllie, ed.: John Wiley and Sons, Inc., New York, p. 312-323.
Watson, K. D., and Morton, D. M., 1968, Eclogite inclusions in kimberlite pipes at Garnet Ridge, northeastern Arizona: Am. Mineralogist, v. 54, p. 267-285.
Wheeler, H. E., 1965, Ozark Precambrian-Paleozoic relations: Am. Assoc. Petroleum Geologists, Bull., v. 49, p. 16471665.
Willard, J. T., 1883-84, Note on a new Kansas mineral: Trans. Kansas Acad. Sci., v. 9, p. 25-26.
Woollard, G. P., 1959, The relation of gravity to geology in Kansas: Kansas Geol. Survey, Bull. 137, p. 63-104.
Wyllie, P. J., ed., 1967, Ultramafic and Related Rocks: John Wiley and Sons, Inc., New York, 464 p.
Yoder, H. S., 1967, Akermanite and related melilite-bearing assemblages: Carnegie Inst. Wash., D.C., Yearbook 66, p. 471-477.
Yoder, H. S., 1968, Anorthite-akermanite and albite-soda melilite reaction relations: Carnegie Inst. Wash., D.C., Yearbook 67, p. 105-108.
Yoder, H. S., and Kushiro, I., 1969, Melting of a hydrous phase: phlogopite: Am. Jour. Sci., v. 267-A, p. 558-582.
Yoder, H. S., and Schairer, J. F., 1968, The melilite-plagioclase incompatibility dilemma in igneous rocks: Carnegie Inst. Wash., D.C., Yearbook 67, p. 101-103.
Zartman, R. E., Brock, M. R., Heyl, A. V., and Thomas, H. H., 1967, K-Ar and Rb-Sr ages of some alkalic intrusive rocks from Central and Eastern United States: Am. Jour. Sci., v. 265, p. 848-870.
Zeller, D. E., ed., 1968, The stratigraphic succession in Kansas: Kansas Geol. Survey, Bull. 189, 81 p. [available online]
Kansas Geological Survey, The Kimberlites of Riley County, Kansas
Placed on web June 16, 2010; originally published in June 1970.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/200/index.html