Kansas Geological Survey, Bulletin 199, pt. 1, originally published in 1970
Originally published in 1970 as part of Kansas Geological Survey Bulletin 199, pt. 1, p. 3-7. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version of the complete bulletin (5 MB) is also available.
Epsomite and hexahydrite occur on the walls, pillars, and ceilings of an underground storage area in the Plattsmouth Limestone Member (late Pennsylvanian). These minerals are confirmed by X-ray diffraction analysis. Pure epsomite is found in an area of low temperature (14-16°C) and high relative humidity (90-91 percent). Hexahydrite is found in an area of higher temperature (20-24°C) and lower relative humidity (38-40 percent). Both field and laboratory evidence indicates that hexahydrite is formed by dehydration of epsomite, which is not stable under laboratory conditions (temperature above 20°C, R.H. below 70 percent). To the authors' knowledge, this is the first reported occurrence of hexahydrite from Kansas.
White efflorescences were collected from several locations in Page Airways' underground storage area, about 1 mile south of the town of Atchison, Atchison County, Kansas. X-ray diffraction data indicate that these are either epsomite or hexahydrite, or a mixture of the two. Because the temperature and humidity of the sample locations in the storage area are not the same, the distribution and occurrence of the efflorescences may offer an opportunity to better understand and study the genesis of these two minerals.
Epsomite or epsom salt (MgSO4 · 7H2O) is noted in a list of Kansas minerals (Mudge, 1881). However, this is the first reported occurrence of hexahydrite (MgSO4 · 6H2O) from Kansas.
The height of the storage area in the Plattsmouth Limestone Member of late Pennsylvanian age (Jewett, O'Connor, and Zeller, 1968) is 12 feet. Chert nodules are common in the limestone, which also contains minor amounts of dolomite (Hill, 1964). The efflorescences cover the walls, pillars, and ceilings of the galleries, and occur locally on the floor.
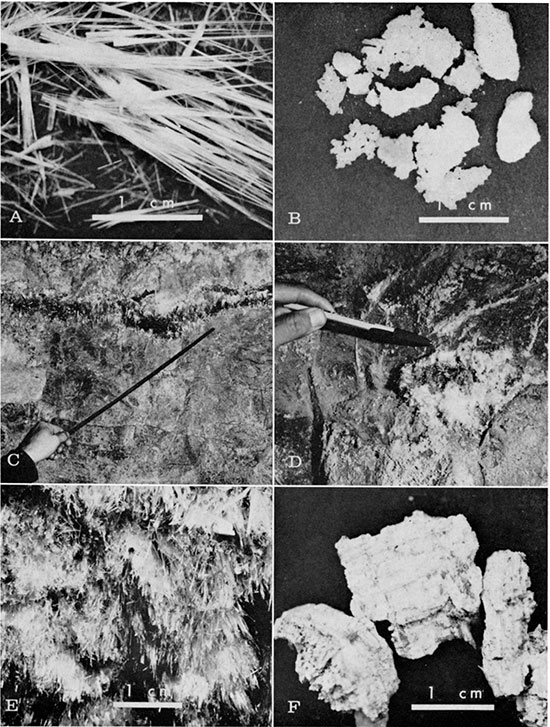
In an area of no temperature and relative humidity control, the efflorescence is epsomite which occurs as fibers or whiskers (Fig. 1, A) and crusts (Fig. 1, B). Epsomite is especially concentrated (1) along the contacts between the clay partings and the limestone (Fig. 1, C); (2) along the contacts between the chert nodules and the limestone (Fig. 1, D); and (3) on the ceilings (Fig. 1, E).
In the area of controlled temperature and relative humidity the efflorescences are hexahydrite, or a mixture of epsomite and hexahydrite. Pure hexahydrite shows earthy or columnar structures (Fig. 1, F). The mixtures are all in powdered form.
Figure 1--Forms of epsomite: A, Fibrous; B, Crusted; C, Epsomite along contact of clay parting and limestone; D, Epsomite along contact of chert nodule and limestone; E, Epsomite on ceiling of gallery; and F, Aggregates of hexahydrite showing columnar structure.

Samples of hexahydrite were collected from the pillars of the storage area proper. Samples consisting of epsomite and hexahydrite were collected from the ceilings of the marginal area. Samples were capped tightly in glass jars, and the laboratory examinations were carried out within one week after collection.
In the controlled area the temperature ranges from 20 to 24°C, with a relative humidity range from 38 to 43 percent. In the uncontrolled area the temperature ranges from 14 to 16°C, with a relative humidity of 90 to 91 percent.
X-ray diffraction data (Table 1, 2) were obtained by packing the powdered samples in aluminum holders. Copper radiation was used with a Philips-Norelco X-ray diffractometer, a curved lithium-crystal focusing monochromator, and a gas-sealed proportional counter. The scanning speed was 0.5°2θ per minute, and chart speed was 0.5 inch per minute. The 20 values were obtained by measuring the center of the half-height of the diffraction peaks. Either the heights of Ka peaks or the average heights of the Ka1 and Ka2 doublets were used for calculation of the relative intensities.
Table 1--X-ray powder data for epsomite.
| 1* | 2† | 1* | 2† | 1* | 2† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d(A) | I | d(A) | I | d(A) | I | d(A) | I | d(A) | I | d(A) | I |
| 5.980 | 20 | 5.99 | 20 | 2.350 | 2 | 2.352 | <1 | 1.679 | <1 | ||
| 5.95 | 6 | 2.258 | 6 | 1.664 | <1 | 1.661 | 3 | ||||
| 5.342 | 45 | 5.35 | 26 | 2.252 | 3 | 2.253 | 8 | 1.658 | 1 | 1.658 | 4 |
| 4.486 | 4 | 4.48 | 14 | 2.222 | 3 | 2.229 | 4 | 1.650 | 3 | ||
| 4.214 | 100 | 4.21 | 100 | 2.204 | 8 | 2.206 | 12 | 1.649 | 2 | 1.646 | 1 |
| 3.781 | 35 | 3.79 | 14 | 2.115 | 2 | 2.115 | 8 | 1.631 | 3 | 1.632 | 4 |
| 3.753 | 12 | 3.76 | 8 | 2.104 | 2 | 2.ll0 | 4 | 1.514 | 1 | ||
| 3.453 | 7 | 3.453 | 16 | 2.039 | 3 | 2.040 | 2 | 1.498 | 2 | ||
| 3.421 | 1 | 3.424 | 2 | 2.016 | 2 | 2.017 | 4 | 1.483 | 2 | ||
| 3.302 | 4 | 3.304 | 4 | 1.966 | 1 | 1.964 | 1.472 | 2 | |||
| 3.178 | 1 | 3.178 | 6 | 1.953 | 2 | 1.955 | 1.453 | 2 | |||
| 2.997 | 10 | 3.000 | 14 | 1.900 | 2 | 1.443 | <1 | ||||
| 2.987 | 10 | 1.893 | 7 | 1.894 | 2 | 1.407 | <1 | ||||
| 2.977 | 5 | 2.977 | 14 | 1.882 | 2 | 1.402 | <1 | ||||
| 2.891 | 15 | 1.877 | 2 | 1.877 | 2 | 1.392 | <1 | ||||
| 2.878 | 50 | 2.880 | 20 | 1.860 | 4 | 1.861 | 2 | 1.386 | <1 | ||
| 2.807 | 6 | 2.812 | 2 | 1.828 | 1 | 1.826 | <1 | 1.374 | <1 | ||
| 2.748 | 10 | 2.748 | 14 | 1.801 | 2 | 1.799 | 4 | 1.357 | 4 | ||
| 2.674 | 20 | 2.677 | 25 | 1.795 | 2 | 1.338 | 2 | ||||
| 2.659 | 30 | 2.659 | 20 | 1.726 | 5 | 1.726 | 3 | 1.328 | 1 | ||
| 2.488 | 1 | 2.493 | 2 | 1.712 | 2 | 1.313 | 3 | ||||
| 2.482 | <1 | 1.702 | <1 | 1.710 | 2 | 1.175 | 1 | ||||
| 2.386 | 5 | 2.389 | 6 | 1.697 | 1 | 1.695 | 2 | ||||
| * Kansas sample. † Synthetic MgSO4 · H2O (Swanson, Gilfrich, and Cook, 1957). |
|||||||||||
Table 2--X-ray powder data for hexahydrite.
| 1* | 2† | 1* | 2† | 1* | 2† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d(A) | I | d(A) | I | d(A) | I | d(A) | I | d(A) | I | d(A) | I |
| 6.038 | 3 | 2.898 | 35 | 2.021 | 5 | ||||||
| 5.798 | 4 | 2.826 | 4 | 2.014 | 9 | 2.00 | 20 | ||||
| 5.454 | 35 | 5.5 | 28 | 2.789 | 11 | 2.77 | 28 | 1.995 | 4 | ||
| 5.102 | 31 | 5.1 | 24 | 2.769 | 15 | 1.978 | 3 | ||||
| 5.002 | 8 | 2.726 | 3 | 1.932 | 3 | ||||||
| 4.879 | 25 | 4.9 | 24 | 2.682 | 13 | 2.67 | 24 | 1.879 | 9 | 1.87 | 24 |
| 4.549 | 5 | 2.596 | 3 | 1.862 | 6 | ||||||
| 4.393 | 100 | 4.4 | 100 | 2.576 | 6 | 2.56 | 8 | 1.819 | 3 | ||
| 4.155 | 9 | 2.522 | 13 | 2.50 | 16 | 1.800 | 4 | 1.80 | 4 | ||
| 4.036 | 38 | 4.04 | 32 | 2.469 | 3 | 1.783 | 4 | ||||
| 3.888 | 6 | 2.441 | 3 | 1.763 | 5 | 1.76 | |||||
| 3.602 | 16 | 3.61 | 20 | 2.338 | 4 | 1.731 | 3 | ||||
| 3.454 | 13 | 3.42 | 16 | 2.316 | 4 | 1.697 | 2 | 1.69 | 4 | ||
| 3.382 | 10 | 2.306 | 4 | 1.653 | 1 | ||||||
| 3.290 | 3 | 2.295 | 6 | 1.6229 | <1 | 1.62 | 4 | ||||
| 3.192 | 14 | 3.20 | 12 | 2.280 | 11 | 2.28 | 24 | 1.5120 | <1 | ||
| 3.094 | 1 | 2.222 | 1 | 1.4998 | <1 | 1.50 | 4 | ||||
| 3.030 | 10 | 2.199 | 3 | 2.20 | 4 | 1.4681 | <1 | 1.46 | 4 | ||
| 2.959 | 9 | 2.068 | 5 | 1.4540 | <1 | ||||||
| 2.936 | 27 | 2.92 | 60 | 2.051 | 1 | 2.05 | 8 | ||||
| * Kansas sample. † ASTM Data File, 1-0354. |
|||||||||||
X-ray diffraction data for Kansas samples are compared with those for epsomite and hexahydrite available in the literature. The data for Kansas epsomite are close to those for synthetic MgSO4 · 7H2O (Swanson, Gilfrich, and Cook, 1957). The hexahydrite, on the other hand, yields more peaks on the diffractometer chart than those previously reported (ASTM, 1967; Tasch and Angino, 1968).
Epsomite is not stable; it partially dehydrates to hexahydrite within 3 hours, and completely converts to hexahydrite within 36 hours in the laboratory (R.H. 60-70 percent; 23°C). No intermediate stage was detected by X-ray diffraction during the dehydration of epsomite (Fig. 2).
Figure 2--X-ray diffraction pattern illustrating changes of epsomite in course of dehydration to hexahydrite. Scanning time for each pattern is 20 minutes. Time at beginning of patterns is expressed as hour and minute after sample was exposed in laboratory.
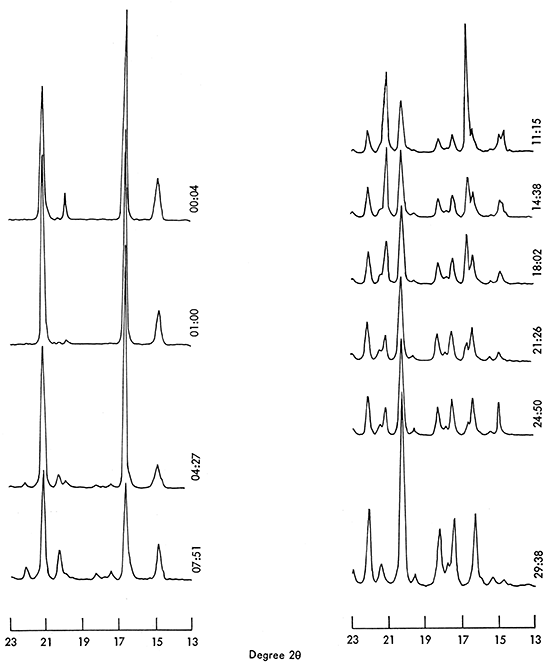
Curves in Figure 2 are representative of a continuously repeated X-ray diffractogram which arbitrarily was made in the scanning range between 13 and 23° 2θ. The scanning speed was 0.5° 2θ per minute with a chart speed of 0.25 inch per minute.
Quantitative spectrographic analyses on the epsomite and hexahydrite indicate that Si, Ca, Fe, and Cu were present in trace amounts in both minerals studied. Na, Mn, and Ni were found only in the hexahydrite sample.
The basic elements necessary in the composition of epsomite and hexahydrite are magnesium and sulfur. Two samples of the Plattsmouth Limestone from Atchison County contained 1.31 and 3.17 percent MgO, 0.09 and 0.14 percent SO3, and 0.06 and 0.12 percent S respectively (Hill, 1964). The trace elements found in samples of epsomite and hexahydrite are also found in the above two limestone samples. The overburden of the storage area consists mainly of shales which contain more sulfides (pyrite and/or marcasite) than the limestone. The sulfides in the shales may have been oxidized to sulfuric acid and subsequently carried downward by meteoric water. Through the interaction of this acidic solution with the limestone, calcium and magnesium sulfates have formed. Because magnesium sulfate is more soluble than calcium sulfate, it may be carried farther by the meteoric water along the capillaries, fissures, joints, and bedding planes. Under favorable conditions, magnesium sulfate is deposited as epsomite, which is unstable both in the area of controlled temperature and humidity and in the laboratory. Epsomite dehydrates rapidly and forms hexahydrite.
E. E. Angino first brought the samples to the attention of the senior author for identification and suggested the study. William Jackson of Page Airways, Incorporated, kindly guided the authors to collecting localities in the mine and supplied the data on temperature and relative humidity for the different sections of the storage area.
American Society Testing Materials, 1967, X-ray powder data file sets 1-5 (revised), inorganic: Am. Soc. Testing Materials, 685 p.
Hill, W. E., Jr., 1964, A geochemical study of the chert in the Plattsmouth Limestone Member of the Oread Limestone of northeastern Kansas: Unpub. Master's dissertation, Dept. Geol., Univ. Kansas, 50 p.
Jewett, J. M., O'Connor, H. G., and Zeller, D. E., 1968, Pennsylvanian System; in The Stratigraphic Succession in Kansas, D. E. Zeller, ed.: Kansas Geol. Survey Bull. 189, p. 21-43. [available online]
Mudge, B. F., 1881, List of minerals found in Kansas: Kansas Acad. Sci. Trans., v. 7, p. 27-29.
Swanson, H. E., Gilfrich, N. T., and Cook, M. I., 1957, Standard X-ray diffraction powder patterns: Natl. Bureau Standard Circ. 539, 70 p.
Tasch, Paul, and Angino, E. E., 1968, Sulphate and carbonate salt efflorescences from the Antarctic interior: Antarctic Jour. U.S., v. 3, p. 239-241.
Kansas Geological Survey, Epsomite and Hexahydrite from an Underground Storage Area, Atchison, Kansas
Placed on web Oct. 4, 2016; originally published in March 1970.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/199_1A/index.html