
Kansas Geological Survey, Bulletin 191, pt. 1, originally published in 1968

Originally published in 1968 as part of "Short Papers on Research in 1967," Kansas Geological Survey Bulletin 191, part 1, p. 9-11. This is, in general, the original text as published. The information has not been updated.
Cheyenne Sandstone from Comanche County, dune sand from near Liberal, Kansas, and caliche or chalk from the Fort Hays Limestone Member of the Niobrara Chalk were used in glass compositions to make reflective glass beads. Both sources of sand were acid-leached to reduce their iron content. Index of refraction and light reflectivity of the experimental glass beads produced were of the same value as those of currently manufactured commercial products.
Several years ago Nixon et al. (1950) recommended that the Cheyenne Sandstone was suitable for industrial use. Nothing has occurred in the passing years to develop the deposit, mainly because of the distance from markets and competing sands from other sources in surrounding states. This is a reexamination of the Cheyenne Sandstone for possible use in the manufacturing of reflective glass beads, a growing market, which can utilize a glass of higher iron content than many of the current industrial glasses. In addition a sample of dune sand from near Liberal, Kansas, was beneficiated and compared with the Cheyenne Sandstone as a potential raw material in the manufacture of glass beads.
The Cheyenne Sandstone in Comanche County is poorly indurated. Thus, no special digging equipment is required to recover it. Hydraulic mining would be possible and could also serve as the first step in removing impurities. Dune sands are easily dug from the ground.
The laboratory beneficiation method for Cheyenne Sandstone was as follows:
The laboratory beneficiation of dune sand was as follows:
In addition to the Kansas sands, local calcium carbonate sources (Fort Hayes limestone and caliche) were used in glass compositions. The limestone and caliche were used without any beneficiation.
Numerous glass compositions were melted, but only a few representative ones are listed in Table 1. Some of the compositions may be uneconomical for the manufacture of glass beads, but they were used mainly to check our laboratory procedures and equipment for making glass beads. Part of the melted glass composition was poured into a graphite mold to form a sample of glass and part was poured into water to form a crushable frit for later processing into beads.
Table 1--Typical glass compositions made with Kansas sands and calcium carbonates.
| Sample no. |
Composition, in percent | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beneficiated Cheyenne Sandstone |
Raw dune sand |
Fort Hays Limestone Member |
Caliche | Li2CO3 | Anhydrous boric acid |
Na2CO3 | K2CO3 | As2O3 | BaCO3 | ZnO | |
| KGS-2A | 61.3 | 12.9 | 4.3 | 0.4 | 4.7 | 15.7 | 0.8 | ||||
| KGS-3B | 42.0 | 0.3 | 3.8 | 12.0 | 0.1 | 17.6 | 3.7 | ||||
| KGSPE-2 | 35.8 | 9.3 | 35.0 | 19.9 | |||||||
If a free-falling particle of glass is heated to its "melting" temperature, the surface tension of the viscous glass will shape the particle into a perfect sphere. All test glass beads were formed by dropping the glass particles into a vertical furnace (Fig. 1) using the flame fusion method.
Figure 1--Sketch of apparatus used in making glass beads.
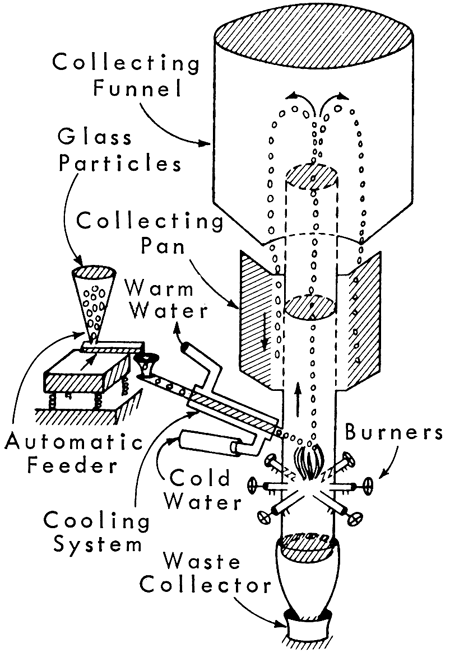
The test glass frits were ground in a porcelain ball mill using alumina balls and screened to -32 +65 mesh. It is important not to have too much variation in particle size. If the particle is too large, it will fall through the flame; if it is too small, the velocity of the hot gases will prevent the particle from reaching the hot flame. For proper fusion, the particle must be in the flame long enough to soften the glass to a point where the surface tension forces can shape the sphere and produce a smooth surface. Too low a temperature will produce only partial spheroidization and a "wrinkled" surface. Each glass composition and particle size range has its best spheroidizing temperature and gas velocity.
After grinding and screening, the glass particles were fed into the furnace about 10 inches above the burner. The feeder tube is water-cooled to prevent the glass particles from sticking at the furnace entrance. The glass particles fuse almost instantaneously in the flames and are carried upward by the velocity of the hot gases, out of the furnace tube, and fall into the collecting funnel, and roll onto a sloping collecting plate that collects the beads and also somewhat classifies them as rounded or non-rounded. A vibrating table can be used for further separation of imperfect beads.
Index of refraction and percent daylight reflection values are listed in Table 2. For ordinary highway usage, beads must have an index of refraction greater than 1.50 and a percentage of daylight reflection greater than 45. Several compositions were made with unbeneficiated dune sand. The higher iron content produced more color in the glass and reduced the percent daylight reflectance value.
Table 2--Physical properties of glass produced from Kansas sands and calcium carbonate.
| Sample no. |
Color | Index of refraction* |
Percent daylight reflection† |
|---|---|---|---|
| KGS-2A | clear | 1.51 | 63 |
| KGS-3B | light blue tint | 1.58 | 50 |
| KGSPE-2 | light green tint | 1.57 | 45.7 |
| *Measured by the comparative oil immersion method. †Test done by Materials Testing Laboratory, Kansas State Highway Commission. MgO is rated as 100% daylight reflection. |
|||
Crushing the frit into proper particle size is a major problem, as the percentage yield of correct particle size is small. An interesting future study would be the quenching temperature variables on the fracture characteristics of glass. There are some indications, from the manner in which thick sheets of tempered glass break, that a "heat treating" technique could be developed that would permit the glass to fracture into particles of uniform size, thus producing glass beads of a uniform size.
Nixon, E. K., Runnels, R. T., and Kulstad, R. O., 1950, The Cheyenne Sandstone of Barber, Comanche, and Kiowa counties, Kansas, as raw material for glass manufacture: Kansas Geological Survey, Bulletin 86, pt. 3, 44 p. [available online]
Kansas Geological Survey, Short Papers on Research in 1967
Placed on web Aug. 16, 2011; originally published in April 1968.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/191_1B/index.html