
Kansas Geological Survey, Bulletin 180, part 2, originally published in 1966

Originally published in 1966 as Kansas Geological Survey Bulletin 180, part 2. This is, in general, the original text as published. The information has not been updated.
Chemical and physical examination of the chert-free portions of the Keokuk Limestone (Mississippian) in Cherokee County, Kansas, showed it to be a high purity (mean 98.31% CaCO3), low silica (mean 0.24%), low iron (mean 0.12%) limestone of commercial quality. It is estimated that about 1 1/4 million tons of the high-calcium limestone are present. Analyses of the limestone are included. Loss on ignition rate was shown to increase when the samples were calcined under a nitrogen atmosphere.
Mississippian rocks are found at the surface in Kansas only in a small area of less than two townships in Cherokee County in extreme Southeastern Kansas (Fig. 1). Here the Keokuk Limestone of Osagian age is a bluish-gray to gray, sometimes buff, medium- to coarse-grained, fossiliferous limestone ranging from 10 to 100 feet in thickness. Fossiliferous chert is irregularly distributed throughout the Keokuk. Within the Keokuk chert-free beds occur, ranging in thickness from 10 to 50 feet.
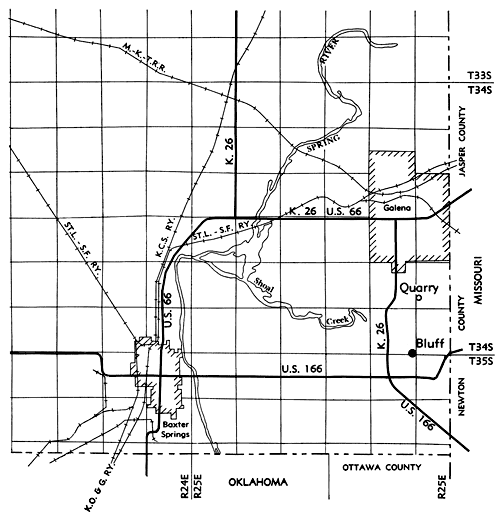
Figure 1--Map showing sample locations, Cherokee County, Kansas. (Based upon General Highway Map, Cherokee County, Kansas, 1963 ed.)
Analyses of chert-free strata in the Keokuk Limestone have shown that the calcium carbonate content is as high as 97 to 98 percent (Runnels, 1951; Runnels and Schleicher, 1956). An extension of these studies seemed warranted because of considerable interest in both the occurrence and use of high-calcium limestone. Nine spot samples and eight channel samples were collected for analysis.
W. E. Hill, Jr., and A. L. Hornbaker aided in sample location and collection. George Shimer made some of the chemical analyses.
Nine spot samples were taken from an outcrop of the chert-free beds in the Keokuk Limestone along a northeastward flowing creek (Fig. 1):
| KGS Sample Number | Location |
|---|---|
| 65146 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65147 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65148 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65149 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65150 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65151 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65152 | SE SE SE Sec. 35, T 34 S, R 25 E |
| 65153 | NW NW NW Sec. 1, T 35 S, R 25 E |
| 65154 | SE NW NW Sec. 2, T 35 S, R 25 E |
Samples 65146 through 65152 were taken at a vertical spacing of 4 to 5 feet along the outcrop. These samples were found to have an exceptionally high calcium carbonate content of 97 to 99 percent (Table 1).
In order to assure good sample representation and to provide a check of the chemical composition of the spot samples 10 channel samples were collected. Of these 10 samples, five were taken at each of two locations. The first location was a bluff cut by a small unnamed northward-flowing creek through one of the chert-free beds (Fig. 1):
| KGS Sample Number | Location |
|---|---|
| 661 | NE NE NE Sec. 2, T 35 S, R 25 E |
| 662 | NE NE NE Sec. 2, T 35 S, R 25 E |
| 663 | NE NE NE Sec. 2, T 35 S, R 25 E |
| 664 | NE NE NE Sec. 2, T 35 S, R 25 E |
| 665 | NE NE NE Sec. 2, T 35 S, R 25 E |
An exposed portion 28.2 feet thick was sampled. The first 5-foot channel sample (661) was taken vertically beginning at the top of a chert-bearing limestone in the Keokuk lying approximately 2 feet above the creek bed. Four 5.8-foot channel samples (662-665) were taken above it.
The second sample collection site was an underground quarry (Fig. 1):
| KGS Sample Number | Location |
|---|---|
| 666 | SW SW SW Sec. 25, T 34 S, R 25 E |
| 667 | SW SW SW Sec. 25, T 34 S, R 25 E |
| 668 | SW SW SW Sec. 25, T 34 S, R 25 E |
| 669 | SW SW SW Sec. 25, T 34 S, R 25 E |
| 6610 | SW SW SW Sec. 25, T 34 S, R 25 E |
Four 5-foot channel samples were taken in the underground quarry, one (666) in the chert-bearing limestone and three (667-669) above it. An additional 5-foot channel sample (6610) was taken on the outside of the underground quarry. Channel sampling was done according to the procedure used by Galle (1964).
Table 1--Chemical composition of high-calcium chert-free beds in the Keokuk Limestone, Cherokee County, Kansas.
| KGS sample number |
CaO | MgO | CaCO3 (Calc.) |
MgCO3 (Calc.) |
SiO2 | Al2O3 | Fe2O3 | TiO2 | P2O5 | SO3 | S | K2O | NaO | LOI 550 |
LOI 550/1000 |
Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 65146 | 54.96 | 0.26 | 98.09 | 0.57 | 0.30 | 0.12 | 0.15 | 0.02 | TR | TR | TR | TR | 0.01 | 0.30 | 43.43 | 99.55 |
| 65147 | 55.26 | 0.37 | 98.63 | 0.34 | 0.17 | 0.04 | 0.11 | 0.02 | TR | Nil | Nil | TR | 0.01 | 0.14 | 43.55 | 99.67 |
| 65148 | 55.38 | 0.39 | 98.84 | 0.21 | 0.16 | 0.03 | 0.10 | 0.04 | TR | TR | Nil | TR | 0.01 | 0.18 | 43.57 | 99.86 |
| 65149 | 55.33 | 0.38 | 98.75 | 0.08 | 0.27 | 0.06 | 0.13 | 0.03 | TR | TR | 0.01 | TR | 0.01 | 0.25 | 43.46 | 99.92 |
| 65150 | 55.39 | 0.29 | 98.86 | 0.32 | 0.10 | 0.02 | 0.14 | 0.01 | TR | TR | Nil | TR | 0.01 | 0.17 | 43.64 | 99.77 |
| 65151 | 55.17 | 0.19 | 98.47 | 0.36 | 0.22 | 0.05 | 0.12 | 0.02 | TR | TR | <0.01 | TR | 0.01 | 0.24 | 43.49 | 99.51 |
| 65152 | 55.13 | 0.32 | 98.40 | 0.50 | 0.19 | 0.03 | 0.12 | 0.01 | TR | Nil | 0.01 | TR | 0.01 | 0.20 | 43.52 | 99.53 |
| 65153 | 55.26 | 0.25 | 98.63 | 0.33 | 0.14 | Nil | 0.10 | 0.03 | TR | TR | Nil | TR | 0.01 | 0.17 | 43.54 | 99.50 |
| 65154 | 54.83 | 0.33 | 97.86 | 0.54 | 0.51 | 0.12 | 0.15 | 0.04 | TR | TR | <0.01 | TR | 0.01 | 0.32 | 43.31 | 99.62 |
| 661 | 55.13 | 0.41 | 98.40 | 0.34 | 0.10 | 0.09 | 0.08 | Nil | 0.01 | TR | 0.01 | Nil | Nil | 0.31 | 43.44 | 99.57 |
| 662 | 55.25 | 0.42 | 98.61 | 0.08 | 0.13 | 0.11 | 0.08 | Nil | TR | TR | 0.02 | TR | TR | 0.33 | 43.40 | 99.72 |
| 663 | 54.97 | 0.21 | 98.11 | 0.21 | 0.33 | 0.15 | 0.16 | Nil | TR | TR | 0.02 | TR | TR | 0.43 | 43.25 | 99.50 |
| 664 | 55.02 | 0.37 | 98.20 | 0.52 | 0.17 | 0.11 | 0.11 | Nil | TR | TR | 0.01 | TR | TR | 0.25 | 43.45 | 99.48 |
| 665 | 55.12 | 0.17 | 98.38 | 0.17 | 0.23 | 0.09 | 0.14 | 0.01 | TR | TR | 0.01 | TR | TR | 0.35 | 43.35 | 99.46 |
| 666* | 44.04* | 0.28 | 78.60 | 0.61 | 19.83* | 0.06 | 0.13 | 0.05 | 0.02 | 0.05 | 0.01 | 0.03 | 0.01 | 0.25 | 34.83* | 99.58 |
| 667 | 55.05 | 0.30 | 98.25 | 0.69 | 0.36 | 0.03 | 0.13 | 0.04 | 0.01 | 0.01 | 0.01 | 0.05 | 0.01 | 0.40 | 43.55 | 99.94 |
| 668 | 54.88 | 0.34 | 97.95 | 0.80 | 0.29 | 0.04 | 0.13 | 0.05 | 0.01 | 0.02 | 0.02 | 0.08 | 0.02 | 0.46 | 43.47 | 99.79 |
| 669 | 54.53 | 0.43 | 97.33 | 1.99 | 0.42 | 0.05 | 0.13 | 0.05 | 0.01 | 0.05 | 0.01 | 0.08 | 0.02 | 0.44 | 43.39 | 99.60 |
| 6610* | 53.28* | 0.28 | 95.09 | 1.17 | 2.44* | 0.12 | 0.18 | 0.05 | 0.02 | 0.01 | TR | 0.15 | 0.02 | 0.51 | 42.39 | 99.45 |
| 52137† | 54.79 | 0.21 | 97.79 | 1.04 | 0.27 | 0.07 | 0.10 | 0.04 | 0.01 | TR | 0.01 | 0.15 | 0.02 | 0.33 | 43.57 | 99.56 |
| Mean | 55.08 | 0.31 | 98.31 | 0.54 | 0.24 | 0.07 | 0.12 | .02 | 0.30 | 43.41 | ||||||
| S | 0.23 | 0.11 | 0.03 | |||||||||||||
| * Samples 666 and 6610 exceeded the limits of three standard deviations and were excluded in the calculations for CaO and SiO2. † KGS sample 52137 (NE Sec. 26, T 34 S, R 25 E) described by Runnels and Schleicher, 1956, rerun and included for purposes of comparison. |
||||||||||||||||
The samples were prepared for analysis by crushing in a mechanical law crusher, followed by quartering and splitting until a sample size of approximately 100 g was obtained. They were further ground by mortar and pestle until each sample passed a 60-mesh screen (250 µ). The sieved samples were rolled and blended until thoroughly mixed. They were then split to approximately 20 g for analysis. Chemical analyses were carried out as described by Hill, et al. (1961).
Samples 661 thru 6610 were assumed to be most representative owing to the channel sampling technique used. The standard deviation of the CaO in these samples was determined (Table 1). Similarly, using the CaO values of the spot samples (65146-65154), a second standard deviation was calculated and found to be essentially the same as that of the channel samples. A Student t test supports the assumption that all the samples were from the same population.
Since the CaO values of the spot samples were within one standard deviation of the CaO values for the channel samples, a more inclusive standard deviation was calculated using the values of CaO for spot and channel samples, excluding samples 666 and 6610. The accepted formula used for standard deviation was:
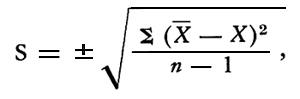
where:
S = standard deviation,
X = sample determination,
![]() = mean of sample determinations,
= mean of sample determinations,
n = number of determinations.
The standard deviation for these samples was found to be 0.23 for CaO. The mean CaO content was 55.08, which is equivalent to 98.31 percent CaCO3. Values from 54.85 to 55.31 percent CaO encompassed all data within one standard deviation.
In order to obtain a representative figure for the two most common impurities, the standard deviation was also figured for silica (SiO2) and iron (Fe2O3). The mean value for SiO2 was 0.24 percent, with a standard deviation of 0.11. The low silica value of the chert-free beds in the Keokuk Limestone suggests their possible use in many industrial processes (e.g., as blast furnace flux).
In determining the standard deviation for Fe2O3, samples 666 and 6610 were included (Table 1). The mean was 0.12 percent, and the standard deviation 0.03. The other constituents of this limestone make up less than 0.5 percent (Table 1) and were not treated statistically.
As part of the study of physical properties of the chert-free limestone in the Keokuk, loss of weight on ignition (LOI) was determined under three different atmospheric conditions: in carbon dioxide, in air, and in nitrogen. A set of three samples (663, 665, and 667) was chosen for the LOI study. These were heated and held at 50°C for intervals of 1 hour, cooled, and weighed. The temperature range was from 500-1000°C. The resulting percent loss of weight on ignition was summed and graphed against temperature (Fig. 2).
Figure 2--Rate of loss of weight on ignition curves for high-calcium chert-free samples from the Keokuk Limestone and for analytical-grade calcium carbonate. A, CO2-enriched atmosphere; B, CO2-air; C, N2. Loss on ignition rate increased progressively from A to B to C.
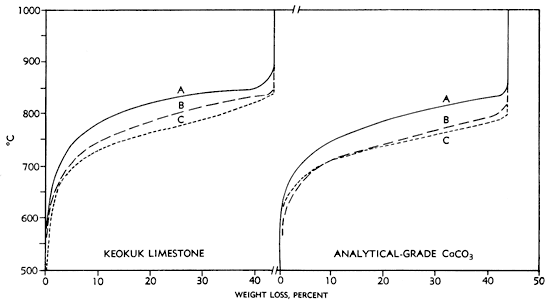
Curve A (Fig. 2) represents the rate of LOI of the chert-free samples of Keokuk Limestone where the samples were heated in a muffle furnace in closed platinum crucibles. In this system the convection volume was reduced to that bounded by the sides and lid of the platinum crucible and the surface of the sample (no more than 17 cc). Since there would have been little dilution of the carbon dioxide (CO2) evolved with air, the sample was essentially in a CO2-enriched atmosphere--the most favorable condition for recarbonation and the poorest for rapid evolution of gas and subsequent loss of weight.
Curve B represents a set of samples heated without lids in a closed muffle furnace in an airCO2 atmosphere. There was a marked increase in the rate of CO2 evolution. The volume which previously was restricted to the platinum crucible was in this case expanded to include the volume of a proportionate part of the inside of the muffle furnace. This procedure allowed a more rapid loss of CO2 and a consequent increase in the rate of weight loss.
Curve C represents the results obtained when a constant flow of nitrogen (N2) was passed through the muffle furnace and over a set of samples in uncovered platinum crucibles. This technique gave the greatest CO2 evolution rate. Sweeping the surface of the limestone with N2 rapidly removes the CO2 and prevents recarbonation as noted by Boynton (1966, p. 140). Rate of loss on ignition of analytical-grade calcium carbonate is shown for purposes of comparison.
These data clearly indicate that if maximum loss of weight on ignition is desired at any given temperature for any given time increment (here 1 hour) then consideration of the use of N2 or some other inert sweeping gas is indicated. Further investigation of the effects of an N2 atmosphere on the rate of weight loss of the limestone seems warranted. Additional study will be necessary before the mechanics acting to produce these effects are fully known.
The Keokuk Limestone in Cherokee County in southeastern Kansas is a limestone of unusually high calcium content. The amounts of chert-free limestone available in this part of the Keokuk Limestone are estimated to be of the order of 1-1 1/4 million tons based upon a 20-foot thickness (personal communication, Allison Hornbaker, 1966). Approximately 20 acres are available for open pit quarry operation. Exploratory drilling will be necessary in order to outline the areal extent of high quality limestone. The chert-free limestone studied here is within 4 miles of rail transportation available in the north part of Galena (Fig. 1). It is 6 miles from Baxter Springs, Kansas, and 13 miles from Joplin, Missouri.
Boynton, R. S., 1966, Chemistry and Technology of Lime and Limestone: 520 p., Interscience Publishers, John Wiley & Sons, N.Y.
Galle, O. K., 1964, Comparison of chemical analyses based upon two sampling procedures and two sample preparation methods: Trans. Kansas Acad. Sci., v. 67, no. 1, p. 100- 110.
Hill, W. E., Jr., Waugh, W. N., Galle, O. K., and Runnels, R. T., 1961, Methods of chemical analysis for carbonate and silicate rocks: Kansas Geol. Surv., Bull. 152, pt. 1, p. 1-30.
Moore, R. C., 1928, Early Mississippian formations in Missouri: Missouri Bur. Geol. and Mines, v. 21, 2d. ser., p. 1-283.Moore, R. C., Frye, J. C., Jewett, J. M., Lee, Wallace, and O'Connor, H. G., 1951, The Kansas Rock Column: Kansas Geol. Surv., Bull. 89, 132 p. [available online]
Runnels, R. T., 1951, Some high-calcium limestones in Kansas: Kansas Geol. Surv., Bull. 90, pt. 5, p. 77-104. [available online]
Runnels, R. T., and Schleicher, J. A., 1956, Chemical compositions of Eastern Kansas Limestones: Kansas Geol. Surv., Bull. 119, pt. 3, p. 81-103 [available online]
Kansas Geological Survey, Analyses of High-Calcium Chert-Free Beds in the Keokuk Limestone, Cherokee County, Kansas
Placed on web June 30, 2010; originally published in Nov. 1966.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/180_2/index.html