
Kansas Geological Survey, Bulletin 109, pt. 1, originally published in 1954

Originally published in 1960 as Kansas Geological Survey Bulletin 109, pt. 1. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (2.4 MB) is also available.
Cellular products made from foamed materials exhibiting pozzolanic action offer the building industry an attractive solution to problems involving stable, lightweight, durable, attractive, and fireproof structural shapes. A wide variety of Kansas raw materials is available to a manufacturer of such products, Three methods of introducing a foam have been generally used: whipping a foam in a suitable slurry, generating gases in the mix, and mixing a slurry and a pre-formed. foam. Subsequent autoclaving is usually employed where structural strengths are desired in the products.
Production and materials costs are in line with other comparable constructional materials presently available.
Figure 1—Diagram illustrating the steps necessary to produce cellulated shapes from raw materials.
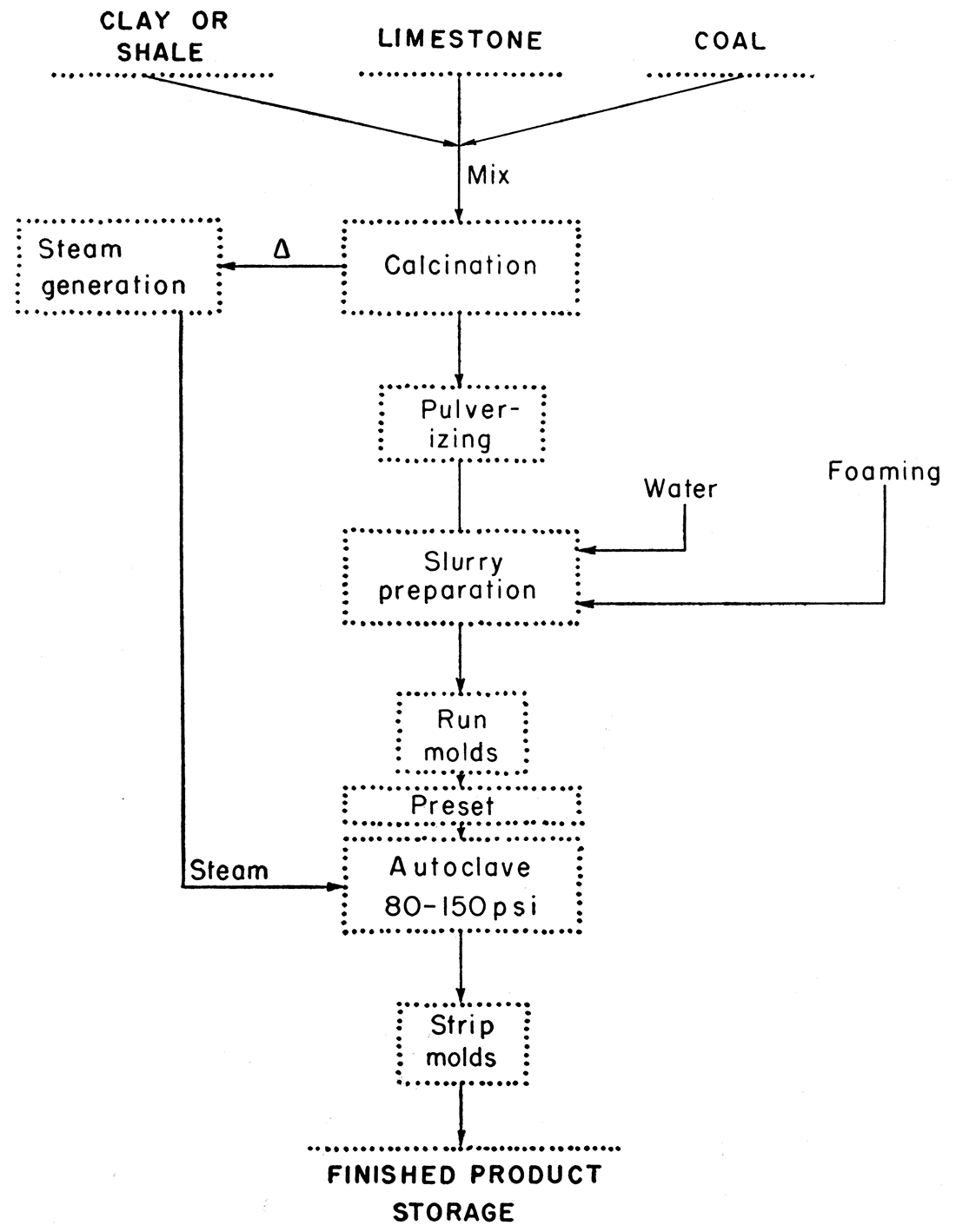
The concept of using pozzolanic materials, substantially set with cellular structures, to form lightweight structural shapes is by no means new, as a review of literature (Anderegg, 1936, 1948; Anon., 1951, 1953; Dilnot, 1952) and patents (U.S. Patent 1,932,971, Oct. 31, 1933; U.S. Patent 2,442,519, June 1, 1948; British Patent 522,271, June 26, 1940; Canadian Patent 370,989, Jan. 3, 1938) reveals. The use of such materials has been developed rather extensively in some European countries, notably Sweden (Anderegg, 1947) and England. Recently definite plans have been evolved to produce structural shapes in the United States.
Basically, structural shapes made from cement-like materials that develop setting and bonding due to pozzolanic action and wherein lightness of weight is achieved by entrapping a multitude of cells offer certain attractive features. In addition to lightness of weight, structural strengths can be obtained by autoclaving, shapes may be reinforced with steel, their thermal conductivities are very low, the physical appearance is attractive, and a wide variety of materials can be employed.
Most of the raw material suitable for use in foamed concrete is abundantly available in Kansas. These include Portland cement, pulverized lightweight aggregates and stack dust from lightweight aggregate plants (Plummer and Hladik, 1951), fly ash, volcanic ash (Carey and others, 1952) , limestone (Runnels, 1951), coal mine refuse, and fine silica sand (Nixon, Runnels, and Kulstad, 1950; Rose, 1950). This investigation was undertaken with the purpose of determining the relative merits of various foaming materials and foaming techniques in mixes containing raw materials available in Kansas and from other sources.
In general there are three basic methods for incorporating a cell structure in a slurry of cementitious materials: (1) the slurry with or without additives can be suitably stirred or whipped in order to entrain air bubbles, (2) certain materials can be added to the slurry which generate gases causing bubble formation, and (3) a foam may be pre-generated and then mixed with the slurry (Abbot, 1952). All these methods have been tried by experimenters with varying degrees of success. In European practice much material has been foamed by the addition to slurries of a special aluminum powder which generates gas, producing a multitude of small bubbles and causing the mass to swell. Recently with the introduction of certain hydrolized proteins it is possible to produce very stable foams that lend themselves admirably to the method of whipping a cell structure in a cementitious slurry or pre-generating a foam and subsequently mixing with a slurry. The latter process has been used with considerable success in England; it has the distinct advantage of being easily controlled so that the final product characteristics can be determined in advance and corrections made as the process is in operation. Furthermore, the foamed slurry is pumpable.
Generally speaking the autoclaved products from foamed slurries can be divided into three classes:
The thermal conductivities (K factor) of these products range from 1.1 for the 40 to 45 pound per cubic foot material to 0.56 for. the 20 pound per cubic foot material. Where low moisture penetration and good freezing resistance are desired, tests indicate these can be achieved by proper cell formation.
Proper autoclaving of structural shapes (Anon., 1953a) substantially reduces the reversible expansion and shrinkage due to wetting and drying as compared to unautoclaved products. This reduction can amount to one-half to two-thirds of the dimensional changes encountered in unautoclaved products.
Tests indicate that it is possible to produce unautoclaved material in the 15 to 20 pounds per cubic foot range with strengths of 75 to 150 psi and K factors of 0.50 to 0.60, which is entirely adequate for many uses.
Some of the materials that can be employed for foaming include Portland cement, hydrated lime, pulverized sand or flint, crushed and calcined pozzolanic shales, crushed cinders, fly ash, coal mine refuse, volcanic ash, lightweight aggregates, and pulverized chat. Tests indicate which materials alone, or in combination, will give satisfactory results.
Obviously, most if not all these materials are quite readily available in Kansas. Several preliminary tests performed on some combinations of these materials are described in the following discussions. Inasmuch as this investigation was somewhat exploratory, the majority of the results reported are qualitative rather than quantitative and as such are intended to point up lines for future detailed work.
For the first series of tests mixtures of limestone and coal washing refuse were calcined, ball milled until all passed a 60-mesh screen, made into a neat cement paste, and cast in small cylinders. The limestone had been crushed until all was less than one-fourth inch; the waste was used as received. Table 1 shows mixtures of refuse and limestone tried. Each mix was calcined at 1700° F., 1900° F., and 2100° F.
Table 1—Preliminary limestone and refuse mixtures calcined for use in series 1 tests
| Mix no. |
Limestone, percent |
Coal refuse, percent |
|---|---|---|
| 1 | 80 | 20 |
| 2 | 60 | 40 |
| 3 | 40 | 60 |
| 4 | 20 | 80 |
| 5 | 50 | 50 |
| 6 | 0 | 100 |
| 7 | 30 | 70 |
From observation of the apparent hardness of cast cylinders, mix 2 was most pozzolanic. The range of temperatures chosen gave no appreciable differences in the degree of set. The 50-50 mix (mix 5) was selected for more detailed tests.
Mix 5 was calcined at 1900° F., ground, made into a neat paste, and autoclaved at 15 psi. After initial set, it was a hard dense mass which showed no signs of cracking.
In another test mix 5 was pulverized in a ball mill using 2,225 grams of mix, 3 grams of Santomerse No.1 (foaming agent), and 1,000 cc water. This slurry, in which a good cell structure of very fine cells had developed, was then run into a mold. This mass when autoclaved at 15 psi had very little strength. Autoclaving at 110 psi for 8 hours, however, resulted in quite good strengths.
Mix 5 used in the proportions of 2,225 grams of mix, 45 grams of Portland cement, 225 grams of minus 28-mesh fired clay aggregate, and 1,000 cc water, when autoclaved at 100 psi for 8 hours, developed fair strength.
It was apparent from this series of tests that suitable mixtures and techniques could be combined with the net result that a strong, lightweight, inert material could be produced. The foaming agent Santomerse would have performed better with the addition of a stabilizing agent, although it did fairly well alone. Higher and longer application of autoclaving pressures also undoubtedly would have helped to produce better strengths. The amount of fuel that still remains in the coal washing waste would be of considerable advantage where it might be desirable to calcine mixes on sintering machines—in many cases little or no additional fuel would be required.
In the course of some experiments with a batch-type sintering grate the mixtures listed in Table 2 were sintered, using 10 percent by weight of a semi-anthracite coal. After sintering, these materials were ball milled until 100 percent passed an 80-mesh screen. Using a high-speed stirrer, the ball-milled material was thoroughly mixed with water to a moderately thick slurry (approx. 125 cc water per 100 grams dry material) after which 0.5 percent aluminum powder was added and stirred thoroughly; then the slurry was run into molds. After setting 2 or 3 minutes the generated gas caused the mass to swell from 1 1/2 to 2 times its original volume. After presetting overnight the samples were autoclaved at 100 psi for 6 hours. All these mixes produced fairly sound, moderately strong cellulated products. The cell shape was definitely directional.
Table 2—Preliminary mixtures of limestone, loess, and shale sintered for use in series 2 tests. [Note: 10 percent by weight of semi-anthracite coal was added before sintering.]
| Mix no. |
Limestone, percent |
Loess, percent |
Shale, percent |
|---|---|---|---|
| 1 | 40 | 40 | 20 |
| 2 | 50 | 30 | 20 |
| 3 | 40 | 20 | 40 |
| 4 | 50 | 20 | 30 |
Several mixtures containing volcanic ash, hydrated lime and/or high early cement were cellulated with aluminum powder. In general these mixes, while developing a cell structure, were inclined to be too soft and friable, even when autoclaved, to show promise as a structural material. One such mix consisting of 20 percent volcanic ash, 30 percent pulverized flint, 20 percent high early strength cement, and 30 percent hydrated lime, when cellulated with 0.5 percent aluminum powder and autoclaved at 100 psi for 6 hours, had a compressive strength of only approximately 100 psi. It should be noted that the cellular structure of this mix was very coarse.
In general, the mixes in which aluminum powder was used to produce a cellular structure were characterized by large irregular cells, directional in shape. Also it was noted that in order to use aluminum powder successfully, very close control of the various operations would be required.
A series of mixes were investigated in connection with a pre-foaming technique. This method requires a generated foam and a cementitious slurry prepared separately. Then these two are mixed and the entire mix run into molds. In general this was a very satisfactory method for producing a cellulated mass.
Table 3—Slurry batches used in tests with pre-generated foam
| Mix no. |
High early Portland cement |
Volcanic ash |
Pulverized silica |
Hydrated lime |
|---|---|---|---|---|
| 1 | 40 | 30 | 30 | |
| 2 | 50 | 50 | ||
| 3 | 60 | 10 | 30 | |
| 4 | 60 | 40 | ||
| 5 | 80 | 20 | ||
| 6 | 50 | 50 |
Two foaming ingredients were tried, Armac 12-D, a fatty amine acetate (90 percent dodecylamine acetate) and Mearl-crete P, which is of the hydrolized protein type. In each case the foam was generated with a high-speed stirrer.
In the test employing Armac 12-D the foam-producing material had the following composition:
| Armac 12-D | 5 parts |
| Water | 100 parts |
| Plaster of Paris | 30 parts |
This gave a foam that was stable enough for later mixing.
Using a slurry batch that consisted of mix 1 shown in Table 3 prepared with 55 percent water, based on total weight of the dry material, increasing amounts of foam were added. Properties of the product after autoclaving for 6 hours at 100 psi are shown in Table 4.
Table 4—Properties of foamed material prepared by adding varying amounts of Armac 12-D foam to mix 1 shown in Table 3
| Mix no. |
Ratio, foam to slurry |
Oven-dry weight, pounds per cubic foot |
Compressive load, pounds per square inch* |
|---|---|---|---|
| 0 | 0 | 81.5 | 4,280 |
| 1 | 1-100 | 69.0 | 1,510 |
| 2 | 2-100 | 61.1 | 3,430 |
| 3 | 3-100 | 60.0 | 2,100 |
| 4 | 4-100 | 52.5 | 500 |
| 5 | 5-100 | 52.0 | 400 |
| 6 | 6-100 | 45.0 | 250 |
| * One specimen, approximately 2 by 2 by 2 inch cubes. | |||
The same foam composition was tried with several other combinations of Portland cement, volcanic ash and/or pulverized silica as well as with a hydrated lime and silica combination (Table 3, mixes 2-6). A thick slurry was made from each by adding water. To each 100 grams of dry materials in these mixes 135 grams of the foam composition was added. In this case the foam composition consisted of 5 grams Armac 12-D, 100 grams water, and 30 grams plaster of Paris. The combination of materials was poured into forms; after the initial set the shapes were removed from the forms and autoclaved at 100 psi for 6 hours. The properties of shapes made from the various mixes are given in Table 5.
Table 5—Properties of shapes made from mixes 2-6 listed in Table 3 after autoclaving
| Mix no. |
Oven-dry weight, pounds per cubic foot |
Compressive load, pounds per square inch* |
|---|---|---|
| 2 | 33.3 | 216 |
| 3 | 36.5 | 486 |
| 4 | 39.0 | 421 |
| 5 | 41.8 | 710 |
| 6 | 28.9 | 347 |
| * One specimen. a 2 by 2 by 2 inch cube. | ||
Mearl-crete P, a dark-brown liquid, is a complete foaming agent requiring no further additions. The mixes listed in Table 6 were recommended by the manufacturer of Mearl-crete P. The water and dry ingredients were mixed thoroughly; then the foaming agent was added and the entire mix stirred vigorously for another 3 minutes. The resulting slurry was run into molds, allowed to preset, and the shapes autoclaved 6 hours at 100 psi. Properties of the processed shapes are given in Table 7. Mixes 1 and 2 were excellent, 3 was only fair, and 4 and 5 were poor from a working viewpoint. Mixes 4 and 5 exhibited bad slumping characteristics before preset.
Table 6—Raw material mixes recommended by the manufacturer of Mearl-crete P, a foaming agent
| Mix no. |
Water, cc |
High early strength Portland cement, grams |
Ground Kaw River -35 mesh sand, grams |
Pulverized silica, grams |
Mearl-crete P foaming agent, cc |
|---|---|---|---|---|---|
| 1 | 151 | 218 | 2.4 | ||
| 2 | 150 | 130 | 86 | 2.5 | |
| 3 | 100 | 130 | 86 | 2.5 | |
| 4 | 80 | 109 | 109 | 2.5 | |
| 5 | 80 | 86 | 130 | 2.5 |
Table 7—Properties of shapes made from mixes listed in Table 6 after autoclaving
| Mix in Table 6 |
Oven-dry weight, pounds per cubic foot |
Compressive load, pounds per square inch |
|---|---|---|
| 1 | 41.9 | 805 |
| 2 | 40.5 | 967 |
| 3 | 55.0 | 637 |
| 4 | 45.0 | 342 |
| 5 | 33.6 | 258 |
Tests of foamed concrete in which the foaming agent was included in the mix indicate that good results can be obtained using an agent like Santomerse. Concrete foamed with aluminum powder, however, was weak and the cell structure was not good, indicating that very close control of the process would be necessary.
In general, tests in which the pre-foaming technique was used yielded better results than those in which the foaming agent was included in the batch. The superiority of the pre-foaming technique was evident in the better and more controllable cell structure, controllable densities, and greater ease in the preparation of batches and the molding of shapes.
Although the primary aim of this investigation was the testing of various foaming techniques, the tests demonstrated that a number of materials readily available in Kansas, such as Portland cement, pulverized lightweight aggregate, fly ash, volcanic ash, limestone, coal mine refuse, and fine silica sand, are suitable for use in foamed concrete.
The role of foamed cementitious materials in conjunction with precast shapes and autoclaving has been demonstrated as being a very useful one; European practice has demonstrated the practicability of such a combination. Such precast shapes include structural and insulating blocks; floor, wall, and roof slabs; beams; and lintels. It is to be noted that reinforcing steel can be incorporated where necessary.
Foamed autoclaved cementitious materials offer the building industry a solution to many of its problems. Several advantages that can be realized from the use of these materials are (1) the raw materials are widely available; (2) processing is relatively simple; (3) the products from such materials exhibit lightness in weight, adequate strength, high thermal insulation, fire proofness, high resistance to moisture penetration and frost action, and extremely low wetting and drying movement, and they can be cut, sawed, drilled, screwed, or nailed; (4) they are a good plastering material; and (5) they are highly inert to normal agencies.
Reported data show that the costs of the raw material prepared for foam additions falls in the range of 7 to 12 cents per cubic foot of product, weighing 40 to 45 pounds per cubic foot. Foaming agent will add another 2 to 5 cents. Direct labor will add another 12 to 15 cents per cubic foot of product. It has been reported that a highly mechanized plant is capable of producing a unit 4 by 8 by 16 inches for a total cost, including overhead, of approximately 6.5 cents which is equivalent to a cost of 21.9 cents per cubic foot. The equivalent cost per cubic foot of 8 by 8 by 16 inch blocks, within the range of 14 to 20 cents per block, is shown in Table 8.
Table 8—Cost per cubic foot of aggregate at various prices per block
| 8 by 8 by 16 inch block cost, cents |
Equivalent ost per cubic foot, cents |
|---|---|
| 14 | 23.6 |
| 15 | 25.2 |
| 16 | 27.0 |
| 17 | 28.6 |
| 18 | 30.4 |
| 19 | 32.0 |
| 20 | 33.8 |
In the field of roof slabs and planks, foamed autoclaved cementitious silicates offer an ideal basic material. The market price for good roofing materials is in general higher than for structural blocks which would indicate that these materials might have their greatest initial usefulness in this field.
While the application of this type of foamed material has not been studied extensively for this particular. use, it has indications of being a good infill component of prefabricated walls.
The investment in a complete efficient plant to produce a finished product from raw material for an average operation would probably represent $2,000,000 to $3,000,000. If a manufacturer did not wish to produce a cementitious material but preferred only to make products, a satisfactory plant would require $400,000 to $700,000 for installing an efficient, economical operation. For the latter type of operation the autoclaves represent 60 to 80 percent of the total cost.
Abbott, J. A. (1952) How low-density solids are made: Chemical Engineering, vol. 59, no. 7, pp. 212-215, 401-404.
Anderegg, F. O. (1936) It's Microporite: Rock Products, vol. 39, no. 5, pp. 48-51.
Anderegg, F. O. (1947) Ytong, a Swedish lightweight building unit: Rock Products, voL 50, no. 8, p. 110.
Anderegg, F. O. (1948) Lightweight concretes; gas, foam and lime concretes: Rock Products, vol. 51, no. 11, p. 91.
Anonymous (1951) Cellular concrete: Architectural Record, vol. 110, no. 2, p. 159.
Anonymous (1953) Cellular concrete: Pit and Quarry, vol. 46, no. 1, pp. 259-260.
Anonymous (1953a) High pressure curing: Rock Products, vol. 56, no. 6, pp. 198- 199.
Carey, J. S., and others (1952) Kansas volcanic ash resources: Kansas Geol. Survey, Bull. 96, part 1, pp. 1-68. [available online]
Dilnot, S. (1952) Cellular calcium silicate products: Rock Products, vol. 55, no. 10, pp. 110-116.
Nixon, E. K., Runnels, R. T., and Kulstad, R. O. (1950) The Cheyenne sandstone of Barber, Comanche, and Kiowa Counties, Kansas, as raw material for glass manufacture: Kansas Geol. Survey, Bull. 86, pt. 3, pp. 41-84. [available online]
Plummer, Norman, and Hladik, W. B. (1951) Lightweight aggregate from Kansas clays and shales: Kansas Geol. Survey, Bull. 91, pp. 1-100.
Rose, K. E. (1950) Silica sand from south-central Kansas for foundry use: Kansas Geol. Survey, Bull. 86, pt. 4, pp. 85-104. [available online]
Runnels, R. T. (1951) Some high-calcium limestones in Kansas: Kansas GeoL Survey, Bull. 90, pt. 5, pp. 77-104. [available online]
Kansas Geological Survey
Placed on web Jan. 10, 2019; originally published March 1, 1954.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/109_1/index.html