
Petroleum: a primer for Kansas, Page 3 of 15
Prev Page--Introduction ||
Next Page--Layered rocks
Clastic rocks are those that are formed by the accumulation and cementation of sedimentary particles derived from weathered fragments of preexisting rocks. Weathering processes, such as freezing and thawing, rain, wind, and other similar events, break down the parent rock into small particles that can then be transported by wind and rain runoff. Streams carry the mud, sand, and gravel from the source area down to its final resting place, be that a stream channel, floodplain, lake, or ultimately the sea. There it accumulates, is buried and compacted by later-arriving sediments, and cemented to form sedimentary rocks. The mud compacts to shale or mudstone, the sands are cemented by silica or calcite to form sandstones, and the gravels become conglomerates. Sandstones, because of the inherent porosity between their grains, often become excellent reservoirs for oil or natural gas. In oil-field terminology, any potentially productive sandstone is called a "sand" (fig. 2a).
Figure 2a--A greatly magnified image of a sandstone as seen in a thin section of the rock under the microscope. The scale is equal to one millimeter. The rock sample was injected with blue-colored epoxy that is seen here filling pores which are interconnected (permeable). After plastic is injected and solidified, the rock sample is cut and polished on a glass slide to a thickness of 35 thousandths-of-an-inch. The "thin section" of the rock is thin enough to permit light to be transmitted through it as in this photomicrograph.
This particular sandstone contains grains of quartz (white), calcite, and feldspar (shades as brown). The grains originally came from other rocks that had been eroded. The sample is exceedingly porous and permeable. Thegrains are loosely packed and there is very little cement filling the space between the grains. The arrow indicates possible pathways for fluid movement.

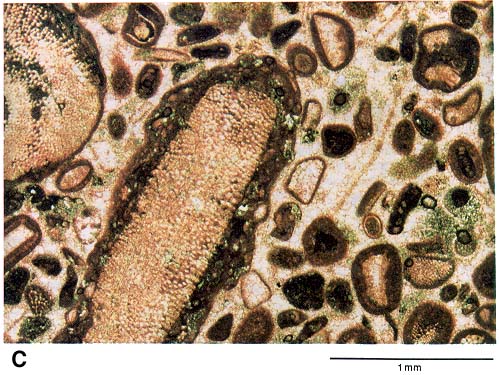
Figure 2b--A thin-section photomicrograph of a Pennsylvanian limestone taken from a core sample of a producing zone in Victory field, haskell County, Kansas. This particular sample comes from an interval that is not a good reservoir rock. Circular grains composed of calcite (finely crystalline, reddish-stained areas in a grain) and dolomite (clear, coarse crystals) are completely cemented by medium crystalline calcite. No porosity is visible.
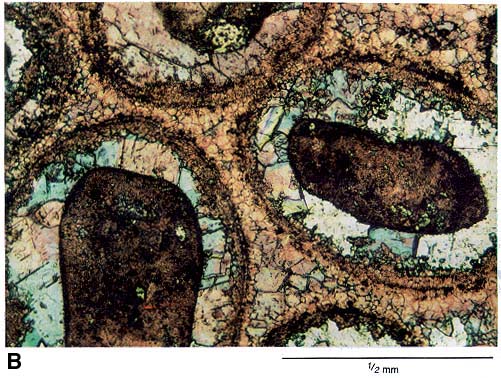
Figure 2c--A thin-section photomicrograph of a Pennsylvanian limestone. The scale bar equals one millimeter. These well-rounded carbinate grains consist of broken shells and other skeletal remains of marine organisms. The rounding is produced by coatings of dense, dark, finely crystalline calcite which formed rims around the particles as they were agitatated by current action in a shallow marine setting. The rounded particles are completely cemented together with finely crystalline calcium carbonate (calcite), and consequently no porosity is present.
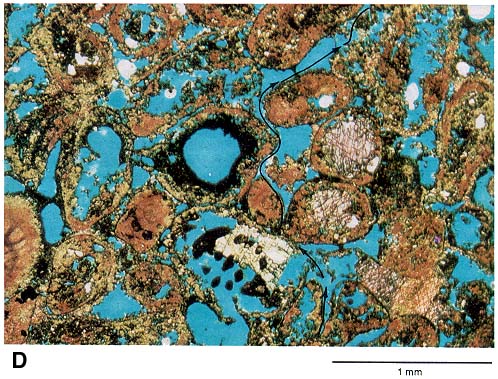
Limestone is composed of calcium carbonate (calcite or aragonite), thus the general rock term "carbonate" is used. Magnesium, a common element in seawater, can replace some of the calcium within the crystal structure of calcite. This often happens by various processes that are not fully understood; the resulting rock is known as "dolomite" (calcium-magnesium carbonate). The process of changing limestone to dolomite produces somewhat smaller crystals, so the resulting rock has tiny pores between the new crystals. This kind of porosity, much like that in sandstone, often contains oil and natural gas (fig. 2d).
Figure 2d--This thin-section photomicrograph came from a sample of a core taken 1 ft (.3 m) below the one shown in figure 2b. The scale bar equals one millimeter. This sample is representative of an excellent carbonate reservoir rock with pore space contributing nearly one-third of the total volume of the rock. In fact, this zone is one of the main reservoirs of Victory field. The carbonate grains artificially stained red on this thin section are poorly preserved remains of coated fossil fragments. Permeable porosity (indicated again by the blue epoxy) occurs within these particles as well as between them. The arrows trace possible avenues of migration for fluid such as oil and gas that now are found in this reservoir rock.
Prev Page--Introduction || Next Page--Layered rocks