


Kansas Geological Survey, Open-file Report 88-39
Great Plains and Cedar Hills Aquifers--Page 17 of 25
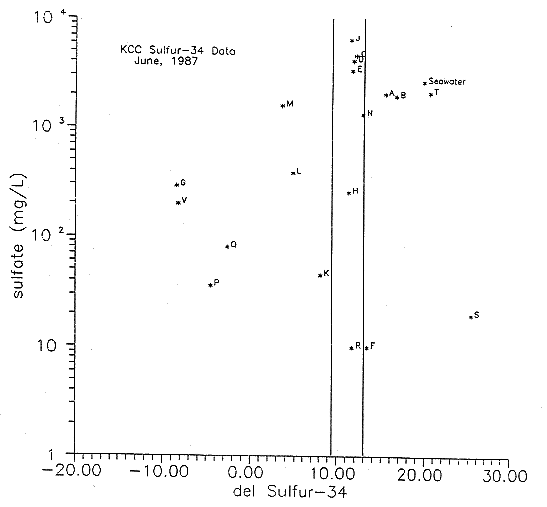
Figure 33. 34S versus Sulfate Concentration of Ground Waters and Oil-Field Brines from Study Area (see Table 4 for Labels).

Disseminated sulfide minerals or organic sulfur may be the source of the lower concentration of 34S in the various ground waters. Kinetic fractionation during sulfide oxidation by bacteria results in sulfate that is depleted in 34S (Pearson and Rightmire, 1980). In surface waters there is a tendency for sulfate derived from evaporite sources to be enriched in 34S while sulfate derived from the oxidation of organic sulfur or sulfide minerals will be depleted in 34S. Ground waters may be reducing enough that sulfide is the dominant ionic species. Fractionation between coexisting sulfate and sulfide in reducing environments in ground waters may result in values below the evaporite limit frequently seen in surface waters (delta 34S = +10 or greater) (Pearson and Rightmire, 1980; Thode and Monster, 1965).
Several of the waters collected for this study contained noticeable H2S smell both during collection and analysis. The occurrence of lignites and organic layers in the Great Plains aquifer suggests the presence of disseminated sulfide minerals such as pyrite in the sediments. The minerals may be a source of sulfide that could be oxidized by oxygen-rich ground water or sulfide-oxidizing bacteria resulting in an overall decrease of the 34S content. We will be resampling the Upper Dakota well at the Hays North site, the Hill City site, and possibly at the Gorham site to determine if there was any problem due to time lag between sample collection and sample analysis that would result in the observed values.
Previous page--Oxygen and Deuterium Isotopes ||
Next page--Monitoring Wells
Start of Report ||
Report Contents