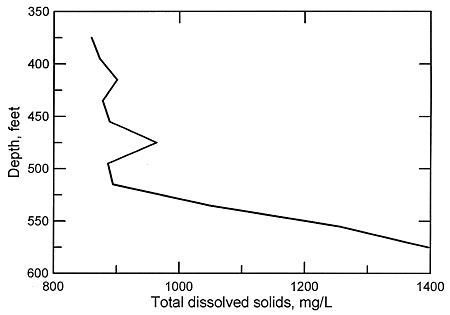
Figure 4. Depth profile of total dissolved solids concentrations in Dakota aquifer waters from a test hole in Ellis County.

The substantially greater permeability of the sandstone units within the Dakota aquifer in comparison with the shales can allow a faster rate of flushing of salinity by fresher regional flow. The general inverse correlation of the particle size of the Dakota sediments with TDS concentrations in areas where some salinity exists, primarily in the confined aquifer, means that substantial local differences can occur in both the vertical and areal distribution of water quality depending on the particular sandstone-to-shale ratio. Consequently, often the better the water-yielding characteristics of the aquifer, the better the water quality within a given area. In some locations where there is a thick sandstone body below low permeability rocks in the Dakota aquifer, the water can be fresher in the sandstone than in the overlying, less permeable units.
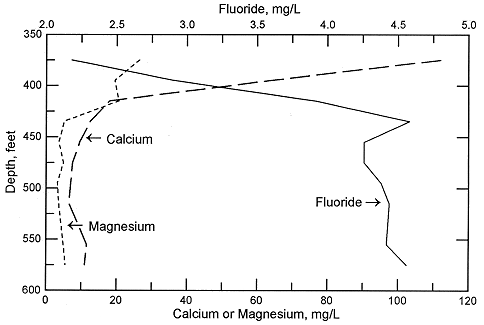
In the confined aquifer, recharge passing through the overlying units can have appreciably higher calcium, magnesium, and sulfate concentrations than in fresh to slightly saline portions of the upper Dakota aquifer. An example of the changes with depth in these dissolved constituents in the Dakota Formation is illustrated in Figures 5 and 6 for the same test hole in Ellis County as for Figure 4. The decrease in calcium and magnesium and the increase in sodium content caused by the softening process is marked in the upper part of the aquifer. The increase in dissolved sodium with depth is greater than that of chloride in the upper part of the formation, whereas the increase in chloride is greater towards the bottom (Figure 5). The fluoride concentration follows a pattern with depth opposite to that of the calcium because the low calcium concentration within the aquifer waters allows fluoride-containing calcium minerals to dissolve. Fluoride then decreases with depth at the bottom of the Dakota aquifer and into Permian strata where calcium concentrations are much greater in the saline water. Note the parallels of the constituent concentration changes with depth in Figures 5 and 6 to the changes along the cross section (Figures 2 and 3) from the recharge area in southwestern Kansas to the saline water in the confined aquifer in central Kansas.
Figure 5. Depth profile of sodium, chloride, and sulfate concentrations in Dakota aquifer waters from the same test hole in Ellis County as for Figure 4.
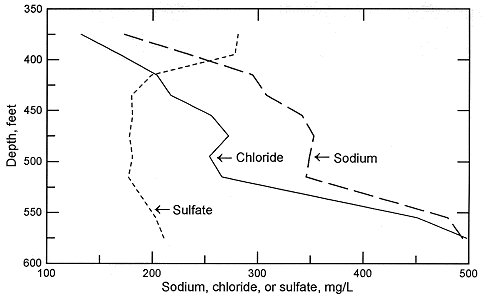
Figure 6. Depth profile of calcium, magnesium, and fluoride concentrations in Dakota aquifer waters from the same test hole in Ellis County as for Figure 4.
Previous Page--Regional Water-Quality Patterns ||
Next Page--Water Quality Effects in Surface Water
Dakota Home ||
Water Quality Index