Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 258, part 2
Prev Page--Introduction and Palynofloras || Next Page--Marine-influenced Sedimentation
![]()
![]()
![]()
Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 258, part 2
Prev Page--Introduction and Palynofloras ||
Next Page--Marine-influenced Sedimentation
![]()
The Dakota Formation of Iowa, Nebraska, and Kansas contains one of the most diverse mid-Cretaceous palynofloras known anywhere in the world. The mid-Cretaceous was a remarkable time for the evolution of plant communities in a greenhouse world. This interval was marked by the literal blossoming and explosive radiation of the angiosperms, one of the most important episodes in the history of life on earth. Abundant and well-preserved palynomorph assemblages have been routinely recovered from unoxidized lignitic and mudrock units in the formation, and these samples have provided the primary basis for correlation of the Dakota Formation, a predominantly nonmarine sedimentary succession. Palynostratigraphy became essential for correlating strata within the Dakota Formation, as the better-known and more refined Cretaceous marine biostratigraphy (based on mollusks, foraminifera, nannofossils) is largely inapplicable to the nonmarine facies succession that comprises the bulk of the formation.
Initial reports of the rich palynofloras in the Dakota type area were based on assemblages derived primarily from upper Dakota strata of western Iowa, and these floras provided evidence for a lower to middle Cenomanian correlation of these strata (Ravn, 1981; Ravn and Witzke, 1994, 1995). These reports suggest that lower Dakota strata in the type area are most likely of Albian age, but sampling was inadequate to fully demonstrate an Albian correlation at that time. Renewed sampling during the 1990s and early 2000s resulted in the recovery of rich palynomorph assemblages from lower Dakota strata in Iowa, Nebraska, and Kansas that contained well-defined Albian palynofloras. Preliminary assessments of lower Dakota palynostratigraphy were presented by Witzke, Ravn, et al. (1996), Witzke, Ludvigson, Ravn, et al. (1996), and Witzke and Ludvigson (1998), but the full taxonomic listing of the constituent palynofloras and their biostratigraphic significance have not yet been presented. Additions and corrections to the four-part biostratigraphic scheme proposed for the Dakota Formation by Witzke, Ravn, et al. (1996) and Witzke, Ludvigson, Ravn, et al. (1996) are noted in this paper. Within the context of these four biostratigraphic subdivisions (units 1-4), all identified palynotaxa recovered from the Dakota Formation of Iowa, Nebraska, and Kansas are listed here for the first time. Publication of the full systematic treatment of these palynofloras with locational and stratigraphic details must await further study.
Numerous leaf-compression floras also have been discovered in the Dakota Formation of Iowa, Kansas, and Nebraska, but only a few of these have been studied (e.g., Upchurch and Dilcher, 1990). All known compression floras are dominated by angiosperms (especially platinoids and magnoliaceans at most localities), but fern and gymnosperm (taxodiacean) compressions are also known. By contrast, the Dakota palynofloras are dominated by pteridophyte (fern) spores, probably because the angiosperms are relatively stingy in their production of pollen. The biological relationships of most Cretaceous palynotaxa are known with varying degrees of confidence, but absolute certainty of these relationships requires exceptional preservation of palynomorphs associated with reproductive bodies of known plant compressions. The accompanying tables (tables 1-4) are intended to list spore and pollen taxa according to their most likely relationships with major plant groups. In some cases, these relationships are not clearly known or are controversial. For example, although Foveosporites and Reticulatisporites are listed with the pteridophytes, some authors have suggested relationships with lycophytes or bryophytes, respectively. Regardless, the pteridophyte dominance of the Dakota palynofloras remains evident (fig. 3).
Figure 3--Relative palynospecies diversity within major vascular plant groups in Dakota palynostratigraphic units 1 through 4 (% of total vascular palynoflora for each unit). Data tabulated from tables 1-7. A relative increase in angiosperm-pollen diversity through the Dakota succession is recognized. Absolute diversity of other algal, fungal, and marine palynomorph species is also shown. n--total number of species identified (number in parentheses includes additional unidentified species).
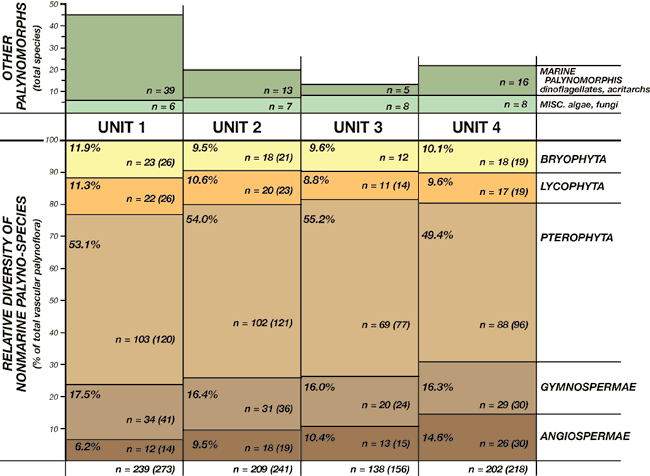
Pteridophyte taxa include probable representatives of the osmundaceans, schizaeaceans, gleicheniaceans, matoniacaeans, marsiliaceans, polypodiaceans, and others. Water ferns and tree ferns likely are represented. Bryophyte (mosses and liverworts) and lycophyte (lycopods and selaginellaceans) spores also are abundant and diverse in the Dakota assemblages. Fungal hypha and algal spores are present in many assemblages.
Pollen grains from gymnosperms and angiosperms generally are less abundant than the pteridophytes, but the diversity of these forms is notable. Gymnosperm pollen suggests the presence of pines, podocarps, taxodiaceans, and araucariaceans in the region. Additional gymnosperms include cycadophytes, various incertae sedis, and certain "advanced" gymnosperms (gnetaleans, erdtmanithecales). The diversity of gymnosperm pollen, however, is not reflected in the Dakota compression floras, perhaps suggesting that many palynotaxa were part of the regional pollen rain. Angiosperm pollen shows an overall increase in diversity and complexity upward through the Dakota Formation (fig. 3), the only significant stratigraphic change in diversity through the Dakota succession. This in-crease in diversity likely reflects part of the ongoing mid-Cretaceous explosive angiosperm radiation. The biological relationships of many of the Dakota angiosperm pollen taxa remain problematic. Primitive angiosperms are included in the floras (e.g., Asteropollis). Compression floras suggest that the angiosperms were dominated by forms with magnoliacean and platinoid leaf types, but a variety of other types are also present.
The floral composition and sedimentary character of the Dakota Formation indicate humid subtropical to warm temperate climates in the region during the mid-Cretaceous. Abundant mosses and lycophytes suggest that wet habitats were common in the Dakota depositional environments. The common occurrence of algal spores is especially noteworthy, indicating that wetland and ponded settings were common in the Dakota depositional systems. Sedimentary features, including sideritic paleosols (saturated wetland soils), kaolinitic mudstones (indicative of humid weathering), and abundant lignites and carbonaceous shale (swampy wetlands), all support moist to wet environments during most or all of Dakota deposition. Of note, there are no sedimentary features suggesting arid or dry conditions (e.g., calcareous paleosols). Nevertheless, large vertical features within some plinthic paleosols and the presence of tree rings in wood indicate seasonality, probably resulting from changes between wetter and drier seasonal cycles. Overall, the Dakota environments probably were characterized by heavily vegetated, moist to swampy forests that covered much of the expansive low-lying coastal plain along the eastern margin of the Western Interior seaway. These forests were probably marked by small to large trees of gymnosperms and angiosperms with a dense understory of ferns. Many angiosperms likely grew as small trees, shrubs, and vines in these environments.
Four biostratigraphic units are recognized within the Dakota succession of Iowa, Nebraska, and Kansas, as discussed herein. These units provide a relatively coarse biostratigraphic scheme, especially when compared with the more detailed coeval marine zonation based on ammonites, inoceramids, and foraminifera (e.g., Kauffman et al., 1993). Nevertheless, the Dakota palynostratigraphic scheme has enabled general correlation of the terrestrial, fluvial, and estuarine facies of the Dakota Formation within the context of the Albian-Cenomanian depositional cycles of the Western Interior. The three major stratigraphic sequences represented within the Dakota succession (Brenner et al., 2000) can now be correlated and identified using this palynostratigraphic scheme. The accompanying tables (tables 1-4) summarize all nonmarine palynotaxa recognized within the Dakota Formation of the region. These units are remarkably diverse, each containing between 130 and 200 nonmarine species. Depending on how certain indeterminate species are tabulated, the actual recovered diversities may be much higher (perhaps as high as 230 species in units 1 and 2). Many taxa on these lists are long-ranging forms of limited biostratigraphic utility, and many taxa span all four Dakota palyno-units. However, each unit contains a number of palynomorphs of biostratigraphic significance (most are underlined or highlighted on the accompanying tables), marking important first or last occurrences in the succession and providing a basis for local, regional, and inter-regional correlation. The Dakota Formation is further constrained by its bounding and lateral stratigraphic relationships with marine shale and marl units of the Kiowa, Graneros, and Greenhorn formations.
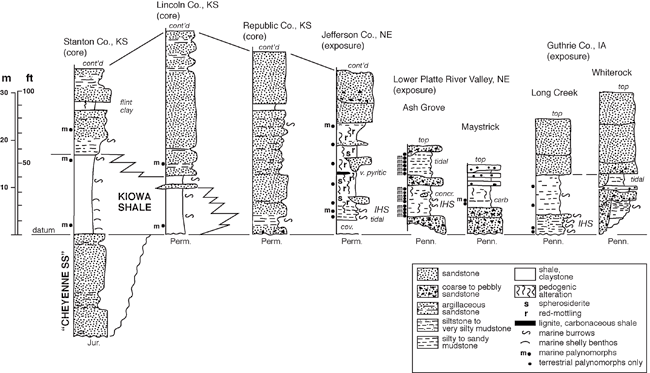
Dakota Palynostratigraphic Unit 1 directly corresponds to Dakota Sequence D0 of Brenner et al. (2000), a stratigraphic interval that is known to interfinger westward with marine facies of the Kiowa shale. Most palynomorphs were recovered from the lower half of this sequence (see fig. 4), with the most extensive sampling from the Ash Grove quarry exposures and drillcore in the lower Platte River valley of Nebraska (fig. 1; see Witzke et al., 1996a; Joeckel et al., 2005). Unit 1 has produced abundant and diverse palyno-assemblages (table 1) from a series of samples taken from the lower Dakota outcrop belt of eastern Nebraska (Cass, Sarpy, Jefferson counties) and central Iowa (Guthrie County), and from cores in central and western Kansas (Lincoln, Stanton counties). These samples include Dakota strata variously referred to the Nishnabotna Member, lower Terra Cotta Member, and Longford Member. This palynostratigraphic unit was first defined by Witzke, Ravn, et al. (1996) and Witzke, Ludvigson, Ravn, et al. (1996), but some additions and modifications are included here. Unit 1 is characterized by occurrences of palyno-taxa not known to range above the early Late Albian Kiowa-Skull Creek depositional cycle elsewhere in the Western Interior of North America including Eucommiidites minor, Disaltriangulisporites irregularis, Foveosporites labiosus, Couperisporites complexus, Interulobites exuperans, and Stoverisporites lunaris. Witzke, Ravn, et al. (1996, p. 17) included D. perplexus and possibly Cicatricosisporites goniodontos and C. patapscoensis on this list, but these species are now known to range into Unit 2 (but are still considered to be Albian indicators). In addition, numerous other Albian indicators are present in Unit 1 (forms not known to range into the Cenomanian) including Densoisporites velatus, Staplinisporites caminus, Cicatricosisporites potomacensis, Disaltriangulisporites mutabilis, D. perplexus, and others (see underlined species on table 1). Witzke, Ravn, et al. (1996) considered Impardecispora marylandensis to be an Albian indicator, but this species is now known to range into the Cenomanian (units 3, 4).
Table 1--Dakota Palynostratigraphic Unit 1; early Late Albian; Cass, Sarpy, Jefferson counties, Nebraska; Guthrie County, Iowa; Stanton, Lincoln counties, Kansas; identifications by Robert L. Ravn. Biostratigraphically significant taxa (first or last occurrence) are underlined. Those taxa that are restricted to the palynostratigraphic unit are shown in bold type.
|
BRYOPHYTA
Aequitriradites spinulosus LYCOPHYTA
Camarozonosporites ambigens |
PTEROPHYTA
Antulsporites distaverrucosus |
GYMNOSPERMAE
Abietineaepollenites sp. indet. ANGIOSPERMAE
Asteropollis asteroides MISCELLANEA
Lecaniella foveata |
Figure 4--Representative stratigraphic sections, lower Dakota Formation, Sequence D0, Kiowa-Skull Creek marine cycle, Iowa, Nebraska, and Kansas (~ Palynostratigraphic Unit 1). Positions of palynostratigraphic samples are noted. Dakota strata are replaced westward in Kansas by marine-shale facies of the Kiowa Formation. The location of the KGS Stanton County core along the Kansas-Colorado border is given in Macfarlane et al. (1993, p. 10-11), while locations for the KGS Jones #1 core; the KGS Kenyon core in Republic County, Kansas; the Jefferson County, Nebraska, study area; the lower Platte River valley study area in eastern Nebraska; and the Guthrie County study area in western Iowa are all shown in fig. 1. Symbol for leaf fossils as in fig. 5. Abbreviations: m--marine palynomorphs; IHS--inclined heterolithic strata, carb--carbonaceous, concr--concretion, Jur--Jurassic, Perm--Permian, Penn--Pennsylvanian, cont'd--continued above.
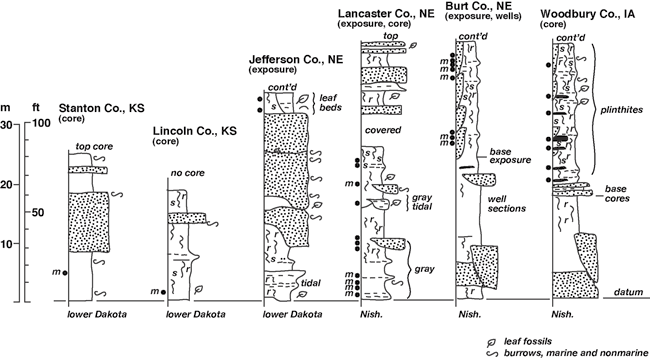
Dakota Palynostratigraphic Unit 2 directly corresponds to Dakota Sequence D1 of Brenner et al. (2000), which encompasses the middle strata of the Dakota Formation. This stratigraphic interval has been variously assigned to the lower Woodbury and upper Terra Cotta Members and locally includes strata assigned to the upper Nishnabotna Member in Iowa. Unit 2 has produced abundant and diverse palyno-assemblages (table 2) from samples taken in the Dakota outcrop belt of eastern Nebraska (Jefferson, Lancaster, Burt counties), and cores from western Iowa (Woodbury County) and Kansas (Lincoln, Stanton counties). The most extensive sampling was undertaken in brick pit and exploration core sections in the Yankee Hill area of Lancaster County, Nebraska; the Missouri River bluff exposures in Burt County, Nebraska; and brick-pit exploration cores at Sargeant Bluff, Woodbury County, Iowa (fig. 5; also see White et al., 2005). Palyno-stratigraphic Unit 2 is characterized by the highest occurrences of typical Albian palynomorphs, including Cicatricosisporites apicanalis, C. goniodontos, C. patapscoensis, C. potomacensis, Disaltriangulisporites mutabilis, D. perplexus, Impardecispora apiverrucata, I. excavata, Januasporites spiniferus, Plicatella jansonii, Punctatisporites couperi, Scopusporis lautus, Cedripites canadensis, Eucomiidites troedssonii, Podocarpidites multesimus, Quadricolpites reticulatus, and others (see underlined species on table 2). No Cenomanian indicators are known to occur in the unit. It is presently uncertain if any of the contained species are biostratigrapically unique to Unit 2 in the Western Interior, but many last occurrences of Albian species are recorded within the unit. Unit 2 is a relatively coarse biostratigraphic interval defined by the last occurrences of Albian species above Unit 1.
Figure 5--Representative stratigraphic sections, middle Dakota Formation, Sequence D1, Muddy-Mowry marine cycle, Iowa, Nebraska, and Kansas (~ Palynostratigraphic Unit 2). The location of the KGS Stanton County core is given by Macfarlane et al. (1993, p. 10-11), while locations for the KGS Jones #1 core in Lincoln County, Kansas; the Jefferson County, Nebraska, study area; the Yankee Hill study area in Lancaster County, Nebraska; the Burt County, Nebraska, study area; and portions of the type Dakota area in Woodbury County, Iowa, are shown in fig. 1. Symbols as in fig. 4. Abbreviations: Dak--Dakota Formation; Nish--Nishnabotna Member.
Proposed Muddy-Mowry Cycle. The Unit 2 palynoflora shares much in common with that of the upper Albian Muddy Sandstone of Wyoming (Ravn, 1995). The Muddy Sandstone interval and equivalent strata in the central Western Interior basin are marked by widespread progradation of sandstone and nonmarine facies, which interstratify with several pulses of marine transgression (e.g. Holbrook et al., 2002, 2006). The base of the Muddy Sandstone and equivalent strata across the Western Interior is recognized to mark a regional unconformity and sequence boundary (SB3) across the basin, from Canada to New Mexico, from Kansas to Montana (e.g., Scott et al., 1998; Scott et al., 2001; Scott et al., 2004; Oboh-Ikuenobe et al., 2007). The Muddy Sandstone is overlain by siliceous marine shale and siltstone strata of the Mowry Shale in the central Western Interior, and the contact between the two is considered to be comformable (Oboh-Ikuenobe et al., 2007). The "Huntsman Shale," which lies above the Muddy/'J' sandstone may represent an eastern tongue of the Mowry Shale in eastern Colorado and adjoining areas of Nebraska and Kansas (Hamilton, 1994; Graham, 2000). The Muddy-Mowry sequence is capped by a widespread sequence boundary (SB4) across the Western Interior (e.g., Scott et al., 2004). Collectively, the Muddy-Mowry interval and correlative strata are now identified as a discrete and widely recognized multi-phased stratigraphic sequence across the Western Interior basin. Internally, this sequence is marked by complex smaller-scale cyclicity and additional higher-order sequences (e.g., SB3.2) (e.g., Holbrook et al., 2006; Scott et al., 2004; Graham, 2000).
Although the Mowry had been considered to be a lower Cenomanian unit in some previous reports, recent biostratigraphic and chronostratigraphic studies now support an uppermost Albian age (Oboh-Ikuenobe et al., 2007). As such, Dakota Palynostratigraphic Unit 2 apparently can be correlated directly with the Muddy-Mowry interval farther to the west in the central Western Interior. Like the Muddy-Mowry interval, the correlative middle Dakota succession that includes Unit 2 in Iowa, Kansas, and Nebraska has also been recognized as a discrete sequence, which is separated from overlying Cenomanian strata of the upper Dakota by an erosional surface marked by channeling and widespread soil development (e.g., Hamilton, 1994; Scott et al., 1998; Witzke et al., 1999; Brenner et al., 2000; Gröcke et al., 2006; Witzke, 2007). Even though widespread sequence boundaries constrain the Muddy-Mowry and middle Dakota interval in the Western Interior, most previous studies have variably included this interval within either an expansive Greenhorn or Kiowa-Skull Creek marine cycle. It was most recently included with the Kiowa-Skull Creek cycle by Scott et al. (2004). In keeping with recent sequence stratigraphic studies of the Dakota Formation and other mid-Cretaceous strata of the Western Interior, it seems desirable to separate this stratigraphic interval from the underlying Kiowa-Skull Creek cycle as well as from the overlying Cenomanian transgressive phases of the lower Greenhorn marine cycle. Therefore, upper Albian strata coeval with Palynostratigraphic Unit 2 are considered to relate to a separate multi-phased marine depositional cycle in the Western Interior here termed the "Muddy-Mowry cycle." Further evidence for marine-influenced deposition in middle Dakota strata coincident with this cycle is presented later in this report.
Table 2--Dakota Palynostratigraphic Unit 2; latest Albian; Lancaster, Jefferson, Burt counties, Nebraska; Stanton County, Kansas; Woodbury County, Iowa; identifications by Robert L. Ravn. Biostratigraphically significant taxa (first or last occurrence) are underlined. Those taxa that are restricted to the palynostratigraphic unit are shown in bold type.
|
BRYOPHYTA
Aequitriradites spinulosus LYCOPHYTA
Camarozonosporites ambigens |
PTEROPHYTA
Antulsporites distaverrucosus |
GYMNOSPERMAE
Abietineaepollenites sp. ANGIOSPERMAE
Cupuliferoidaepollenites minutus MISCELLANEA
Multicellaesporites sp. |
Dakota Palynostratigraphic Unit 3 corresponds to the basal portion of upper Dakota stratigraphic sequence D2 of Brenner et al. (2000). Unit 3 includes strata of the upper Woodbury Member in Iowa and eastern Nebraska and the Janssen Member of Kansas, and it locally includes facies assigned to the upper Terra Cotta Member in areas of Nebraska and Kansas. Unit 3 has been most extensively sampled for palynomorphs in cores from Sioux and Woodbury counties, Iowa, and from exposures in eastern Nebraska (Thurston, Jefferson counties); Kansas cores have not been sampled extensively (fig. 6). Compared to the other three Dakota palynostratigraphic units, the recovered palynoflora of Unit 3 is slightly less diverse (table 3). This seeming change in diversity is probably an artifact of sampling and preservation, and likely does not represent an actual decline in floral diversity in the Dakota environments. A number of characteristic Cenomanian species make their first appearance at or near the base of the unit, including Lycopodiacidites arcuatus, Dictyophyllidites impensus, Foveosporites cenomanianus, Foveogleicheniidites confossus, Rugubivesiculites convolutus, and Artiopollis indivisus. Several additional species were regarded as Cenomanian indicators by Witzke et al. (1996a, p. 18), but these are now known to range downward into Unit 2 (Balmeisporites glenelgensis, Petalosporites quadrangulus, Triporoletes pluricellulus). Witzke et al. (1996b) defined the top of Unit 3 at the last-appearance datum of Neoraistrickia robusta (see also Ravn and Witzke, 1995). This interval (below the top of N. robusta) was correlated with the lower Cenomanian by Ravn and Witzke (1995). Not previously recognized, a number of additional species (table 3, underlined taxa) also make their last appearance within Unit 3, including Triporoletes simplex, Baculatisporites comaumensis, Cicatricosisporites mediostriatus, Impardecispora trioreticulosa, Ischyosporites disjunctus, Januasporites spiniferus, and Lobatia involucrata.
Table 3--Dakota Palynostratigraphic Unit 3; Early Cenomanian; Woodbury, Sioux counties, Iowa; Jefferson, Burt, Thurston counties, Nebraska; identifications by Robert L. Ravn. Biostratigraphically significant taxa (first or last occurrence) are underlined. Those taxa that are restricted to the palynostratigraphic unit are shown in bold type.
|
BRYOPHYTA
Aequitriradites spinulosus LYCOPHYTA
Camarozonosporites ambigens |
PTEROPHYTA
Antulsporites distaverrucosus |
GYMNOSPERMAE
Balmeiopsis limbatus ANGIOSPERMAE
Artiopollis indivisus MISCELLANEA
Inapertisporites sp. |
Figure 6--Representative stratigraphic sections, upper Dakota Formation, Sequence D2, lower Greenhorn marine cycle, Iowa, Nebraska, and Kansas (~ Palynostratigraphic Units 3 and 4). The location for the Amoco No. 1 Bounds core along the Kansas-Colorado border in Greeley County, Kansas, is given by Scott et al. (1998), while the locations of the KGS Jones #1 core in Lincoln County, Kansas; the KGS Kenyon core in Republic County, Kansas; the Jefferson County, Nebraska, study area; portions of the type Dakota area in Thurston and Dakota counties, Nebraska; and Woodbury County, Iowa, are shown in fig. 1. The IGS Hawarden core in Sioux County, Iowa, is given by Witzke and Ludvigson (1994). Symbols as in figs. 4 and 5. Abbreviations: humm--hummocky, I. prefragilis--Inoceramus prefragilis.
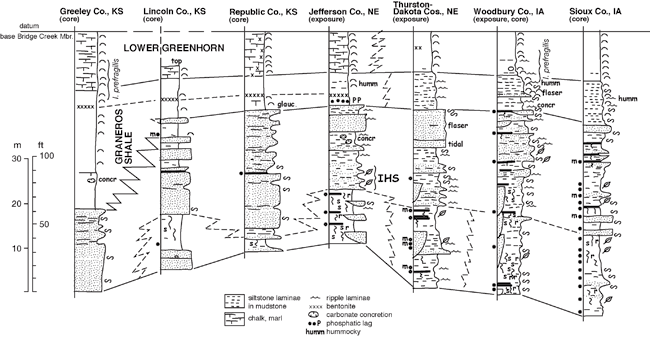
Dakota Palynostratigraphic Unit 4 is known primarily from a series of lignite samples in uppermost Dakota strata (both core and outcrop sections) from northwestern Iowa (Woodbury, Sioux counties; summarized in Ravn and Witzke, 1995). Additional samples from eastern Nebraska (Thurston, Jefferson counties) and north-central Kansas (Republic, Lincoln counties) are now included within Unit 4. All samples were derived from strata of the upper Woodbury Member in Iowa and Nebraska, and the Janssen Member in Kansas. Cores from farther west in Kansas (Greeley, Stanton counties) show correlative strata to be entirely within marine shale facies of the Graneros Shale (fig. 6). The position of Unit 4 below upper Cenomanian marine strata in Iowa and Nebraska, and the probable lateral equivalency of Unit 4 with middle Cenomanian marine strata in western Kansas, support the correlation of Unit 4 with the middle Cenomanian (see also Ravn and Witzke, 1995).
A few spore species make their first appearance in Unit 4 (table 4), including Cicatricosisporites crassiterminatus and C. paradorogensis. The angiosperms show a marked increase in diversity over earlier assemblages, and a number of angiosperm pollen species, including morphologically complex forms, make their first appearance within Unit 4, including Doyleipollenites robbinsiae, Ephedripites ambiguus, Foverotricolporites rhombohedralis, Liliacidites dividuus, Montiapollis hallii, and Tricolpites patens. Witzke, Ravn, et al. (1996) indicated that Foveogleicheniidites confossus and Dictyophyllidites impensus also make their first appearance with Unit 4, but these two species apparently range downward into upper Unit 3. The first-appearance datums of M. hallii and C. crassiterminatus have been used to subdivide unit 4 (Witzke, Ravn, et al., 1996; Ravn and Witzke, 1995). Unit 4 also shows a general upward loss of a number of species common in older Albian and lower Cenomanian assemblages (e.g., Nicholsipollis mimas, Cycadopites fragilis, Cicatricosisporites mediostriatus, Impardescispora trioreticulosa, Cingulatriletes congruens, etc.). Strata of Unit 4 show the highest proportion of marine-influenced sedimentary facies (coastal, estuarine) within the Dakota Formation. The increased diversity of angiosperm taxa seen within this unit may suggest that coastal settings may have provided particularly favorable habitats for angiosperm diversification.
Table 4--Dakota Palynostratigraphic Unit 4; Middle Cenomanian; Woodbury, Sioux counties, Iowa; Thurston, Jefferson counties, Nebraska; Lincoln County, Kansas; identifications by Robert L. Ravn. Biostratigraphically significant taxa (first or last occurrence) are underlined. Those taxa that are restricted to the palynostratigraphic unit are shown in bold type.
|
BRYOPHYTA
Aequitriradites spinulosus LYCOPHYTA
Camarozonosporites ambigens |
PTEROPHYTA
Antulsporites distaverrucosus |
GYMNOSPERMAE
Araucariacites cf. australis ANGIOSPERMAE
Artiopollis indivisus MISCELLANEA
Ariadnaesporites spinocaperatus |
Marine palynomorphs (dinoflagellate cysts, acritarchs) are locally associated with assemblages of nonmarine palynomorphs at various stratigraphic positions within the Dakota Formation of Iowa, Nebraska, and Kansas. The initial discovery of Dakota marine palynomorphs was limited to uppermost Dakota strata (Unit 4; see Ravn and Witzke, 1995), primarily in places where Dakota strata interfinger with marginal-marine shale and siltstone units of the lower Greenhorn marine cycle. The subsequent discovery of common marine palynomorphs in basal Dakota strata (Unit 1) of the lower Platte Valley in eastern Nebraska (fig. 4, table 5) indicated that marine-influenced sedimentation also characterized other portions of the Dakota succession, including strata equivalent to the maximum transgressive phases of the Kiowa-Skull Creek marine cycle (Witzke, Ravn, et al., 1996; Witzke et al., 1999). Although Witzke, Ravn, et al. (1996) indicated that marine palynomorphs were absent from palynostratigraphic units 2 and 3, subsequent samples from eastern Nebraska (Lancaster, Burt, Thurston counties) revealed that marine palynomophs occur at a number of stratigraphic positions within units 2 and 3 as well (as first reported by Witzke et al., 1999). These occurrences provide indisputable evidence for recurring episodes of localized marine influence during Dakota deposition, most likely associated with estuaries that encroached eastward at times of relative sea-level rise in the Western Interior seaway (see subsequent discussions).
Table 5--Late Albian marine palynomorphs; Kiowa-Skull Creek depositional cycle; basal Dakota Formation and equivalent strata, eastern estuarine and shallow-marine facies. p--Platte River valley, eastern Nebraska; j--Jefferson County, Nebraska (eastern estuarine facies of basal Dakota Formation); k--Kansas cores (Kiowa Formation, basal Dakota Formation); m--western Manitoba-eastern Saskatchewan cores and exposures (upper Swan River Formation, Skull Creek Member of lower Ashville Formation, Pense Formation); identifications by Robert L. Ravn. Taxa from lower Dakota Formation of Kansas-Nebraska shown in bold.
| Achomosphaera? sp. | k |
| Alterbidinium? sp. | m |
| Apteodinium sp. | k |
| Ascodinium sp. | k |
| Baltioladinium jaegeri | m |
| Baltisphaeridium sp. | p,j |
| Callaiosphaeridium? sp. | m |
| Circulodinium distinctum | k |
| C. sp. | m |
| Chlamydophorella sp. cf. C. discreta | k |
| C. nyei | m |
| C?. sp. | k |
| Cometodinium sp. cf. C. whitei | p,j |
| C.? sp. | m |
| Coronifera oceanica | k |
| Crassosphaera sp. | m |
| Cribroperidinium edwardsii | m |
| C. spp. | k,m |
| Cyclonephelium sp. cf. C. compactum | k,m |
| Cymatiosphaera sp. | m |
| cf. Dinopterygium cladoides | k |
| Exochosphaeridium? sp. | k |
| Fromea amphora | m |
| F. fragilis | m |
| indet. gonyaulacoid dinocyst | p |
| Heterosphaeridium difficile | k |
| Hystrichodinium pulchrum | k,p |
| Impletosphaderidium spp. | k,m,p,j |
| Kiokansium williamsii | m |
| K. sp. | k |
| Leiosphaeridia sp. | p |
| indet. leiosphere spp. | p |
| Litosphaeridium arundum | m |
| cf. Lophosphaeridium sp. | p |
| Michystridium spp. | k,m,p |
| Odontochitina operculata | k,m |
| O. rhakodes | k |
| Oligosphaeridium albertense | m |
| O. complex | k,m |
| O. pulcherrimum | k |
| O. totum | m |
| Ovoidinium verrucosum | m |
| Palaeoperidinium cretaceum | k,m |
| Pseudoceratium interiorense | m |
| P. polymorphum | m |
| Pterodinium sp. cf. P. aliferum | m |
| P. sp. | m |
| Pterospermella sp. cf. P. hartii | k |
| P. sp. | k,m |
| Senoniasphaera sp. | k |
| indet. skolochorate dinocyst | p,j |
| Spinidinium sp. | m |
| Spiniferites ramosus | m |
| S. sp. | k,m |
| Striphrosphaeridium anthophorum | m |
| Subtilisphaera sp. | k |
| ?Systematophora sp. | k,p |
| ?Trichodinium sp. | k |
| Veryhachium sp. | k,m,p,j |
| Xiphophoridium alatum | k |
| indet. angular dinocyst | p |
| indet. alate spinose dinocyst | p |
None of the recovered dinoflagellate cysts and acritarchs from the Dakota Formation apparently is of any particular biostratigraphic utility. Nevertheless, the occurrences of marine palynomorphs in the Dakota Formation (tables 5-7) likely can be used to characterize various estuarine and brackish-water biofacies associations. Certain acritarch genera occur in many of the Dakota marine palynomorph assemblages, especially Cymatiosphaera, Dictyotidium, Micrhystridium, Veryhachium, Baltisphaeridium, and Leiosphaeridia. Several genera of dinoflagellate cysts commonly co-occur within many of these marine-influenced Dakota assemblages, including Cribroperidinium, Hystrichodinium, Impletosphaeridium, Odontochitina, Fromea, Cyclonephelium, and indeterminate skolochorate dinocysts. Some dinoflagellate cysts may be restricted to particular Dakota palynostratigraphic units, possibly including Cometodinium (Unit 1), Cleistosphaeridium (Unit 4), and Oligosphaeridium (Unit 4).
Table 6--Latest Albian marine palynomorphs, Muddy-Mowry depositional cycle, middle Dakota Formation, eastern estuarine facies. n--eastern Nebraska, k--central Kansas; identifications by Robert L. Ravn.
| Cribroperidinium sp. | k |
| Cymatiosphaera sp. | n |
| cf. Dinopterygium cladoides | k |
| Dictyotidium sp. | n |
| cf. Gorgonisphaeridium sp. | n |
| Hystrichodinium cf. pulchrum | n |
| Impletosphaeridium? sp. | k,n |
| Leiosphaeridia sp. | n |
| Micrhystridium spp. | n |
| Odontochitina operculata | n |
| indet. ?pilomate cyst | n |
| indet. skolochorate dinocyst | n |
| Veryhachium spp. | k,n |
Table 7--Cenomanian marine palynomorphs, lower Greenhorn depositional cycle, upper Dakota Formation, eastern estuarine facies western Iowa, eastern Nebraska, central Kansas; identifications by Robert L. Ravn.
| Baltisphaeridium sp. |
| Circulodinium distinctum |
| Cleistosphaeridium sp. |
| Criboperidinium? sp. |
| Cyclonephelium sp. |
| Dictyotidium? sp. |
| Fromea? sp. |
| Impletosphaeridium sp. |
| indet. leiosphere sp. |
| Micrhystridium sp. |
| Multiplicisphaeridium sp. |
| Odontochitina costata |
| Oligosphaeridium sp. |
| indet. skolochorate dinocyst |
| Trichodinium sp. |
| Veryhachium spp. |
Low-diversity Albian-Cenomanian marine-influenced palynomorph associations described by Oboh-Ikuenobe et al. (2004, 2007) from the Western Interior include dinoflagellate cysts (Cyclonephelium, Palaeoperidinium, Cribroperidinium) and acritarchs (Pterospermella). They interpreted these assemblages to represent nearshore and probably brackish-water biofacies. Of note, Cyclonephelium, Palaeoperidinium, and Pterospermella have not been recovered in Dakota strata of Iowa or eastern Nebraska, but these taxa have been found in more seaward areas of western Kansas and Manitoba, where nonmarine Dakota-Swan River facies interfinger with marine shale facies of the Kiowa, Skull Creek, and Graneros formations (tables 5, 7). This supports a shallow-marine to nearshore habitat for these forms, but these taxa apparently were not associated with the large estuarine systems that pushed eastward into the Dakota coastal plain during times of regional marine transgression. The above-noted dinoflagellate-acritarch associations from the Dakota Formation of Iowa and Nebraska are interpreted to represent biofacies associated with the eastern estuaries, and all of these taxa were likely brackish-water tolerant to varying degrees.
Prev Page--Introduction and Palynofloras || Next Page--Marine-influenced Sedimentation
Kansas Geological Survey
Placed online Feb. 12, 2010
http://www.kgs.ku.edu/Current/2010/Ludvigson/03_corr.html
email:webadmin@kgs.ku.edu