Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 253, part 3
Prev Page--Introduction, Materials and Methods || Next Page--Conclusions and References
Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 253, part 3
Prev Page--Introduction, Materials and Methods ||
Next Page--Conclusions and References
![]()
Water chemistry in the Hays area is a predominantly calcium-mixed-anion-type water. The anions of bicarbonate, sulfate, and chloride are the main contributors to the specific conductance (fig. 3; Appendix B). Nitrate-N concentration near or above the drinking-water limit of 10 mg/L (U.S. EPA, 2003) occurred in several of the city wells (fig. 2, Appendix B).
Figure 3 portrays the correlation of anions contributing to specific conductance. This relationship in water chemistry is often observed in semi-arid environments with high evapotranspiration (Townsend and Macko, 2005; Hem, 1985). Nitrate-N also follows this trend if well C-24 is not included in the analysis (table 2), suggesting that evapoconcentration of water with fertilizer may be a source of the high nitrate. However, δ15N values for the water samples do not support this conclusion (fig. 4). Well C-24 has higher specific conductance, chloride, and bicarbonate values and a much lower nitrate-N concentration than the other wells. These values are indicative of reducing water chemistry. Because of the very low nitrate-N value (Appendix B), well C-24 was not included in fig. 3.
Figure 3--Contribution of bicarbonate, sulfate, and chloride anions and nitrate-N concentration to specific conductance values. Increasing values suggest impacts of evapoconcentration on ground water in the area (table 2). Well C-24 values are not plotted in fig. 3.
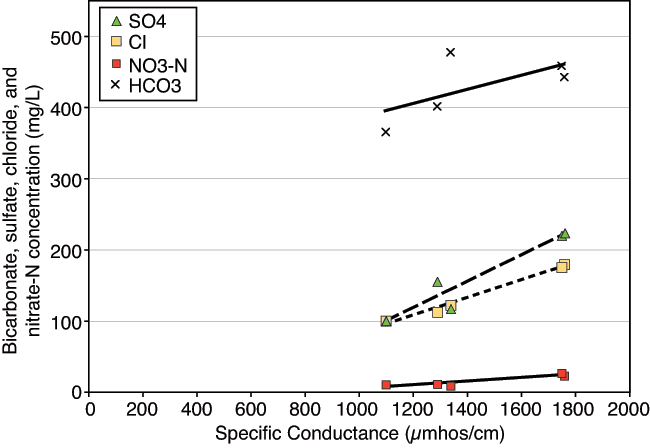
Figure 4--Range of nitrate-N and δ15N values observed in this study. The δ15N values are all greater than +10 ‰, which is the lower limit of the animal waste and probable denitrification and/or volatilization enrichment zone on the graph. The line at 2 mg/L nitrate-N is the background level for pristine water (Mueller and Helsel, 1996). The line at 10 mg/L is the U.S. EPA drinking-water limit for nitrate-N (U.S. EPA, 2003).
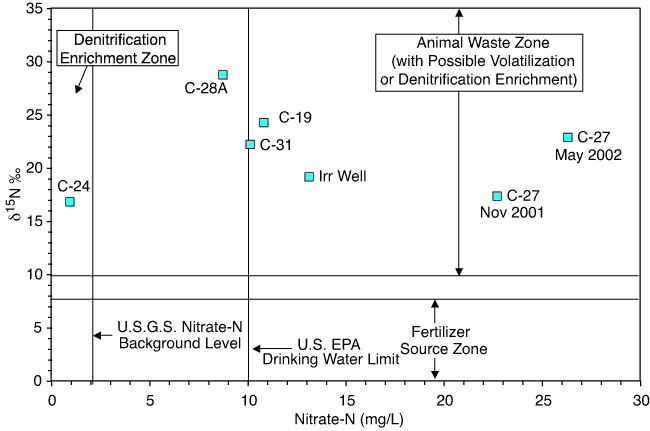
Table 2--Trend-line R2 values for anion and nitrate-N plot with specific conductance in fig. 3.
| Without Well C-24 (5 samples) | ||
|---|---|---|
| Trend line | Calculated trend line | R2 |
| Nitrate-N | y = 0.0256x - 21.294 | 0.854 |
| Sulfate | y = 0.1851x - 105 | 0.913 |
| Chloride | y = 0.1234x - 42.317 | 0.985 |
| Bicarbonate | y = 0.099x + 285.06 | 0.415 |
| With Well C-24 (6 samples) | ||
| Trend line | Calculated trend line | R2 |
| Nitrate-N | y = 0.0024x + 15.233 | 0.012 |
| Sulfate | y = 0.2121x - 145.68 | 0.946 |
| Chloride | y = 0.1764x - 107.58 | 0.936 |
| Bicarbonate | y = 0.117x + 260.68 | 0.677 |
Figure 2 illustrates the range of nitrate-N and chloride values from 1995 to 2002 (City of Hays manager, personal communication, 2002). Overall, the nitrate-N concentration shows an increasing trend, particularly in well C-27, which is surrounded on the west and south by farmed land (fig. 1). In well C-24 chloride concentrations are much higher and nitrate-N values are generally lower than in the other wells. This well is not routinely used and has a reducing water quality (Appendix A). The δ15N value for this well (discussed in next section) indicates that denitrification may have occurred.
Table 3 shows the results of the Mann-Kendall test for increasing or decreasing trend of nitrate-N and chloride values in ground-water samples from all city wells sampled in the Hays area from 1995 to 2000, plus the results from this study. The nitrate-N and chloride values from this study were included for wells C-19, C-27, C-31, C-24, and C-28A. All wells sampled in this study, except for well C-24, showed a statistically significant increasing trend (shown by a positive τ value) for nitrate-N (table 3). These results, plus the significance levels (shown by the p value) for three of the other city wells (C-32, YE-1, and YE-2), suggest that nitrate-N is increasing throughout the area, although the rate of nitrate concentration increase is not the same for all of the wells.
Three of the wells from this study (C-19, C-24, and C-27) plus two other city wells (C-21 and C-30) showed a statistically significant increasing trend for chloride (table 3). Well C-24 showed a significant chloride increase but not for nitrate-N. These results are related to the observed reducing water quality at well C-24.
Table 3--Kendall test for trend results for City of Hays wells (1995-2002). Wells in the study and statistically significant p values shown in bold.
| Well | Chloride | Nitrate-N | ||||
|---|---|---|---|---|---|---|
| No. of samples |
tau (τ) | p value | No. of samples |
tau (τ) | p value | |
| C-19 | 20 | 0.394 | 0.0115 | 20 | 0.658 | 0.0001 |
| C-24 | 18 | 0.477 | 0.0058 | 19 | 0.164 | 0.3445 |
| C-27 | 18 | 0.346 | 0.0426 | 18 | 0.673 | 0.0001 |
| C-28A | 20 | -0.1526 | 0.3539 | 20 | 0.521 | 0.0115 |
| C-31 | 21 | -0.195 | 0.2 | 21 | 0.314 | 0.0496 |
| C-32 | 20 | -0.078 | 0.6205 | 20 | -0.468 | 0.0042 |
| C-17 | 20 | 0.247 | 0.1261 | 20 | -0.211 | 0.2047 |
| C-21 | 18 | 0.412 | 0.0172 | 18 | 0.098 | 0.5954 |
| C-29 | 19 | 0.175 | 0.2866 | 20 | 0 | 1 |
| C-30 | 20 | 0.389 | 0.0165 | 19 | 0.0233 | 0.9161 |
| C-33 | 19 | -0.175 | 0.2822 | 19 | 0.152 | 0.3814 |
| YE-1 | 20 | 0.311 | 0.5148 | 20 | 0.558 | 0.0006 |
| YE-2 | 18 | -0.242 | 0.1714 | 20 | 0.347 | 0.0346 |
The nitrate-N observed in the wells most likely comes from several sources. As shown in fig. 1, the Irrigation Well is on the south side of the river and the other wells are located to the north. At the time of this study (2002) all of the sampled wells except C-24 and C-28A had nitrate-N concentrations measured above the drinking-water limit of 10 mg/L (fig. 2; Appendix A). Measured δ15N values are all greater than +10‰ and appear to be derived from animal waste, possibly affected by denitrification or volatilization enrichments (fig. 4). The relationship between high nitrate-N concentrations and enriched_δ15N values has been observed in other studies in Kansas and other states and is usually related to an animal-waste source (Townsend et al., 2001; Fogg et al., 1998). Possible sources include animal waste from feedlots, pastures, manure used as fertilizer in the past, or leaking septic or wastewater-treatment systems. Movement of nitrate-rich water from the surface to ground water could occur through macropore flow (described in next section) or possibly through poor well construction. No well survey of old or abandoned wells was done during this study, but work in other parts of Kansas indicates that poor well construction may permit movement of contaminated water to ground water (Townsend et al., 2001).
Well C-24 has a higher specific conductance reading and higher chloride and sulfate concentrations than the other wells, suggesting that this well has reducing water chemistry (Appendix A). This well also has a very low nitrate-N value, an enriched δ15N value, and a higher bicarbonate value, which suggests that potential denitrification reactions have occurred in the ground water resulting in the observed enriched δ15N (Herbel and Spalding, 1993).
Soil cores collected near the M-319 monitoring wells and at the agricultural irrigation site have δ15N values that show an overall increase with depth, while the other three cores show either little change or decreased values (fig. 5b; Appendix B). Percent total nitrogen values for all of the soil cores decrease with depth. The decreasing percent total nitrogen values and increased δ15N values are similar to findings from other researchers (figs. 5a, 5b; Appendix B; Broadbent et al., 1980; Karamanos et al., 1981; Shearer et al., 1978; Rennie et al., 1976). The decreasing percent total nitrogen at all sites and the increased δ15N values at depth for several of the cores suggests that microbial action is impacting the nitrogen content of the soil and contributing to the observed increasing δ15N values at some sites.
Figure 5--Soil chemical properties of percent total nitrogen (5a), δ15N (5b), nitrate-N (5c), ammonium-N (5d), total percent carbon (5e), and δ13C (5f) for the five sampling sites.
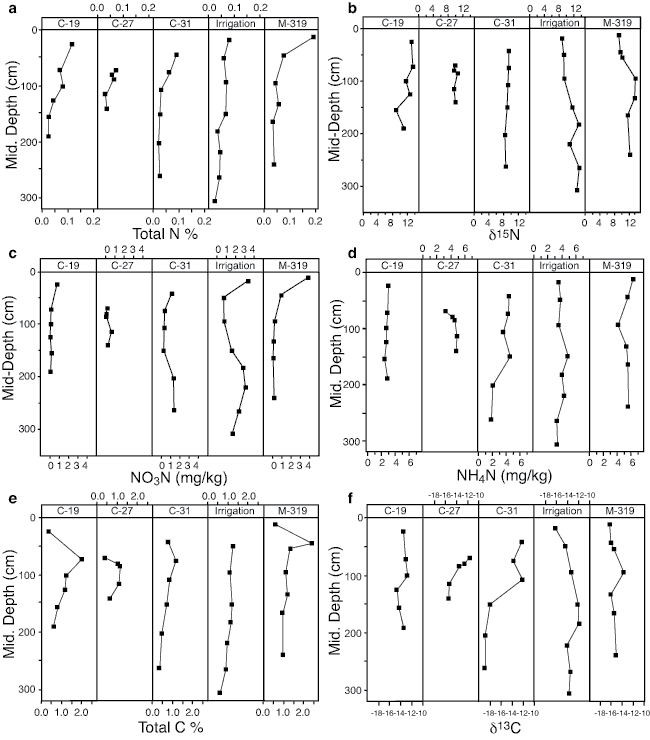
Although organic nitrogen is probably being utilized by microbes, the organic nitrogen likely is not being converted into a form that is moveable by soil water. A C:N > 15 generally means that nitrogen is not available for plant use and is mineralized (Ihori et al., 1995). In the study area a mean C:N of 16.7 suggests that the organic nitrogen portion of the soil is not available for use by plants and may not be available as the labile phase in soil water (Appendix B).
Nitrate-N and ammonium-N concentrations from the cores vary with depth but not as much as the percent total nitrogen (figs. 5a, 5c, 5d). Calculated range, mean, and median nitrate-N concentrations in mg/L for the Detroit and Roxbury soils are shown in table 4. Except for those found at the irrigated site, the nitrate-N values are generally less than the quantity observed in the measured ground-water samples. The mean δ15N value of all soil cores is +10.7‰, which is also considerably below the value observed in the ground water (Appendices A and B).
Denitrification or volatilization processes in the soil zone may have contributed to the enrichment of the δ15N values with depth, but it is unlikely that the observed nitrate-N in the unsaturated zone is the primary source of nitrate-N in the ground water because both the nitrate-N and δ15N values are much lower than those observed in the ground water (Appendices A and B).
Table 4--Comparison of calculated nitrate-N values from soils with observed ground-water nitrate-N values.
| Site ID | Soil Type | *Minimum Bulk Density, Minimum Moisture |
Calculated Nitrate-N (mg/L) | *Maximum Bulk Density, Maximum Moisture |
Calculated Nitrate-N (mg/L) | Ground-water Value (NO3-N mg/L) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | Median | Range | Mean | Median | |||||
| C-31 | Detroit silt loam | 1.33 mg/cm3 19% | 1.9-9.6 | 5.6 | 5.2 | 1.8 mg/cm3 31.4% | 1.6-7.9 | 4.6 | 4.3 | 10.1 |
| C-27 | Detroit silt loam | 1.33 mg/cm3 19% | 0.5-4.4 | 1.7 | 1.2 | 1.8 mg/cm3 31.4% | 0.4- 3.6 | 1.4 | 1.0 | 26.3 |
| Irrigation Well | Roxbury silt loam | 1.36 mg/cm3 18.5% | 5.3-24.4 | 15.0 | 14.9 | 1.69 mg/cm3 28.8% | 4.3-19.5 | 12.0 | 11.9 | 13.1 |
| C-19 | Roxbury silt loam | 1.36 mg/cm3 18.5% | 0.2-12.3 | 1.5 | 0.7 | 1.69 mg/cm3 28.8% | 0.15-4.5 | 1.2 | 0.6 | 10.8 |
| M-319 | Roxbury silt loam | 1.36 mg/cm3 18.5% | 0.5-28.1 | 6.4 | 1.2 | 1.69 mg/cm3 28.8% | 0.44-22.4 | 5.2 | 1.2 | Sample not collected |
| * Data from NRCS national soil survey characterization data (http://ssldata.nrcs.usda.gov/). | ||||||||||
The δ13C values in the soil cores reflect the occurrence of C4 plants as the primary source of carbon in the soil profile (Appendix B). Although the percent total organic carbon decreases with depth (as did the percent total nitrogen discussed previously), suggesting some bacterial degradation, the δ13C values do not generally show enrichment with depth except at the Irrigated Well area (figs. 1, 5e, 5f).
δ13C values and δ15N values for the sampled cores and ground water are compared in fig. 6. The soil cores were collected to approximately 3-m depth, whereas the water table is at 7-8 m.
Figure 6--δ13C and δ15N values for both soils and ground water in the study area. Graph indicates separation of carbon and nitrogen sources between soil and ground-water values.
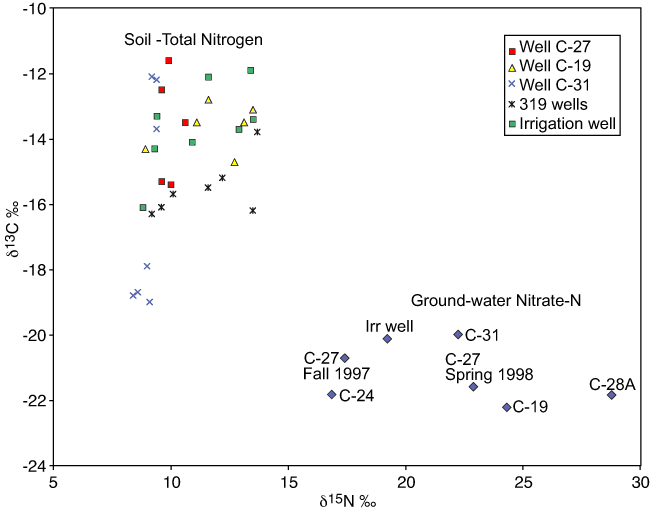
The δ13C values are enriched for the soils and depleted for the ground water indicating the possibility of different sources for the dissolved organic carbon. As discussed previously, the estimated soil nitrate-N values for the soils converted to mg/L (table 4) show an overall mean of 5.7 mg/L. The maximum calculated values for the area are a 28.1 mg/L surface sample taken near the M-319 monitoring wells and a 24.4 mg/L sample from the Irrigated Well area (fig. 1; table 4; Appendix B), with a decrease of nitrate-N and percent total nitrogen with depth at all sites. This suggests that nitrate-N in the soil profile is not the major source of nitrogen to ground water.
Sources of nitrogen and carbon to ground water enriched in δ15N and depleted in δ13C may be related to waste from animals utilizing C3 plants as a food source or from previous land use, such as farming of C3 plants. The soils collected for this study are enriched in δ13C and depleted in δ15N, which is expected with current C4 plants (grasses and crops) and microbial utilization of nitrogen. However, if soil organic carbon is the primary source of carbon to ground water, the δ13C values would be expected to be more similar in value.
Carbon isotopes in the soils represent current C4 plants, typical of grasses near the wells in the city areas and the sorghum in the irrigated field and fall in a higher range of values (-10 to -19‰). The δ13C of organic carbon in ground-water samples may represent C3 plant sources, such as wheat, trees, and shrubs, or perhaps human or animal waste (-22 to -30‰) (Gearing et al., 1991; Boutton, 1996; Mortatti et al., 2004). At this point, the 13C data are inconclusive as to the exact sources of the carbon. However, the data do suggest two separate sources for the carbon in the soil and ground water.
Prev Page--Introduction, Materials and Methods || Next Page--Conclusions and References
Kansas Geological Survey
Updated July 10, 2008
http://www.kgs.ku.edu/Current/2007/Townsend/03_result.html
email:webadmin@kgs.ku.edu