Kansas Geological Survey, Open-file Report 2017-9
KGS Open File Report 2017-9
May 2017
On October 23, 2015, Kansas Geological Survey researchers Drs. K. David Newell and John H. Doveton sampled natural gas evolving from an unused water well in southeastern Cowley County, Kansas. Robert Bowers, President of Millennium Oil and Gas, Inc. of Dexter, Kansas, assisted in the sampling.
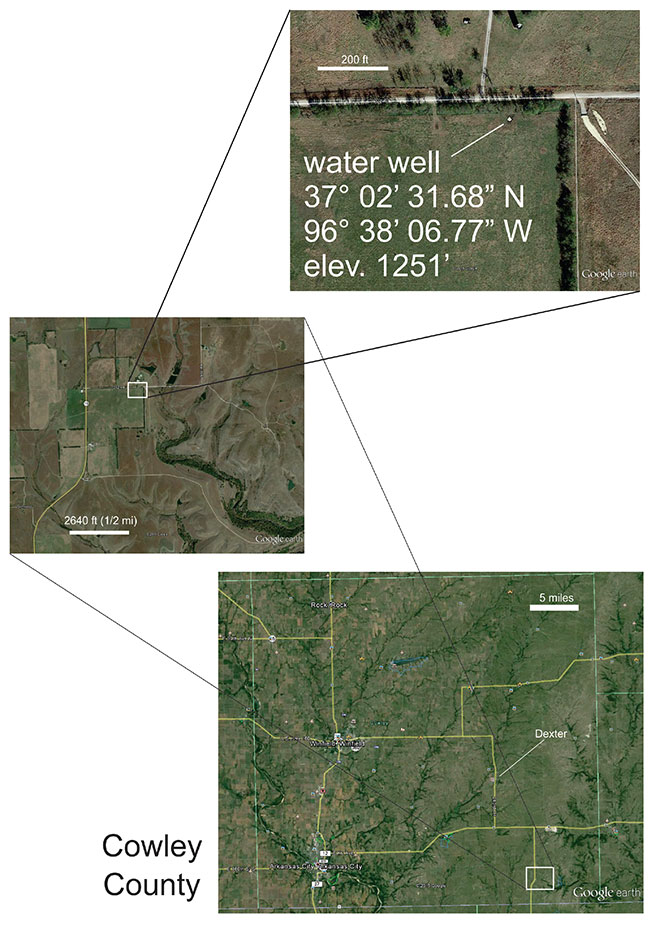
The location of the water well is N2 NE NE NW, sec. 02-T.35S.-R.07E. (see fig. 1). Samples were taken at atmospheric pressure in two recappable 500 ml glass bottles using a low-pressure sampling technique described in Jenden et al. (1988). The gas samples were sent to Isotech Laboratories in Champaign, Illinois, for compositional and isotopic analysis.
Figure 1--Location for water well in N2 NE NE NW sec. 02-T.35S.-R.07E.

The water well was drilled in 1964 to a depth of 194 ft (Robert Bowers, Millennium Oil and Gas, personal communication). The rancher contracting for the drilling never used the well for water for his cattle herd because the well produced natural gas. The well was never plugged or cased. No other water wells on the ranch had any problem with production of natural gas. Geologically, the well was drilled in to the upper part of the Council Grove Group, which crops out at the surface at the well locality.
A metal pipe was placed in the upper 10 feet of the well by Robert Bowers circa 2010 and a cement pad (approximately 8 ft in diameter and 1 ft thick) was poured, which secured the pipe, but the entire well was not cased to total depth. If the water-well pipe is closed in, the gas eventually evolves to the surface from around the pipe and bubbles out below the concrete pad, thus no accurate shut-in pressure measurement can be obtained. A slight pressure can immediately be obtained by placing a hand over the pipe to block its gas flow. Flow of the gas is continuous and a hydrocarbon odor is noticeable. No artesian water flows from the well but a bubbling sound can be heard if one's ear is placed over the open pipe.
An approximate flow rate was determined by noting how quickly a gallon plastic bag over the mouth of the sampling bottle filled with gas (see fig. 2). Approximately 10 gallons of gas were emitted from the well every three minutes. This volume, by unit conversion, corresponds to approximately 64 cubic feet of gas per day. Assuming the well has emitted gas at a constant rate from when it was drilled in mid-1964, the well is calculated to have produced 1,226,550 cubic ft of gas by the end of October 2015.
Figure 2--Gas sampling of the water well by K. David Newell (Kansas Geological Survey, in ball cap) and Robert Bowers (Millennium Oil and Gas, Inc.). Gas from the pipe fittings atop the water well flows through the plastic tube to the glass bottle. An open corner of the gallon plastic bag is duct-taped to the neck of the 500-ml glass bottle. The bag expands as gas fills the bottle. At least seven expansions and releases of gas from the plastic bag are allowed before the plastic tube is withdrawn from the bottle but retained inside the plastic bag. The glass bottle (green) is then capped by manipulation of its ceramic plug (white, attached to the neck of the bottle by wire, reinforced by a red rubber gasket) from the outside of the plastic bag. Once the bottle is capped, the plastic bag can be removed from the sampling bottle. Photograph by John H. Doveton.

Isotech Laboratories, Inc. (Champaign, Illinois) analyzed the gas on December 9, 2015. The following chemical components (by mole %) were reported:
| Carbon Monoxide | (not determined) |
| Helium | 0.405 |
| Hydrogen | (not determined) |
| Argon | 0.0349 |
| Oxygen | 0.13 |
| Nitrogen | 17.88 |
| Carbon Dioxide | 0.22 |
| Methane | 76.11 |
| Ethane | 3.54 |
| Ethylene | (not determined) |
| Propane | 1.05 |
| Propylene | (not determined) |
| Iso-butane | 0.168 |
| N-butane | 0.329 |
| Iso-pentane | 0.0726 |
| N-pentane | 0.0392 |
| Hexanes + | 0.0251 |
| Calculated total BTU/cubic ft @ 60 deg F & 14.73 psia = 883; calculated specific gravity = 0.667. |
| Methane δ13C = -53.78 ‰ |
| Methane δD = -190.3 ‰ |
| Ethane δ13C = -34.78 ‰ |
| Propane δ13C = -30.92 ‰ |
Assuming the oxygen in the gas sample represents residual atmospheric contamination in the sample bottle, the oxygen (as well as other atmospheric chemical species in their atmospheric ratio to oxygen) can be mathematically removed using the ratio of these atmospheric component gases to atmospheric oxygen (see Weaver, 1966).
| Helium | 0.408 |
| Argon | 0.0293 |
| Nitrogen | 17.50 |
| Carbon Dioxide | 0.22 |
| Methane | 76.58 |
| Ethane | 3.56 |
| Propane | 1.06 |
| Iso-butane | 0.169 |
| N-butane | 0.331 |
| Iso-pentane | 0.0731 |
| N-pentane | 0.0394 |
| Hexanes + | 0.0253 |
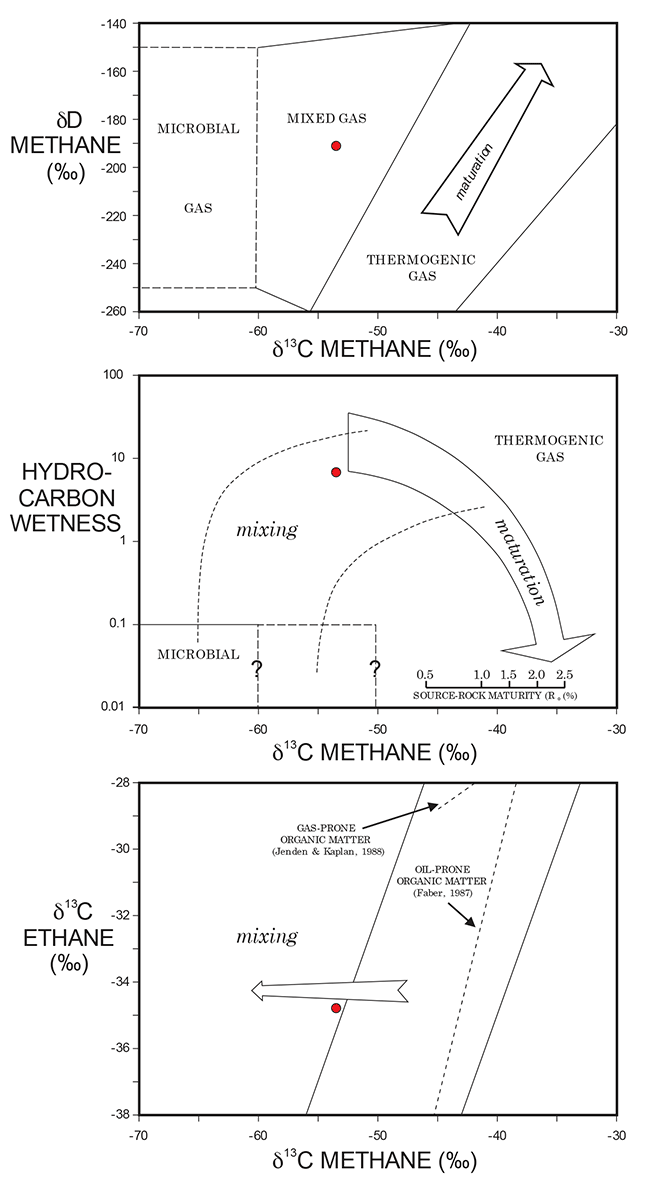
The methane gas in the sample likely represents mixing between two separate methane gases respectively produced by biogenic and thermogenic processes, according to the classifications of Kansas gases by Jenden et al. (1988) (see fig. 3). Due to the presence of heavier-molecular- weight hydrocarbons, the hydrocarbon gas in the water well likely indicates leakage from a deeper hydrocarbon reservoir.
Figure 3--Isotope composition fields (from Jenden et al., 1988), with water-well values (red dot) plotted.
In May 2003, Priority Analytical Laboratory (Wichita, Kansas) analyzed natural gas from Pennsylvanian Layton Sandstone (2115 to 2124 ft depth) at the Millennium Oil & Gas #1 Barnett well (SE NW SW, sec. 24-T.34S.-R.06E.; 5 1/2 miles to the west-northwest of the water well). This natural gas registered the following composition (Robert Bowers, Millennium Oil & Gas, personal communication):
| Carbon Monoxide | (not determined) |
| Helium | 0.12 |
| Hydrogen | 0 |
| Argon | (not determined) |
| Oxygen | 0.05 |
| Nitrogen | 18.31 |
| Carbon Dioxide | 0 |
| Methane | 58.28 |
| Ethane | 10.63 |
| Ethylene | (not determined) |
| Propane | 7.25 |
| Propylene | (not determined) |
| Iso-butane | 0.91 |
| N-butane | 2.03 |
| Iso-pentane | 0.49 |
| N-pentane | 0.58 |
| Hexanes + | 1.35 |
| Calculated total BTU/cubic ft @ 60 deg F & 14.73 psia = 1163; calculated specific gravity = 0.85. |
Assuming the oxygen in the gas sample represents residual atmospheric contamination in the sample bottle, the oxygen (as well as other atmospheric chemical species in their atmospheric ratio to oxygen) can be mathematically removed using the ratio of these atmospheric component gases to atmospheric oxygen (see Weaver, 1966). The resultant composition is
| Helium | 0.12 |
| Nitrogen | 18.17 |
| Methane | 58.42 |
| Ethane | 10.66 |
| Propane | 7.27 |
| Iso-butane | 0.91 |
| N-butane | 2.04 |
| Iso-pentane | 0.49 |
| N-pentane | 0.58 |
| Hexanes + | 1.35 |
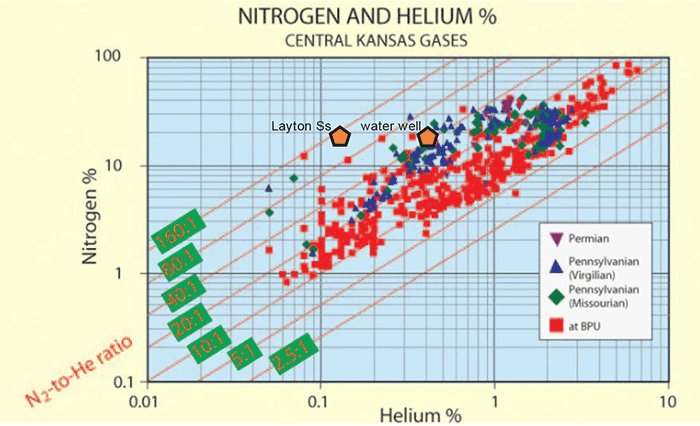
The N-to-He ratio for the water-well gas is 42.95. Its hydrocarbon wetness is 6.42%. The Layton Sandstone sample has an N-to-He ratio of 151.0; its hydrocarbon wetness is 28.51%. The N-to-He ratio for the natural gas from the water well is comparable to what is seen in Permian strata in Kansas (i.e., approximately 40:1; see Newell, 2007), but the percentage of helium (0.41%) is slightly lower than other Permian gases (mostly from central and western Kansas), that have helium percentages around 0.8 to 1.1% (see fig. 4).
Figure 4--Helium and nitrogen percentages for the water-well and nearby natural gas from the Layton Sandstone, compared to other Kansas gases (from Newell, 2007).
Faber, E., 1987, Zur isotopengeochemie gasformiger kohlenwasserstoffe: Erdo, Ergas, Kohle, v. 103, p. 210-218.
Jenden, P.D., and Kaplan, I.R., 1989, Origin of natural gas in Sacramento basin, California: American Association of Petroleum Geologists, Bulletin, v. 73, p. 431-453.
Jenden, P.D., Newell, K.D., Kaplan, I.R., and Watney, W.L., 1988, Composition and stable isotope geochemistry of natural gases from Kansas, Midcontinent, U.S.A.: Chemical Geology, v. 71, p. 117-147.
Newell, K.D., 2007, Geochemical trends in gas quality in Kansas: Kansas Geological Survey, Open-File Report 2007-8. [available online]
Weaver, E.C., 1966, Air; in, The Encyclopedia of Chemistry, 2nd. ed., G.L. Clark and G.G. Hawley, eds.: Van Nostrand Reinhold, New York, p. 31-32.
To view this report, you will need the Acrobat PDF Reader, available free from Adobe.
Kansas Geological Survey
Placed online May 4, 2017
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/OFR/2017/OFR17_9/index.html