Prev--Late Quaternary Palynology and Paleobotany || Next--Paleoindian sites in Kansas
Opal phytoliths from the epidermal cells of grass leaves occur with pollen and spores in atmospheric-dust deposits, soils, paleosols, and sediment of the Cenozoic Era in the Great Plains. Phytoliths can be traced to three groups of subfamilies of grass and to two photosynthetic pathways. Circular, rectangular, and elliptical forms (pooid class) occur in C3 grasses that thrive in cool temperatures and high available soil moisture. Saddle-shaped bodies (chloridoid class) occur in C4 grasses that flourish in warm temperatures where available soil moisture is low. Cross- and dumbbell-shaped phytoliths are dominant in C4 grasses that thrive in warm temperatures and high available soil moisture. All three classes of phytoliths occur with palynomorphs in sediment of the Great Plains and are important indicators of past climates and environments.
Grasses are probably the most widely distributed flowering plants on the earth today. The more than 600 genera and 7,500 species range from the warm tropics to within the Arctic Circle and include such diverse forms as bamboo, sugar cane, cereal grains, domestic grasses, and the tall and short grasses of the prairies (Gould and Shaw, 1983). The grasses reproduce sexually and asexually; asexual reproduction by development of stolens, rhizomes, and tillers is especially prevalent during years of drought (stress).
Commonly, the grasses and other flowering plants are classified mainly on the structure of the plant including emphasis on the structure of the flower and seed. Unfortunately, as with fossils of other organisms, the remains of the entire plant, or of distinctive parts, are extremely rare in the geologic record.
Grass-opal phytoliths from the epidermal cells of the leaf, culm, root, and inflorescence are released as discrete particles after the plant dies. Phytoliths are resistant to dissolution and accumulate in atmospheric dust, soil, paleosol, and sediment. Because of their size, phytoliths are transported along with pollen and spores and, if processed carefully, will be recovered with them.
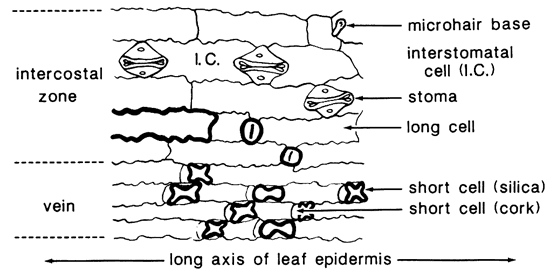
Taxonomists have long recognized the importance of the micromorphology of the leaf in the classification of grasses (Prat, 1936; Metcalfe, 1960). Distribution of epidermal cells in the grass leaf is illustrated by a spodogram of the corn leaf (Zea mays L.) in fig. 1. The organic matter has been removed by ashing at 450° C (842° F); only silicified opaline cells remain. The silica cells and one long cell have been nearly filled with bodies of opal-A (Jones and Segnit, 1971). which are recognized by marked negative relief (bold outlines) when mounted in Canada balsam (n = 1.54). The stoma and other cells have been encrusted with opal, so the relief is lower. Most phytoliths occur over the veins, but some cells in the intercostal zone are silicified also.
Figure 1--Spodogram of Zea mays L. (from Twiss and others, 1969, as adapted from Metcalfe, 1960).
In describing the micromorphology of 345 genera of grasses, Metcalfe (1960) used the orientation, location, concentration, size, and shape of the different types of cells and silica bodies as viewed on the surface of the leaf epidermis and in transverse section of the leaf. Even where the diagnostic vegetative characters are in growth position, classification is still difficult. Metcalfe (1960, p. xli) emphasized that
we are not dealing with a problem comparable with classifying postage stamps which conform to specific designs drawn up in agreement with a printing finn. We have before us biological material that does not conform exactly to immutable designs.
Consequently, Metcalfe (1960) arranged the descriptions of genera and species in alphabetical sequence and urged that this information be combined with that on exomorphology and the data supplied by cytologists, embryologists, and other specialists. He concluded (Metcalfe, 1960, p. lix) that "... it is only by a synthesis of these specialized approaches that any real advance in the taxonomic treatment of the Gramineae is to be expected." Relating discrete phytolith bodies from soil and sediment to the plant from which they came is even more difficult.
Ehrenberg (1847) recognized grass-opal phytoliths in several samples of atmospheric dust, some of which were collected on the H.M.S. Beagle by Charles Darwin. Ehrenberg believed these bodies to be microorganisms and proposed 10 genera and 90 species of Phytolitharia. This classification was genetic so that widely different-shaped bodies were placed under one genus. He also was instrumental in classifying diatoms, and many forms retain his names. Baker (1959a, 1959b, 1960a, 1960b) identified several shapes of phytoliths in atmospheric dust and soils and traced them to grass plants but did not propose a classification.
Relying heavily on the works of Prat (1936, 1948a, 1948b, 1951) and Metcalfe (1960), Twiss et al. (1969) proposed a morphological classification of discrete opal phytoliths that is related to grass taxonomy (fig. 2). The shapes of the phytoliths fall in four classes and 26 types that can be traced to three groups of subfamilies of grass. Class 4, the elongate class, consists of silicified long cells, from 30 to 150 micrometers long, and is characteristic of the grasses generally but cannot be used to identify subfamilies or tribes. Classes 1, 2, and 3 consist of silicified short cells and silica bodies from 10 to 35 micrometers long, which are representative of three groups or subfamilies; festucoid (pooid), chloridoid, and panicoid.
Figure 2--Classification of grass-opal phytoliths (adapted from Twiss and others, 1969).
|
I) FESTUCOID CLASS 1a. Circular 1b. Rectangular 1c. Elliptical 1d. Acicular, variable focus 1e. Crescent, variable focus 1f. Circular crenate 1g. Oblong 1h. Oblong, sinuous II) CHLORIDOID CLASS 2a. Chloridoid 2b. Thin chloridoid III) PANICOID CLASS 3a. Cross, thick shank 3b. Cross, thin shank 3c. Dumbbell, long shank 3d. Dumbbell, short shank 3e. Dumbbell, long shank, straight or concave ends 3f. Dumbbell, short shank, straight or concave ends 3g. Dumbbell, nodular shank 3h. Dumbbell, spiny shank 3i. Regular, complex dumbbell 3j. Irregular, complex dumbbell 3k. Crenate IV) ELONGATE CLASS (no subfamily characteristics) 4a. Elongate, smooth 4b. Elongate, sinuous 4c. Elongate, spiny 4d. Elongate, spiny with pavement 4e. Elongate, concave ends |

|
Sase and Kondo (1974) studied phytoliths from 23 wild-grass species and phytoliths recovered from the A-horizon of buried volcanic-ash soils in Hokkaido (Japan). They expanded the classification to seven classes by introducing the sasaoid class for bamboo, the fan-shape class, and the point-shape class. In the panicoid class, they added several types that are strings of complex dumbbells of types 3i and 3j of Twiss et al. (1969). Sase and Kondo (1974) stated that the silica bodies of the elongate, fan-shape, and point-shape classes were "nonspecific to taxonomic groups" of grasses. They determined that sasaoid phytoliths from bamboo were dominant in paleosols of the last 6,000 yrs, but earlier soils contained festucoid phytoliths.
Brown (1984) concentrated on 112 grass species common in central North America in developing a phytolith key based entirely on eight major shape classes. The eight classes are I, plates; II, trichomes; III, double outline; IV, saddles; V, trapezoids; VI, bilobates; VII, polylobates; and VIII, crosses. Each class is subdivided to accomodate the great range of forms and the key can be expanded as needed. This system includes many more shapes than any other classification system.
Brown (1984) also reported important exceptions to the classification of Twiss et al. (1969). Festicoid (pooid) types occur in nearly all grasses. Panicoid types form in species of Danthonia (subfamily Arundinoideae) and chloridoid saddles occur in Phragmites australis, the only species of tribe Arundineae native to the United States (Gould and Shaw, 1983, p. 129). All workers (Prat, 1948a; Metcalfe, 1960; Twiss et al., 1969; Brown, 1984) have cautioned that morphological classifications of discrete silica bodies can be related to grass taxonomy only with caution.
Because opal phytoliths occur in plants other than the grasses, Bertoldi de Pomar (1971) and Rovner (1971) proposed morphological classifications that include other monocotyledonous and dicotyledonous plants. All forms occur as discrete bodies and have been recovered from soil and sediment. As yet, no comprehensive classification of individual silica bodies derived from plants exists, so that any study of opal phytoliths in soils and sediment must include a careful study of the above references.
The grasses, as do other higher plants, possess two photosynthetic pathways: the Calvin-Benson cycle or C3 pathway and the Hatch-Slack cycle or C4 pathway. The C3 and C4 designations refer to whether the first fixed-carbon compound contains three or four carbon atoms. Both cycles are dark reactions which do not require light to occur and involve the formation of starch from carbon dioxide by a series of chemical reactions (Gould and Shaw, 1983, p. 48).
In the C3 photosynthetic pathway, carbon dioxide from the atmosphere enters the grass leaf through the stomate and passes through the epidermis to the mesophyll which contains the chloroplasts. Here, the CO2 is catalyzed and phosphoglyceric acid (PGA), containing three carbon atoms, is produced as the first fixed-carbon compound (Gould and Shaw, 1983, p. 48). The chlorenchymal cells contain chlorophyll and are distributed throughout the interior of the leaf and enclose the vascular bundles. This C3 pathway occurs almost universally in photosynthetic plants.
In the C4 photosynthetic pathway, carbon dioxide entering the leaf through the stomate into the mesophyll reacts with "phosphoenolpyruvate (PEP) carboxylase in the chlorenchymal cells to form the four-carbon molecule oxaloacetate (OAA)" (Gould and Shaw, 1983, p. 48). OAA is converted to form malic and aspartic acids which are transported into the bundle-sheath cells. Here the acids are decarboxylated and CO2 is released to the C3 cycle in the bundle-sheath cells.
The C3 grasses flourish in cool seasons or high elevations where available soil moisture is high. The warm-season C4 grasses occur in either areas of low or high soil moisture. Although the pathways are identified by the structure of the mesophyll, only opal phytoliths from the epidermis remain after the plant dies.
Twiss (1980, 1983) suggested that grass-opal phytoliths could serve as indicators of C3 and C4 pathways in grasses. The festucoid (pooid) class of phytoliths (fig. 2) occurs dominantly in taxa of Subfamily Pooideae which use the Calvin-Benson (C3) pathway and are most abundant in cool, moist climates. The chloridoid class (fig. 2) occurs in the arid to semi-arid, warm-season C4 grasses belonging to Subfamily Chloridoideae and requires less available soil moisture. The panicoid class (fig. 2) occurs in the warm-season, more humid C4 grasses of Subfamily Panicoideae. According to Gould and Shaw (1983, p. 110), more than 97% of native U.S. grass species (1,026 of 1,053) are divided equally among three subfamilies Pooideae, Chloridoideae, and Panicoideae.
In the United States, Subfamily Pooideae contains nine tribes and all utilize the C3 pathway. Gould and Shaw (1983) reported that of the 68 genera, 55 occur in the tribes of Poeae (17), Aveneae (30), and Triticeae (8); the dominant phytoliths are round, elliptical, crescent shaped, and long and narrow (festucoid and elongate classes). They also listed Stipeae as containing festucoid, cross-shaped, and dumbbell-shaped phytoliths, and Brachyelytreae, Nardeae, and Monermeae as containing dumbbell-shaped phytoliths. Species of this subfamily are most abundant in the northern United States and in high elevations where available soil moisture is high during the growing season.
The Subfamily Panioideae is represented in the United States by 32 genera and 325 species; both C3 and C4 pathways occur (Gould and Shaw, 1983, p. 118). All genera of tribe Andropogoneae are C4 grasses and contain panicoid phytoliths; however, Zea mays contains festucoid (pooid) types 1d and 1e (fig. 2) in the intercostal areas (Twiss and others, 1969). Tribe Paniceae is represented by 25 genera (20 are native) in the United States; some are C3 and some are C4. Even the genus Panicum contains 104 C3 species and 137 C4 species (Brown, 1977). The distribution and types of phytoliths in the Paniceae have not been reported in detail so that it is not known whether all species and genera contain panicoid phytoliths, or more importantly, whether the taxonomic associations are correct. The Subfamily Panioideae comprises the majority of grasses in the tropical and subtropical regions of the world; in the United States these grasses are most abundant in the east and south but do extend throughout the Great Plains.
The eight tribes of the Subfamily Chloridoideae are all C4 and are represented in the United States by 42 genera and 310 species of native grasses (Gould and Shaw, 1983, p. 120). These grasses are most abundant in the hot, arid climates near the tropics of Cancer and Capricorn. They are an old group and according to Hartley and Slater (1960), may have originated in tropical or subtropical Africa at least as early as the Oligocene. In the United States this group is most abundant in the Southwest (Gould and Shaw, 1983, p. 120). Saddle-shaped phytoliths are common in members of the Eragrosteae and Chlorideae (Brown, 1984), which account for 34 genera. Gould and Shaw (1983) have included tribe Aristideae with the Chloriodeae, but Brown (1984) lists eight species of Aristida that contain bilobate (panicoid) phytoliths, and Twiss et al. (1969) listed one species as containing both festucoid (pooid) and panicoid forms.
Although some exceptions do exist, festucoid, chloridoid, and panicoid phytoliths are related to the photosynthetic pathways used by the majority of grasses. Fortunately, the exceptions occur in rare groups, many of which are restricted to specialized environments. The C4 grasses can be split into two groups: chloridoid and panicoid. Chloridoid phytoliths represent those grasses which are abundant in relatively warm and dry areas with low available soil moisture. Panicoid phytoliths occur in grasses which flourish in warm and more moist areas where the available soil moisture is higher.
The specific gravity of opal phytoliths is below 2.3, so that recovery from soil and sediment can be accomplished by gravity separation after some preliminary treatment. Several techniques have been used (Twiss et al., 1969; Rovner, 1971; Carbone, 1977), but the technique of Fredlund and others (1985) is especially effective. From 2 to 250 grams of soil or sediment can be processed by 1) dissolution of carbonates with dilute hydrochloric acid, 2) dispersal and removal of some clay minerals with a solution of sodium pyrophosphate or sodium hexametaphosphate, and 3) flotation of phytoliths and other light constituents out of the heavier mineral fraction with zinc bromide (specific gravity 2.35), a water-soluble heavy liquid. Included in the light fraction are opal phytoliths from grasses and other plants, diatoms, siliceous sponge spicules, carbonized plant remains, and other light silicate minerals. The concentrate is weighed and aliquots are mounted in Canada balsam (n = 1.54) on petrographic glass slides, mounted on SEM stubs, and stored in vials.
Wilding et al. (1977) urged that the weight of the biogenic opal concentrate must be compared to the weight of the total soil or sediment. Biogenic opal is ubiquitous in atmospheric dust, soils and paleosols, and sediment of the Cenozoic Era. The quantity ranges according to geography, climate, geomorphology, plant species, maturity of the plant, and type of soil and sediment.
A large proportion (perhaps up to 80-90%) of plant opal cannot be identified and must be classed as "unclassified" forms. Wilding et al. (1977) reported that opal encrustations and epidermal hairs from dicotyledonous and moncotyledonous plants have been recovered from soils. Opaline trichomes, bulliform cells, silica cells, long cells, and large proportions of unidentified epidermal fragments of grasses have been recovered from atmospheric dust, soils, and ancient sediment (Wilding et al., 1977).
In reporting types of grass phytoliths, the following major classes are proposed: 1) sasaoid (Sase and Kondo, 1974), 2) pooid (festucoid of Twiss et al., 1969; some double outline, some trapezoids of Brown, 1984), 3) chloridoid (saddles of Brown, 1984), 4) panicoid (bilobates, polylobates, and some crosses of Brown, 1984), 5) elongate (plates of Brown, 1984), 6) fan-shape (Sase and Kondo, 1974), 7) point-shape (Sase and Kondo, 1974), and 8) unidentified. These major classes can be further subdivided using the types proposed by Sase and Kondo (1974), Twiss et al. (1969), and Brown (1984); any number of additional types can be added as needed. For paleoenvironmental interpretations, the types in the first four classes are most useful.
Careful sampling and processing reveal that opal phytoliths occur widely in the soil and sediment of the Quaternary and Tertiary periods and especially in the Great Plains.
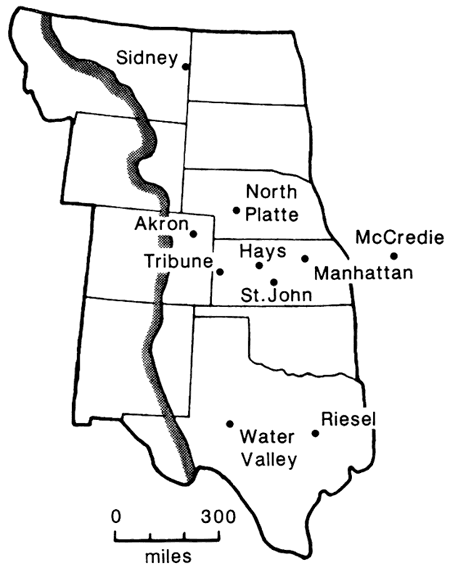
Collections of monthly deposits of dust from nine stations in the Great Plains (fig. 3) from 1963 to 1967 commonly contained grains of biogenic opal which were mostly from grasses (Smith and Twiss, 1965; Twiss et al., 1969; Smith et al., 1970; Twiss, 1983). The quantity of grass phytoliths ranged from 35% at North Platte, Nebraska, Tribune, Kansas, and Hays, Kansas, to 2% at Manhattan, Kansas, and Riesel, Texas, and at each station were most abundant in the spring and summer. The abundance of phytoliths and phytolith fragments correlated positively with the several wind parameters, number of dusty days, dust-deposition rates, median silt diameter, and abundances of quartz, smectite, and illite (Smith et al., 1970).
Figure 3--Dust-deposition network in the Great Plains (Twiss, 1983).
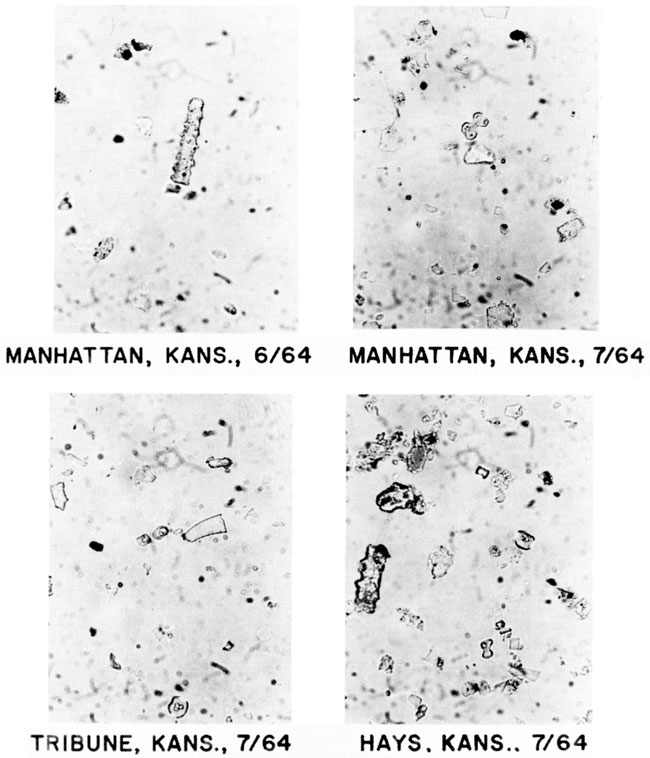
Photomicrographs of the silt-plus-sand fractions of the dust are shown in fig. 4. Most particles in the photos are biogenic opal and some can be positively identified as grass-opal phytoliths. Although the types of phytoliths have yet to be counted, a relationship does seem to exist between phytolith type and extant vegetation. Tribune, Kansas, is in the short-grass prairie and chloridoid and elongate phytoliths are common. Hays is in a region of mixed short and tall grasses; the dust deposits contain chloridoid and panicoid phytoliths that represent the two groups of C4 grasses. Manhattan, in the tall-grass region of the Flint Hills, contains panicoid phytoliths in the dust deposits.
Phytoliths in association with pollen grains, spores, freshwater diatoms, and silt-sized mineral grains can travel several hundreds of kilometers in the atmosphere (Ehren? berg, 1847; Folger et al., 1967; Folger, 1970). Grass phytoliths have been recovered from deep-sea sediments many kilometers from land (Ehrenberg. 1854; Kolbe, 1955; Diester-Haass et al., 1973; Parmenter and Folger. 1974).
Figure 4--Photomicrographs of grass phytoliths in atmospheric-dust deposits from Kansas: elongate (4c) phytolith (55 µm long) at Manhattan, June 1964; panicoid (3f) phytolith (18 µm long) at Manhattan, July 1964; chloridoid (2a, 2b) phytoliths (11 µm long) at Tribune, July 1964; and chloridoid (2a, 2b), panicoid (3d), elongate (4d), and unclassified phytoliths at Hays, July 1964.
Wilding et al. (1977) cited many examples of biogenic opal in soils and reported that opal phytoliths constitute the major part in nonaquatic environments ranging from less than 0.1 to 3% of the total soil. Some soils and alluvium in the Flint Hills region near Manhattan. Kansas, were examined (Twiss et al., 1969). In all samples, phytolith fragments and elongate phytoliths are dominant, followed by panicoid forms (fig. 5). The surface vegetation is dominated by tall grasses belonging to Subfamily Panicoideae.
Figure 5--Photomicrographs of grass phytoliths in extant soils and alluvium near Manhattan, Kansas. Gravity concentrates of biogenic opal that consist mostly of elongate phytoliths (115 µm long) and unclassified phytolith fragments.
The scanning-electron microscope, because of its magnifying power, depth of field, and tilt capabilities of the sample holder, is an effective instrument for examining the three-dimensional form of phytoliths (fig. 6). By studying the shape variations of type-3a phytoliths (fig. 2), Pearsall (1978, 1982) and Piperno (1979. 1980, 1984) have made advances in distinguishing maize phytoliths in soils from other types of panicoid grasses.
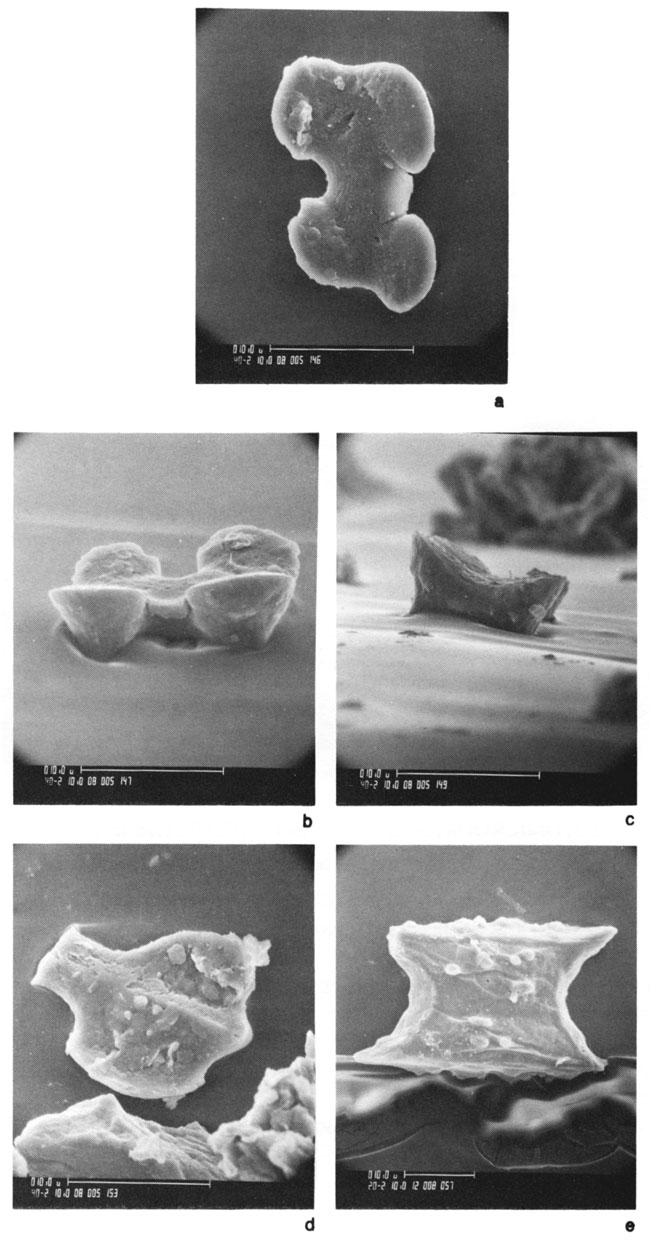
Figure 6--SEM photos of grass phytoliths in extant soils of the Flint Hills region in Kansas: a, b, and c) top view, side view, and end view of panicoid (3f) phytolith from the Hastings soil; d) broken panicoid (3e) phytolith in Hastings soil, and e) elongate (4e) interstomatal phytolith in Crete soil.
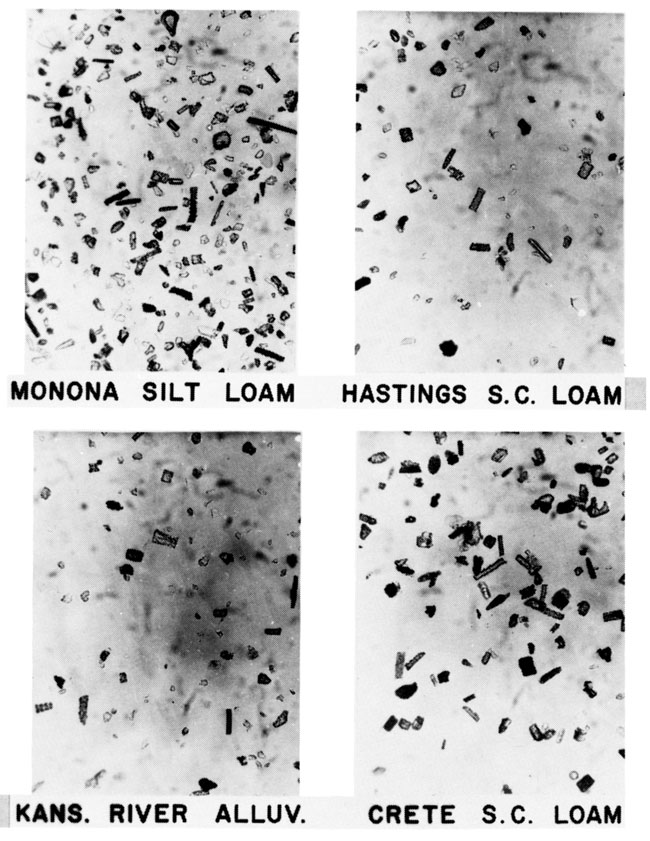
Gravity concentrates of phytoliths from two unnamed paleosols near Manhattan, Kansas, are shown in fig. 7. The Derby buried soil contains pooid and chloridoid phytoliths in addition to the panicoid, elongate. and unclassified fragments which occur in the Derby loamy sand. The surface vegetation consists dominantly of tall grasses of the Subfamily Panicoideae. The SEM photo (fig. 8) shows chloridoid, panicoid, and unclassified phytoliths in the buried soil below the Monona silt loam. All phytoliths contain small (about 300Å) spheres of opal-A (Jones and Segnit, 1971 ) that can be observed with highest magnification of the SEM (fig. 8). The SEM is useful in demonstrating that unidentified opal fragments possess this fine structure and are therefore biogenic.
Figure 7--Photomicrographs of grass phytoliths in buried soils near Manhattan, Kansas. Gravity concentrates of biogenic opal in unnamed paleosols below the Derby and Monona soils. The Derby buried soil contains pooid (festucoid; 1a, 1g), chloridoid (2a), panicoid (3f), elongate (4a, 4c), and unclassified phytoliths. The Monona buried soil contains panicoid (3d), elongate (4a, 4c), and many unclassified phytoliths.
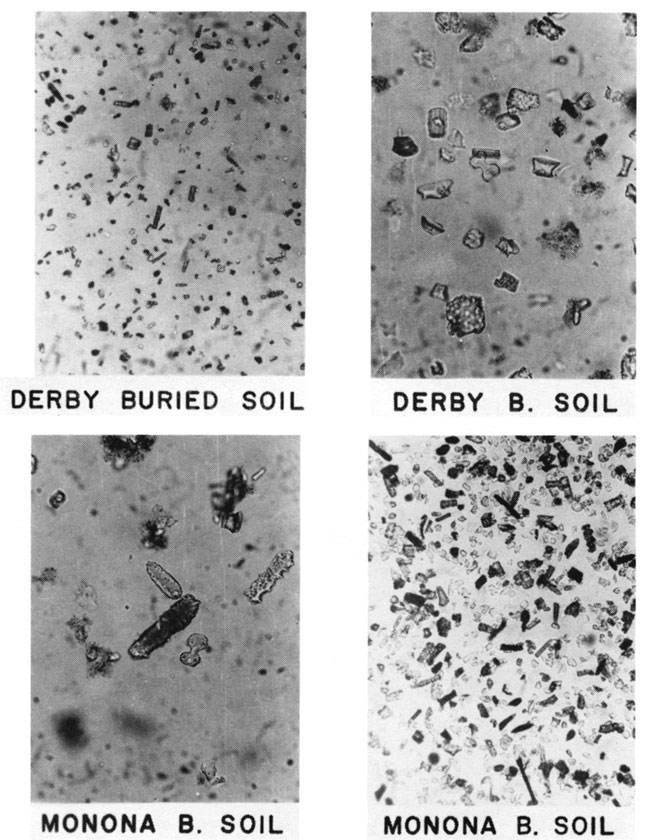
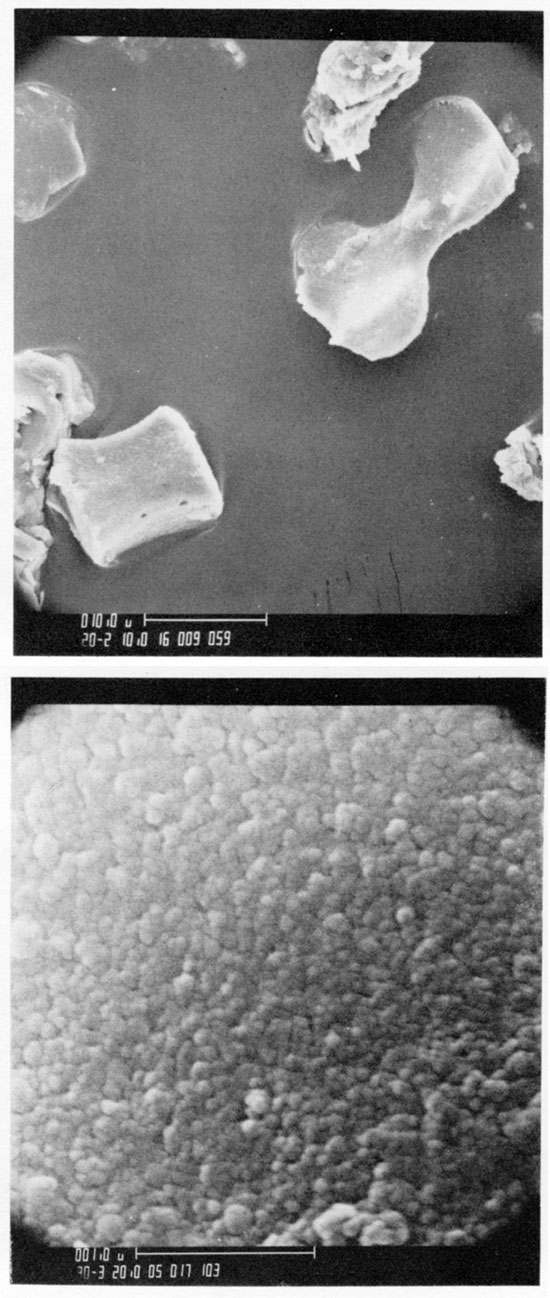
Figure 8--SEM photo of unnamed paleosol below the Monona silt loam in Geary County, Kansas. Gravity concentrate of biogenic opal contains chloridoid (2a), panicoid (3e), and unclassified opal phytoliths. Fine structure of biogenic opal shows opal spheres that are about 300Å in diameter without the conducting coating.
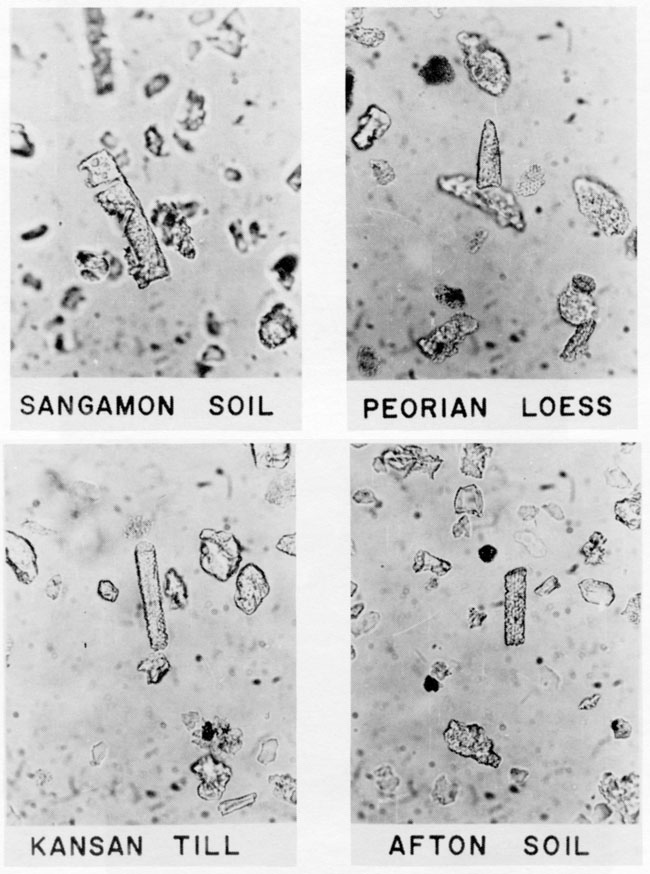
Four samples of Pleistocene sediment from the Iowa Point section in northeast Kansas contained severely corroded elongate and unclassified fragments of grass phytoliths (fig. 9). The paleosols and loess contained more phytoliths than the Kansas till. Others have reported phytoliths in sediment much older (Wilding et al., 1977; Baker, 1960b; Abbott, 1975).
Figure 9--Photomicrographs of grass phytoliths in Pleistocene sediment from the Iowa point section in Doniphan County, Kansas. Phytoliths are severely eroded elongate forms and unclassified phytolith fragments.
Pollen spectra are essential for interpreting the history of plant groups and species, plant communities, and climate (Moore and Webb, 1978, p. 2). Pollen diagrams of stratigraphic sections or boreholes are commonly arranged in groups from left to right, with arboreal types first, followed by nonarboreal types consisting of shrubs, then herbs, and finally spores. Gramineae usually are listed separately in pollen diagrams and their relative abundance can range from a trace to several percent to the total composition. Unfortunately, grass pollen cannot be traced to tribes or subfamilies of Gramineae.
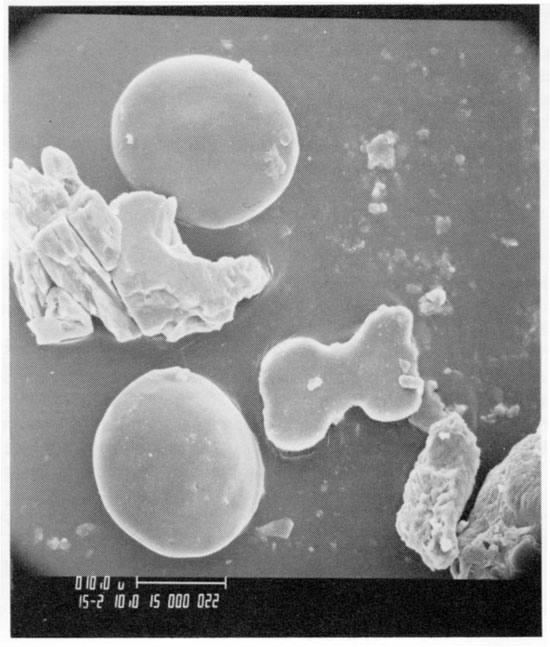
Because pollen, spores, and opal phytoliths have low specific gravities (<2.3) and are approximately the same size (10-150 micrometers), they are transported together by wind and water and occur together in soil and sediment. An SEM photograph (fig. 10) of a gravity separate of the Kansas till from the Iowa Point section in northeast Kansas shows two spherical pollen grains, a dumbbell-shaped (panicoid) opal phytolith, and three fragments of unidentified opal residue. The pollen grains are not diagnostic and are grouped under the Gramineae. The panicoid phytolith (fig. 2, type 3f) is probably from a C4 grass that flourished in warm seasons where available soil moisture was high. The combination of data from pollen. spores, and opal phytoliths can be powerful in interpreting the paleoenvironment.
Figure 10--SEM photo of gravity concentrate of Kansas till from the Iowa point section in Doniphan County, Kansas. Two spherical grains of grass pollen, a panicoid phytolith, and three unclassified opalized epidermal fragments are all from grass.
Few workers have reported palynological and opal-phytolith data together. Salgado-Labouriau (1978) reported panicoid phytoliths as discrete bodies and as epidermal fragments in pollen and spore rain in central Brazil. Kurmann (1981, 1985) identified opal phytoliths, pollen grains, and fungal spores in Kansas soils in order to correlate them with local vegetation. These results were applied to those of a paleosol. Pollen analysis was used to distinguish woodland sites from prairies but was unsuccessful in distinguishing tall-grass and short-grass prairie as was the phytolith analysis. Her results demonstrated the importance of combined phytolith and palynological analysis in characterizing woodland and grassland communities.
Fredlund et al. (1985) tabulated the abundance and type of opal phytoliths from a vertical loess section at the Eustis ash pit in Nebraska. They found that pooid (festucoid) phytoliths were the most abundant regular forms followed by significant vertical changes in the chloridoid and panicoid types. They concluded that increases in the proportion of chloridoid phytoliths in paleosol complexes indicated that the soil-forming periods must have been warmer and drier than the periods of loess accumulation. The data from opal phytoliths correlated well with the data from pollen analysis. Also they included an excellent review of the present status of opal-phytolith analysis.
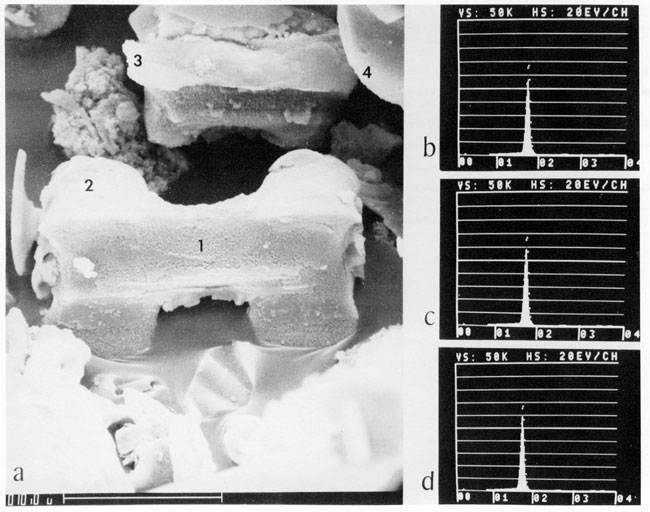
Some reasons why data from pollen and spore analyses have not been reported with those of phytolith analyses are differences in 1) processing of soil and sediment samples, 2) mounting media, 3) methods of examination, and 4) training and experience of scientists. Palynologists frequently treat samples with hydrofluoric acid to remove silicate minerals and siliceous residue from the pollen and pore concentrate, and this destroys opal phytoliths (fig. 11).
Figure 11--Morphology and composition of opal epidermal fragments from the lamina of Little Bluestem, Schizachyrium scoparium (Andropogon scoparius). Numerals in a) are locations of energy dispersive x-ray fluorescence analyses shown in (b). (c), and (d). Dots in b, c, and d are from location 1; solid peaks in b, c, and d are XRF analyses from 2, 3. and 4. respectively. The silicon peaks indicate that the epidermal fragments are nearly pure silica in the form of opal-A.
Because pollen and spores have refractive indices that range from 1.55 to 1.60, palynologists use mounting media of low refractive index (n = 1.40-1.47) to emphasize characteristic structural features (Faegri and Iversen, 1975, p. 111). The indices of the mounting media overlap those of phytoliths (n = 1.41-1.47) (Wilding et al., 1977, p. 472) so that phytoliths are barely visible in microscope mounts. Conversely, petrographic mounting media have indices near 1.54, which renders pollen and spores nearly invisible.
Palynologists commonly use phase-contrast microscopy to enhance the structure of the grains of pollen and spores. Most phytolith workers examine biogenic opal with a polarizing microscope which aids in mineral identification and observation of the external form of the opal. Generally, a worker lacks proficiency in one of these types of microscopy.
Finally, palynologists are specialists in the structure and formation of pollen grains and spores and in their dispersal and preservation. Those individuals interested in phytoliths often have been trained in other disciplines such as geography, geology, soil science, and archeology, and many have little knowledge of palynology. Opal phytoliths are not mentioned in standard texts in pollen analysis, paleobiology, or micropaleontology.
Using grass phytoliths for documenting paleoenvironments and paleoclimates is in its infancy. Although problems in classification of phytoliths and the distribution of these forms in specific grass taxa still remain unsolved, some generalizations can be made. Chloridoid phytoliths occur in C4 grasses that thrive in warm, relatively dry regions where the available soil moisture is low. Panicoid phytoliths occur dominantly in warm-season C4 grasses in regions where available soil moisture is high. Pooid phytoliths are abundant in cool-season C3 grasses but also occur in the intercostal zones of the epidermis of panicoid and chloridoid grasses. Perhaps ratios of classes of phytoliths may be the most useful for documentation.
Diester-Haass et al. (1973) proposed a climatic index that is the ratio of chloridoid phytoliths to the total number of chloridoid and panicoid phytoliths based on a total count of 100. A high index, close to 100, indicates an arid climate whereas a low index suggests a humid climate; the range gives the relative abundance of the two classes of C4 grasses in the source area.
Where pooid, chloridoid, and panicoid phytoliths occur, an index of the ratio of chloridoid to the total count of the three classes can be used as an aridity index. The ratio of C3 to C4 grasses can be approximated by comparing the ratio of pooid phytoliths to the total count of the three classes.
Studies are needed in which phytoliths from Holocene soils and sediment are compared to those that occur in extant vegetative regions. If these studies are successful. then phytoliths can be used with more confidence in interpreting environments throughout the Cenozoic Era.
Progress has been made in the morphological classification of discrete phytoliths from leaves of grasses. These forms reflect the photosynthetic pathway used by the grass and even whether the plant flourished in an arid, warm region or in a moist, warm region. Grass-opal phytoliths can add additional climatic information to pollen and spore analyses. Reconstructing paleoenvironments and paleoclimates is a multidisciplinary task; the value of opal phytoliths has yet to be realized.
Abbott, W. H., 1975, Miocene opal phytoliths and their climatic implications: South Carolina Division of Geology, Geological Notes, no. 2, p. 43-47.
Baker, G., 1959a, Opal phytoliths in some Victorian soils and "red rain" residues: Australian Journal of Botany, v. 7, p. 64-87.
Baker, G., 1959b, Fossil opal-phytoliths and phytolith nomenclature: Australian Journal of Science, v. 21, p. 305-306.
Baker, G., 1960a, Phytoliths in some Australian dusts: Proceedings of the Royal Society of Victoria, v. 72, p. 21-40.
Baker, G., 1960b, Fossil opal phytoliths: Micropaleontology, v. 6, p. 79-85.
Benoldi de Pomar, H., 1971, Ensayo de clasificacion morfologia de los silicofitolitos (Essay of morphological classification of silicophytoliths): Ameghiniana, Revista de la Asociacion Palentologica Argentina, v. 8, p. 317-328.
Brown, D. A., 1984, Prospects and limits of a phytolith key for grasses in the central United States: Journal of Archaeological Science, v. 11, p. 345-368.
Brown, W. V., 1977, The Kranz syndrome and its subtypes in grass systematics: Torrey Botanical Club, Memoirs, v. 23, 97 p.
Carbone, V. A., 1977, Phytoliths as paleoecological indicators: Annals of the New York Academy of Sciences, v. 288, p. 194-205.
Diester-Haass, L., Schrader, H. J., and Thiede, J., 1973, Sedimentological and paleoclimatological investigations of two pelagic-ooze cores off Cape Barbas, nonhwest Africa: Meteor Forschung-Ergebnisse, v. 16, p. 19-66.
Ehrenberg, C. G., 1847, Passatataub und Blutregen: Deutsche Akademie die Wissenschaften der Berlin Abhandung, p. 269-460.
Ehrenberg, C. G., 1854, Mikrogeologie, das Erde und Felsen schaffende Wirken des unsichtbar Kleinen selbstangigen Lebens auf der Erde: L. Voss, Leipzig, 317 p.
Faegri, K., and Iversen, J., 1975, Textbook of pollen analysis (3rd edition): Blackwell Science Publications, Oxford, 295 p.
Folger, D. W., 1970, Wind transpon of land-derived mineral, biogenic, and industrial matter over the Nonh Atlantic: Deep-Sea Research, v. 17, p. 337-352.
Folger, D. W., Burckle, L. H., and Heezen, B. C., 1967, Opal phytoliths in a Nonh Atlantic dust fall: Science, v. 155, p. 1,243-1,244.
Fredlund, G. G., Johnson, W. C., and Dort, W., Jr., 1985, A preliminary analysis of opal phytoliths from the Eustis ash pit, Frontier County, Nebraska: Institute for Tertiary-Quaternary Studies, TER-QUA Symposium Series, v. 1, Nebraska Academy of Sciences, Lincoln, p. 147-162.
Gould, F. N., and Shaw, R. B., 1983, Grass systematics: Texas A & M University Press, College Station, 397 p.
Hartley, W., and Slater, C., 1960, Studies on the origin, evolution, and distribution of the Gramineae; III. The tribes of the Subfamily Eragrostoideae: Australian Journal of Botany, v. 8, p. 256-276.
Jones, J. B., and Segnit, R. E., 1971, The nature of opal, Part 1: Geological Society of Australia, Journal, v. 18, p. 57-68.
Kolbe, R. W., 1955, Diatoms from equatorial Atlantic cores; in, Reports of the Swedish Deep-Sea Expedition, 1947-1948, Sediment Cores from the Nonh Atlantic Ocean, Hans Pettersson, ed.: Goteborg, Elanders Boktryckeri Aktiebolag, v. 7, p. 151-184.
Kurmann, M. H., 1981, An opal-phytolith and palynomorph study of extant and fossil soils in Kansas: M.S. thesis, Kansas State University, Manhattan, 81 p.
Kurmann, M. H., 1985, An opal-phytolith and palynomorph study of extant and fossil soils in Kansas (U.S.A.): Palaeogeography, Palaeoclimatology, Palaeoecology, v. 49, p. 217-235.
Metcalfe, C. R., 1960, Anatomy of the monocotyledons; I. Gramineae: Clarendon Press, Oxford, 731 p.
Moore, P. D., and Webb, J. A., 1978, An illustrated guide to pollen analysis: Halsted Press, New York, 133 p.
Parmenter, C., and Folger, D. W., 1974, Eolian biogenic detritus in deep-sea sediments--a possible index of equatorial Ice Age aridity: Science, v. 185, p. 695-698.
Pearsall, D. M., 1978, Phytolith analysis of archeological soils-evidence for maize cultivation in formative Ecuador: Science, v. 199, p. 177-178.
Pearsall, D. M., 1982, Phytolith analysis-applications of a new paleoethnobotanical technique in archeology: American Anthropologist, v. 84, p. 862-871.
Piperno, D. R., 1979, Phytolith analysis of archeological soils from central Panama: M.S. thesis, Temple University, Philadelphia, 99 p.
Piperno, D. R., 1980, Phytolith evidence of maize cultivation during the Early Ceramic (Monagrillo) Period: Paper presented at the 45th annual meeting of the Society for American Archaeology, Philadelphia, p. 85-86.
Piperno, D. R., 1984, A comparison and differentiation of phytoliths from maize and wild grasses-use of morphological criteria: American Antiquity, v. 49, p. 361-383.
Prat, H., 1936, La Systematique des Graminees: Annals des Sciences Naturelles (Series 10, Botanique), Paris, v. 18, p. 165-258.
Prat, H., 1948a, General features of the epidermis in Zea mays: Annals of the Missouri Botanical Garden, v. 35, p. 341-351.
Prat, H., 1948b, Histo-physiological gradients and plant organogenesis: The Botanical Review, v. 14, p. 603-643.
Prat, H., 1951, Histo-physiological gradients and plant organogenesis (Part II): The Botanical Review, v. 17, p. 693-746.
Rovner, I., 1971, Potential of opal phytoliths for use in paleoecological reconstruction: Quaternary Research, v. I, p. 343-359.
Salgado-Labouriau, M. L., 1978, Pollen and spore rain in central Brazil: Proceedings of the First International Conference on Aerobiology, Federal Environmental Agency, Berlin, p. 89-110.
Sase, T., and Kondo, R., 1974, The study of opal phytoliths in the humus horizon of buried volcanic-ash soils in Hokkaido: Research Bulletin of the Obihiro Zootechnical University, v. 8, p. 465-483.
Smith, R. M., and Twiss, P. C., 1965, Extensive gaging of dust-deposition rates: Transactions of the Kansas Academy of Science, v. 68, p. 311-321.
Smith, R. M., Twiss, P. C., Krauss, R. K., and Brown, M. J., 1970, Dust deposition in relation to site, season, and climatic variables: Soil Science Society of America, Proceedings, v. 34, p. 112-117.
Twiss, P. C., 1980, Opal phytoliths as indicators of C3 and C4 grasses: Geological Society of America, Abstracts, v. 12, p. 17.
Twiss, P. C., 1983, Dust deposition and opal phytoliths in the Great Plains; in, Man and the Changing Environments in the Great Plains, W. H. Caldwell, C. B. Schultz, and T. M. Stout, eds.: Nebraska Academy of Sciences, Transactions, v. 11, p. 73-82.
Twiss, P. C., Suess, E., and Smith, R. M., 1969, Morphological classification of grass phytoliths: Soil Science Society of America, Proceedings, v. 33, p. 105-115.
Wilding, L. P., Smeck, N. E., and Drees, L. R., 1977, Silica in soils--quartz, cristobalite, tridymite, and opal; in, Minerals in Soil Environments, J. B. Dixon and S. B. Weed, eds: Soil Science Society of America, p. 471-552.
Prev--Late Quaternary Palynology and Paleobotany || Next--Paleoindian sites in Kansas
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version updated March 31, 2010. Original publication date 1987.
URL=http://www.kgs.ku.edu/Publications/Bulletins/GB5/Fredlund/index.html