
Kansas Geological Survey, Chemical Quality Series 8, originally published in 1979
Next Page--Appendix

Originally published in 1979 as Kansas Geological Survey Chemical Quality Series 8. This publication is also available as an Acrobat PDF file (2.8 MB).
In western Kansas the demand from the domestic, agricultural, and industrial sectors for groundwater continues to grow steadily. Yet, many areas are faced with declining water tables, and some regions are confronted with water-quality problems arising from natural sources or human activity. All areas are subject to increasing cost for the production of groundwater. Thus, it becomes essential to understand both the quality and quantity relationships of the aquifer systems in this part of the state. Consideration should also be given to cycling or re-use of water, where feasible, in order to extend the lifetime of the resource and minimize pumping cost.
Groundwater from shallow alluvial valley systems tends to be of lower quality than water from the Ogallala Formation in northwestern Kansas. Frequently the existence of the poorer quality groundwater in these drainage ways is accompanied by the presence of saline soils. Irrigation waters from tail-water pits and the associated groundwaters in upland areas were found to be of comparable quality for their inorganic constituent levels, suggesting the possible re-use of tail waters in these regions. Future evaluation of tail-water quality in shallow alluvial valley systems and the accumulation of agrochemical organic residues in tail waters is needed.
Other areas covered by this series of studies are Greeley, Wichita, Scott, Lane, and southern Wallace counties (Kansas Geological Survey Chemical Quality Series 2); Hamilton, Kearny, Finney, and northern Gray counties (Chemical Quality Series 4); Stanton, Grant, Haskell, Morton, Stevens, Seward, Meade, and southern Gray counties (Chemical Quality Series 6); and Ford County and the Great Bend Prairie--Kiowa, Edwards, Pratt, Kingman, Stafford, Barton, Rice, and Reno counties (Chemical Quality Series 7). The final sampling in this program will be the Equus Beds area (1979).
Field work in northwestern Kansas during July 1978 yielded groundwater samples from 315 pumping irrigation wells and samples from tail-water pits associated with eight of those wells. The area covered by this sampling includes Cheyenne, Decatur, Gove, Logan, Rawlins, Sheridan, Sherman, Thomas, Wallace, and the western half of Graham counties.
A general increase in the amount of dissolved solids is found for waters associated with portions of the alluvial systems of the South Fork of the Republican River, Beaver Creek, and Sappa Creek in the northern half of the study area; and the Smoky Hill River and Hackberry Creek in the southern portion of the study area. Calcium-bicarbonate- to calcium-magnesium-bicarbonate-type waters are generally associated with upland areas where production is from the Ogallala Formation, but a transition toward sulfate-type waters is observed for many of the shallow alluvial systems in the study area. Waters from tail-water pits are of comparable quality to those of the producing wells.
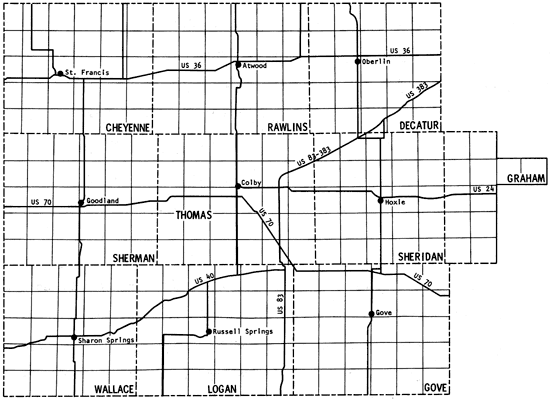
The present study is the fifth part of a program established in 1974 to obtain a chemical-quality data base for irrigation waters of western Kansas. Previous study areas are shown in Figure 1 (Hathaway and others, 1975, 1977, 1978a, 1978b), together with the location of the current one. Figure 2 is a general map of the study area. The present area (~9 1/3 counties) contains the region covered by Groundwater Management District No. 4 as well as two of the wells in southern Wallace County that were sampled in the 1974 study.
Figure 1--Map showing coverage of this report and related past reports in this series.
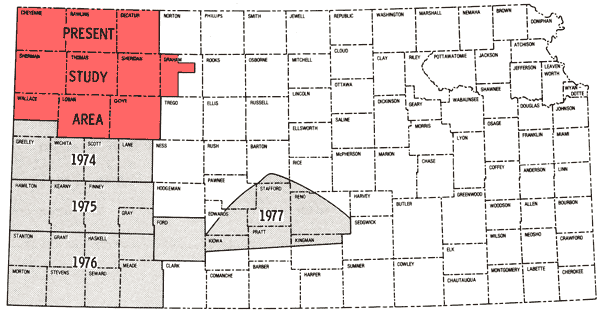
Figure 2--Location of the study area.
The Ogallala Formation of Pliocene age is the major source of irrigation water in the study area. The alluvial valley systems of the Smoky Hill, Saline, Solomon, and Republican rivers, and Beaver, Sappa, Prairie Dog, and Hackberry creeks also serve as sources of groundwater. Wells in the alluvial deposits are generally shallow. From historical data it has been noted that the general quality of the groundwater in these areas is poorer than that of water derived from the Ogallala Formation. Historical chemical-quality data exist for about 15 percent of the wells sampled in this study.
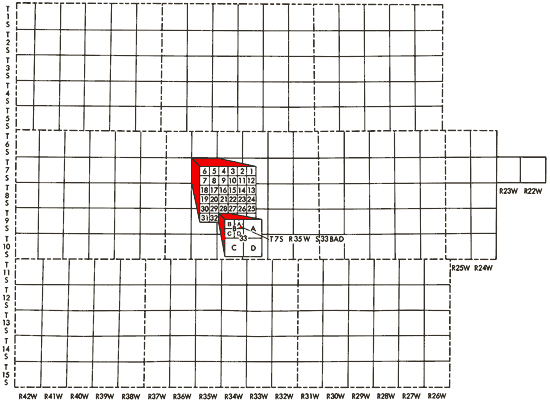
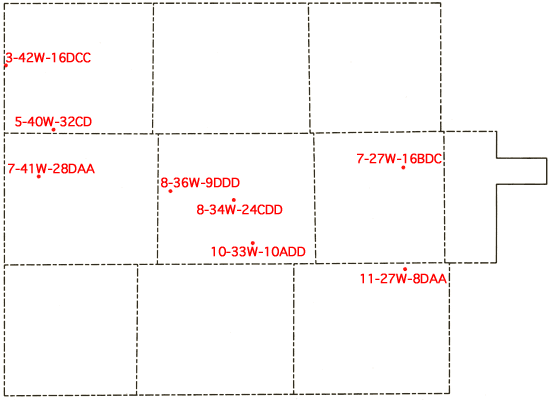
Well locations used in this report are based upon the Bureau of Land Management numbering system. The location is composed of the township, the range, and the section number followed by letters designating the subdivision of the section in which the well is located (Figure 3).
Figure 3--Ilustration of the Bureau of Land Management numbering system used for well locations in this report.
Detailed discussions of the general geologic features of the counties making up the present study area are found in a number of Kansas Geological Survey Bulletins (Prescott, 1953a, 1953b, 1955; Hodson, 1963, 1969; Hodson and Wahl, 1960; Johnson, 1958; Bayne, 1956; Walters, 1956; Frye, 1945; Elias, 1931; and Pearl and others, 1972).
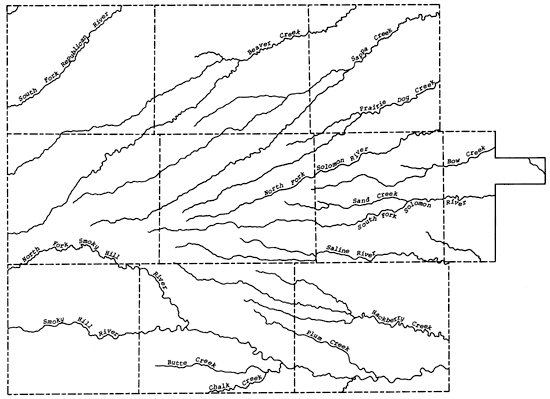
The present study area falls within the High Plains section of the Great Plains physiographic province. The general slope of the land surface is from west to east, with the upland plain area being a relatively flat to gently rolling surface that may exhibit numerous small depressions locally. Within the study area a general eastward flow of the groundwater is observed. The upland plain is highly dissected in the study area by the valley systems of the Republican, Saline, Solomon, and Smoky Hill rivers and Beaver, Sappa, Prairie Dog, and Hackberry creeks, which all trend in an easterly direction. Along the tributary and river valleys of the Smoky Hill River in Wallace, Gove, and Logan counties, the 14orth Fork of the Republican River in Cheyenne County, and the South Fork of the Solomon River in western Graham County, the Ogallala Formation has been eroded away, exposing the consolidated Upper Cretaceous bedrock units. Locally the Ogallala Formation may be cemented by calcium carbonate or silica into mortar beds that are generally exposed along the drainage ways in the study area. Figure 4 shows the principal drainage systems of the study area.
Figure 4--Drainage systems of the study area.
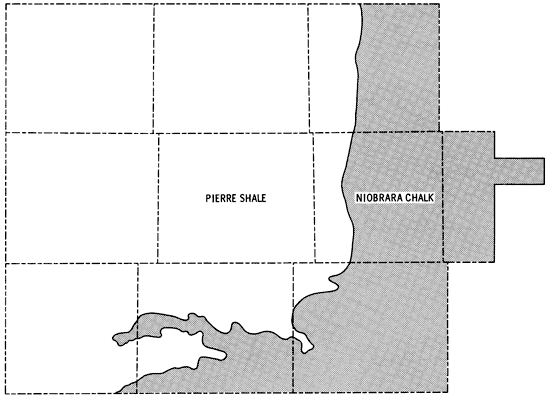
Bedrock in the study area is comprised of consolidated rocks of Upper Cretaceous age. In about the eastern third of the study area the Niobrara Chalk is the bedrock unit, but this gives way to Pierre Shale as one moves westward (Figure 5). Elias (1931) and Johnson (1958) have described faulting and folding in exposed units of the Upper Cretaceous rocks in Wallace and Logan counties. Dissolution of carbonate members followed by subsidence or slumping have been suggested as important factors in accounting for the disturbances in the Upper Cretaceous. The observed folding together of the Ogallala Formation and the underlying Pierre Shale in Wallace County may indicate the presence of other mechanisms as well. Exposures of -L-he Pierre Shale and Niobrara Chalk are prominent along the Smoky Hill River valley in eastern Wallace, Logan, and southern Gove counties.
Figure 5--Generalized bedrock geology of the study area.
Soils on the upland areas are derived from the loess mantle that overlies the Ogallala Formation, whereas the soils of the sloping landscapes of the drainage ways are related to the erosion of material of Pliocene and Pleistocene age. Exposures of the more resistant mortar beds in the Ogallala Formation occur along some of these drainage ways. Soils in the southeastern portion of the study area are derived from the Niobrara Chalk and typically exhibit a sparse vegetive covering and are subject to erosion on sloping landscapes. Saline soils are associated most notably with the alluvium of the Smoky Hill River valley.
Figure 6 presents a generalized soils association map of the study area, which has been produced through a combination of data for individual counties (Angell and others, 1978; Angell, in press; Angell and others, 1973; Barker, in press; Bell and others, 1964; Angell, 1978, written communication; Barker, 1978, written communication). This combination of data has involved some grouping of various soil associations presented in the general county soil maps and some regrouping of soil associations used in those reports in order to better reflect the pattern of soils in the natural landscapes for the multi-county area. Table 1 provides brief descriptions of the soil association groups used in Figure 6.
Figure 6--Soil association map.
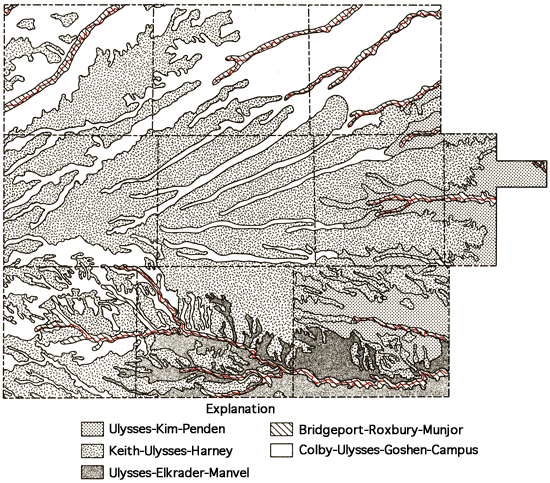
Table 1--Soil Association Groups
|
Groundwater samples were collected from 315 pumping irrigation wells and tail-water pits associated with eight of these wells over a four-day period, July 24-27, 1978. Historical chemical-quality data were found to exist for about 15 percent of the wells sampled, with two of the wells in Wallace County being ones included in the 1974 sampling program. Duplicate sets of samples were collected at 33 sites. Eighteen of the duplicate sets were the result of individual field personnel collecting duplicate sets of samples from wells within their sampling area (Individual Sets). Fifteen duplicate sets of samples were collected from wells located along the boundaries of different sampling areas by two individuals sampling the same well (Overlap Sets). Time intervals between collection of the sets at overlap sites varied from part of one day to three days. In one situation an individual duplicate site also corresponded to an overlap site.
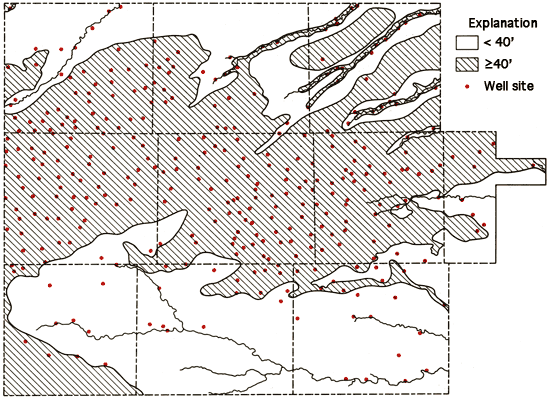
The location of the wells sampled in the study, as well as areas with generally less than 40 feet saturated thickness of unconsolidated sediments (Bayne and Ward, 1969), are shown in Figure 7. From this figure, it is readily apparent that the majority of the wells sampled are in the regions of 40 feet or more of saturated thickness. The others are generally associated with shallow groundwater systems of the valleys of the different drainage ways that dissect the study areal.
Figure 7--Location of wells in the study area and a generalized display of areas with 40 feet or more of saturated thickness.
Sample handling and analytical procedures for most constituents were similar to those described earlier (Hathaway, 1978b). Variations from the analytical routines used previously included the use of an autotitrimeter for the determination of carbonate (CO3) and bicarbonate (HCO3) levels of the water samples at a field laboratory established in Colby, Kansas. Specific conductance and pH measurements were also made at the field laboratory in order to evaluate the consistency of the field measurements made at the time of sampling. Technicon AutoAnalyzer II systems were employed for the analysis of phosphate (PO4) and sulfate (SO4) at the Kansas Geological Survey laboratories in Lawrence. The automated systems for the determination of HCO3, SO4, and PO4 thus replaced the manual systems used in prior studies.
As in the past, fluoride (F) analyses were made at the field laboratory and the determinations of silica (SiO2), calcium (Ca), magnesium (Mg), sodium (Na), potassium (K), strontium (Sr), chloride (Cl), nitrate (NO3), and the trace elements iron (Fe), manganese (Mn), copper (Cu), and nickel (Ni) were all made in the Kansas Geological Survey laboratories.
Samples collected from the eight tail-water pits consisted of a one-liter unacidified sample (for major constituents) and one 250 ml acidified sample (for NO3 and PO4). The sample for NO3 and PO4 was collected into a pre-acidified bottle and filtered upon returning to the Kansas Geological Survey laboratories. This procedure may result in release of PO4 from suspended sediment in the sample, but probably better reflects the potential available PO4 levels of these waters if they are to be recycled onto the fields.
Standard deviations for determinations of the major constituents, based upon the 18 individual and 15 overlap duplicate sets of samples, are presented in Table 2. The magnitudes of the standard deviations noted here for both types of duplicates are comparable to those reported for earlier studies. It is interesting to note that the spread in values of the specific conductance as determined in the laboratory is similar for both types of duplicates and much tighter than those determined by individuals collecting the overlap sets of duplicates. It also appears that use of the Technicon Auto-Analyzer II system for SO4 analyses has reduced the standard deviation for analyses of both types of duplicates by about a factor of two compared to values listed for previous studies.
Table 2--Standard deviations of data for duplicate sets.
| Determination | Individual Duplicate Sets ±σ |
Overlap Duplicate Sets ±σ |
|---|---|---|
| SiO2 | 1.6 ppm* | 2.0 ppm* |
| Ca | 0.3 ppm | 1.0 ppm |
| Mg | 0.2 ppm | 0.6 ppm |
| Na | 1.0 ppm | 0.8 ppm |
| K | 0.08 ppm | 0.2 ppm |
| Sr | 0.04 ppm | 0.1 ppm |
| HCO3 | 1.0 ppm | 0.9 ppm |
| SO4 | 0.8 ppm | 1.3 ppm |
| Cl | 0.04 ppm | 1.0 ppm |
| F | 0.03 ppm | 0.05 ppm |
| NO3 | 0.0 ppm | 1.4 ppm |
| Total Dissolved Solids | 6.7 ppm | 12 PPM |
| Specific Conductance (Field) | 40 μ mho | |
| Specific Conductance (Lab) | 2.6 μ mho** | 4.9 μ mho** |
| *parts per million or milligrams per liter **micro-mhos at 25°C 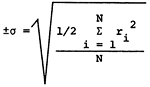 ri = range of analysis of sample N = number of sample pairs |
||
A general compilation of the chemical-quality data for irrigation wells within the study area is given in Appendix A by county and location. A comparison of waters from wells and associated tail-water pits is found in the RESULTS AND DISCUSSION section of this report.
The areal chemical-quality data presented in this report were plotted manually using the 40-foot saturated thickness contour of Map M-5 (Bayne and Ward, 1969) as a general boundary for mapping purposes. Areas with generally less than 40 feet of saturated thickness are represented by open spaces in Figure 7. Chemical-quality data from the 1974 sampling program for southern Wallace County have been used in the production of the current areal maps in order to provide a more complete coverage of the County. This approach seems reasonable in view of the consistency of the chemical-quality data for the two wells in Wallace County that were sampled in both 1974 and 1978.
A general impression of the variation in chemical quality of irrigation waters within the study area can be obtained from the specific-conductance data displayed in Figure 8. Table 3 lists minimum, maximum, and mean values of the major constituents, total dissolved solids, and specific conductance of the irrigation waters.
From Figures 6-8 it is apparent that higher specific-conductance values and higher dissolved-solids levels are generally.associated with shallower wells in the alluvial valleys of the drainage ways in the study area. Wells in the valleys of the South Fork of the Republican River, Smoky Hill River, and Hackberry Creek generally are associated with less than 40 feet of saturated thickness of the aquifer unit. Historical data also show that waters from these valley systems have tended to contain higher dissolved-solids levels than water from wells in upland regions or wells associated with a greater saturated thickness.
Table 3--Summary of ranges and means for chemical-quality data.
| Variable | Min/Max (ppm) | Mean (ppm) |
|---|---|---|
| SiO2 | 17/68 | 46 |
| Ca | 25/343 | 59 |
| Mg | 7.6/145 | 19 |
| Na | 5.9/234 | 34 |
| K | 3.0/24 | 7.3 |
| Sr | 0.4/6.0 | 0.9 |
| HCO3 | 161/518 | 246 |
| SO4 | 7.6/1524 | 71 |
| CL | 1.8/123 | 17 |
| F | 0.4/2.6 | 1.3 |
| NO3 | 0.0/92 | 18 |
| Total Dissolved Solids | 166/2441 | 387 |
| Specific Conductance micro-mhos at 25°C |
310/2900 | 581 |
Figure 8--Specific conductance map. Units = µMho
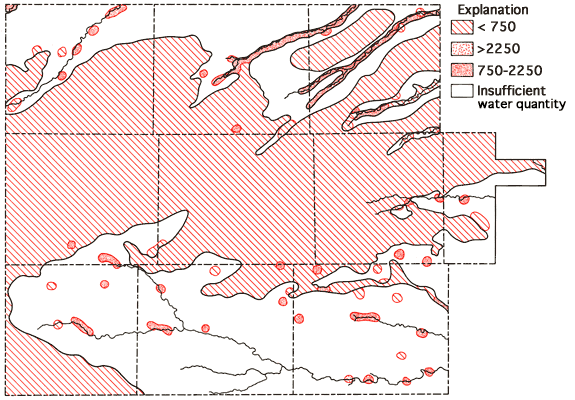
Figure 9 presents Stiff diagrams (Stiff, 1951) for selected wells from the study area. With the aid of these diagrams, it is possible to obtain a general impression of the magnitude and composition of the dissolved salt loads of waters associated with the alluvium systems of the drainage ways as compared to waters derived from the Ogallala Formation. Waters from alluvium of the valley systems all show increased dissolved-solids loads relative to nearby Ogallala waters, with changes in the levels of Ca, Na, Mg, SO4, and Cl being most noticeable. This increase is most pronounced along the lower reaches of the South Fork of the Republican River, Hackberry Creek, and the Smoky Hill River in the study area. Waters from the lower portions of these three drainage systems all exhibit higher sulfate levels than neighboring Ogallala waters, and in fact sulfate tends to become the dominant anion in these waters. Increased SO4 levels in these shallow alluvial systems may be the result of prolonged contact of the water with the gypsum-bearing Pierre Shale, as suggested by Walters (1956) to explain the poorer quality of waters from alluvial and terrace deposits in Rawlins County. Or these levels may result from accumulation in the sediments of the drainage ways of salts generated through natural weathering processes at the surface. Both processes may be important locally. The occurrence of saline soils in parts of the valley systems suggests that salt accumulation, with resultant decrease in water quality, may be of importance locally in these shallow aquifer systems.
Figure 9--Areal display of Stiff diagrams for selected wells.
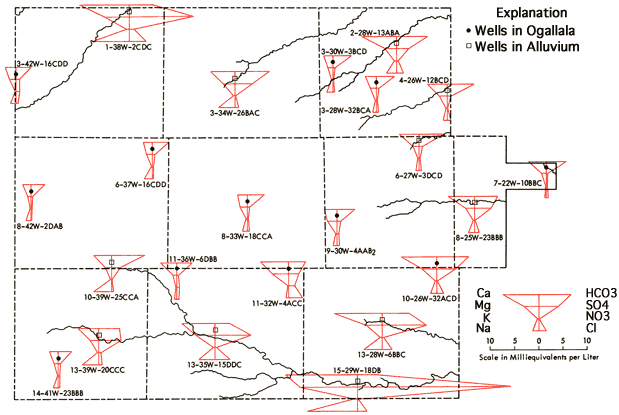
Figure 9 also provides a generalized representation of the transition in chemical quality of waters associated with the Smoky Hill River valley system, in which dissolved solids and sulfate levels both tend to increase as one progresses down the valley system.
The specific-conductance data of Figure 8 suggest the same trend also holds with respect to the dissolved-solids levels in waters associated with the drainage systems of the South Fork of the Republican River, Solomon River, Saline River, Hackberry Creek, Beaver Creek, Sappa Creek, and portions of Prairie Dog Creek in the present study area. Elevated dissolved-solids levels in waters from the alluvium of the drainage systems are frequently associated with the occurrence of saline soils, especially in areas where sulfate becomes the dominant anion in the groundwaters.
The Stiff diagram for well location 11-32W-4ACC, west of Oakley, indicates a higher dissolved-solids level than that of typical Ogallala water from that area. In addition, the Na/Cl ppm ratio for this water is 0.11, a value well below that for rock salt (0.65) or those that appear typical of oil-field brines in Kansas (0.40-0.60). A Na/Cl ppm ratio of 0.30 is observed at 11-30W-31DAB, about 10 miles southeast of Oakley along the North Fork of Hackberry Creek. The Cl levels for 11-32W-4ACC (100 ppm) and 11-30W-31DAB (78 ppm) are higher than those of neighboring wells that are located in the Ogallala Formation (13-44 ppm). The concentrations of Ca, Mg, and SO4 also appear to be anomalously high at 11-32W-4ACC. Thus, the trends in the Cl levels and Na/Cl ratios for these two wells and the Stiff diagram for 11-32W-4ACC all suggest the possibility of a source relatively rich in Ca-Mg-SO4-Cl located west of Oakley that may become mobilized and transported down the North Fork of Hackberry Creek during periods of heavy rainfall and localized flooding.
The areal distribution patterns for many of the major and minor chemical components of the dissolved-solids load of the groundwaters from the study area are similar to the trends noted for the specific-conductance data (Figure 8). An increase in the levels of Ca, Mg, Na, K, Sr, SO4, Cl, and PO4 is generally noted for waters from the alluvial valleys of the drainage ways. Higher PO4 values (>0.25 ppm) are generally found in systems of the northeastern quadrant of the study area, but higher values of Sr (>2 ppm) and SO4 appear to be most common along the Smoky Hill River valley. Potassium levels in excess of 10 ppm are observed for wells in alluvium throughout the study area. In general, the build-up of these chemical species in the alluvial systems appears to be the result of natural processes of weathering, transport to, and accumulation in the soils and waters of the lower valley areas.
In the case of F, levels equal to or greater than 1.7 ppm seem to be more abundant in the northwestern quadrant of the study area; whereas SiO2 values greater than 50 ppm are most common in the northern half of the study area. Nitrate values above 30 ppm appear to be randomly scattered throughout the study area.
Elevated levels of Fe, Mn, Cu, and Ni may result from contamination at the point of sample collection and/or from increased levels of these trace elements in the local groundwater systems. Higher values of Fe and Mn are generally associated with waters from alluvial valley systems. The rusty color of unacidified samples from the Smoky Hill River valley in Gove County suggests that more than simple contamination is responsible for the levels of Fe and Mn observed in these samples. The Fe levels in waters collected in this study from upland areas generally are observed to be less than 50 ppb (parts per billion or micrograms per liter), which is in sharp contrast to values as high as 10 ppm that were reported by Frye (1945) for Thomas County. The higher levels of Fe found in the historical data relative to the present study probably reflect insufficient pumping of the wells prior to sample collection rather than actual chemical-quality changes in the waters of the area.
Figures 10 and 11 represent areal groundwater classification by cation and anion types, respectively. Water-type classifications used in this report are based upon percent milliequivalent contributions of the various chemical species to the total number of milliequivalents of cations or anions. Conversion of the chemical-quality data of Appendix A from parts per million to milliequivalents per liter can be achieved by use of the factors listed in Table 4. These milliequivalent-per-liter values reflect the combining capacities of the various chemical species in the waters.
Table 4--Factors for conversion from parts per million to milliequivalents per liter.
| Species | Multiply By |
|---|---|
| Calcium (Ca) | 0.04990 |
| Magnesium (Mg) | 0.08226 |
| Sodium (Na) | 0.04350 |
| Potassium (K) | 0.02557 |
| Strontium (Sr) | 0.02283 |
| Bicarbonate (HCO3) | 0.01639 |
| Sulfate (SO4) | 0.02082 |
| Chloride (Cl) | 0.02821 |
| Fluoride (F) | 0.05264 |
| Nitrate (NO3) | 0.01613 |
Figure 10--Groundwater classification by cation type.
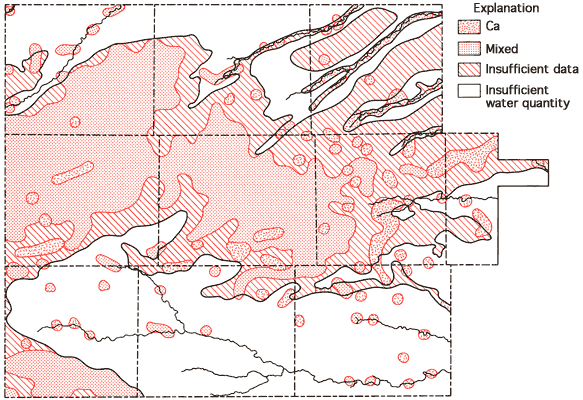
Figure 11--Groundwater classification by anion type.
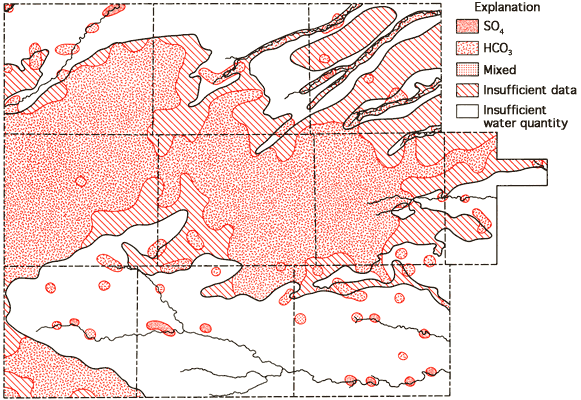
Areas of no control shown in Figures 10 and 11 reflect regions where the sample density is too low to allow extension of the water-type boundaries. Based upon these two figures, it appears that mixed cation-HCO3 waters (generally Ca-Mg-HCO3 are most common in the western twothirds of the study area, with Ca-HCO3-type waters becoming the dominant form in the eastern third of the study area. This change in classification is probably the combined result of a general decrease in the saturated thickness and the transition of the bedrock in this area from the Pierre Shale (western two-thirds) to the Niobrara Chalk (eastern one-third). The increased importance of SO4 in waters of the South Fork of the Republican River, the Smoky Hill River, and Hackberry Creek valleys is also apparent from Figure 11.
Sodium adsorption ratio (SAR) values are computed from the chemical-quality data using the equation
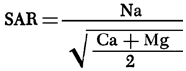
where values in parentheses are the milliequivalents per liter of the specific ions. Figure 12 is a map of SAR values for groundwaters from the study area. SAR values for the study area are observed to be generally less than 1.5, with values in excess of 1.5 most frequently being associated with waters near drainage systems.
Figure 12--Areal display of SAR values for the study area.
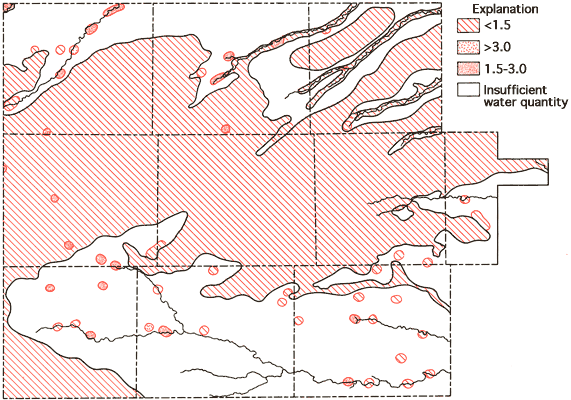
The specific-conductance data (Figure 8) and the SAR data (Figure 12) can be used in conjunction with Figure 13 to evaluate the general suitability of groundwaters for irrigation purposes (U. S. Salinity Laboratory Staff, 1954). Generally speaking, most portions of the study area with 40 feet or more of saturated thickness fall into the medium salinity-low alkali hazard classification and, as such, would not be expected to produce detrimental effects in the soil as a result,of prolonged irrigation practices. In the alluvial valley systems, especially areas of shallow water tables and limited saturated thickness such as the Smoky Hill River and South Fork of the Republican River, compatibility between the soils and groundwater may become a problem when considering long-term irrigation usage.
Figure 13--Diagram showing relationship between specific conductance and SAR in the evaluation of salinity and alkali hazards for irrigation.
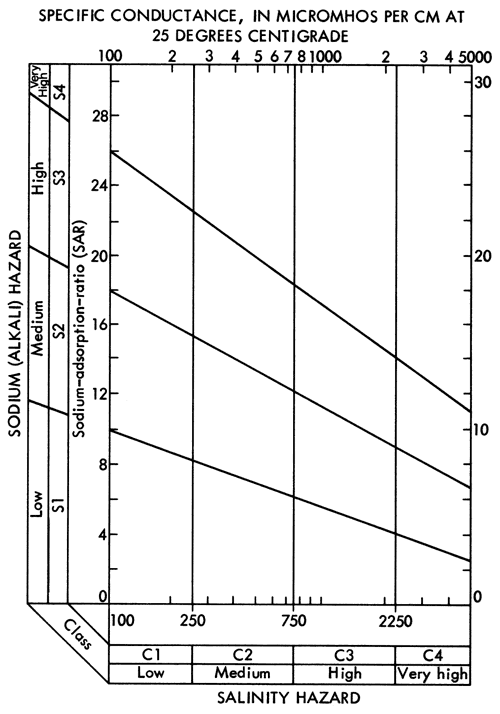
The utilization of tail-water pits to collect residual water in areas where flood irrigation is used has been mandated. The question of re-use of this water then arises since the cost of pumpage of water from the tail-water pits is considerably less than from deep wells. Water samples were collected from eight tail-water pits and their associated irrigation wells in order to evaluate changes in water quality that might occur as a result of irrigation water leaching salts from surface soils. The locations of the tail-water pits are shown in Figure 14, and the chemical-quality data for the tail-water pits and associated irrigation wells are presented in Table 5.
Figure 14--Location of tail-water pits sampled in study.
Two general trends are apparent in the data from Table 5. First increased temperatures of the tail-pit waters relative to the groundwaters are accompanied by increased pH values and the occurrence of carbonate (CO3) in the tail-pit samples. This probably results from loss of dissolved carbon dioxide gas upon warming of the water at the ground surface, allowing conversion of some HCO3 to CO3. Secondly, a consistent increase is observed in K and PO4 levels in tail-pit waters. This may reflect some mobilization of soil amendments during the irrigation process. It has been noted earlier (SAMPLING AND ANALYSIS Section) that the samples for PO4-NO3 analyses were acidified in the field and filtered later in the laboratory. Thus, the increase in PO4 levels in the tail-pit waters may also reflect release of PO4 from suspended matter in the water samples, but should also reflect in a general fashion available PO4 levels in these waters.
Table 5--Chemical-quality data for tail-water pits and associated irrigation wells.
| Location | Sp Cond µmho |
Temp °C |
pH | SAR | SiO2 ppm |
Ca ppm |
Mg ppm |
Na ppm |
K ppm |
Sr ppm |
HCO3 ppm |
CO3 ppm |
SO4 ppm |
Cl ppm |
F ppm |
NO3 ppm |
PO4* ppm |
Total Dissolved Solids ppm |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-42W-16CDD | Well | 465 | 18.5 | 8.1 | 0.82 | 66 | 43 | 16 | 25 | 7.4 | 0.8 | 184 | 0 | 33 | 11 | 1.4 | 42 | 0.2 | 329 |
| 3-42W-16DCC | Pit | 485 | 27.0 | 8.6 | 0.92 | 62 | 43 | 16 | 28 | 8.6 | 0.8 | 174 | 6.5 | 32 | 12 | 1.3 | 42 | 0.4 | 322 |
| 5-40W-31DB | Well | 435 | 15.0 | 8.0 | 1.50 | 63 | 31 | 12 | 39 | 5.8 | 0.7 | 203 | 0 | 21 | 8.9 | 1.3 | 23 | 290 | |
| 5-40W-32CD | Pit | 425 | 27.0 | 8.8 | 1.57 | 56 | 33 | 9.7 | 40 | 7.7 | 0.4 | 170 | 16 | 22 | 8.6 | 1.4 | 21 | 0.6 | 266 |
| 7-27W-16BAA | Well | 415 | 15.0 | 7.7 | 0.51 | 48 | 43 | 19 | 16 | 6.7 | 0.8 | 226 | 0 | 15 | 5.5 | 1.3 | 12 | 280 | |
| 7-27W-16BDC | Pit | 405 | 23.0 | 8.5 | 0.56 | 48 | 42 | 16 | 17 | 9.3 | 0.7 | 215 | 5.3 | 16 | 5.0 | 1.3 | 11 | 0.4 | 262 |
| 7-41W-28DBB | Well | 340 | 15.5 | 8.0 | 1.01 | 59 | 31 | 9.2 | 25 | 4.2 | 0.4 | 178 | 0 | 16 | 3.4 | 1.2 | 10 | 0.1 | 242 |
| 7-41W-28DAA | Pit | 335 | 27.0 | 8.6 | 1.07 | 49 | 32 | 7.6 | 26 | 7.2 | 0.3 | 158 | 8.2 | 18 | 3.7 | 1.1 | 9.6 | 0.7 | 215 |
| 8-34W-24BAD | Well | 480 | 16.0 | 7.7 | 0.59 | 54 | 46 | 20 | 19 | 6.2 | 0.6 | 227 | 0 | 33 | 9.3 | 1.4 | 13 | 0.2 | 293 |
| 8-34W-24CDD | Pit | 460 | 22.0 | 8.6 | 0.60 | 51 | 47 | 18 | 19 | 11 | 0.7 | 195 | 15 | 36 | 9.2 | 1.3 | 9.5 | 0.6 | 300 |
| 8-36W-4DDC | Well | 420 | 16.5 | 7.8 | 0.90 | 54 | 44 | 14 | 26 | 5.0 | 0.7 | 204 | 0 | 28 | 7.9 | 1.6 | 18 | 0.1 | 304 |
| 8-36W-9DDD | Pit | 410 | 22.0 | 8.6 | 0.99 | 49 | 38 | 11 | 27 | 7.7 | 0.4 | 178 | 5.3 | 25 | 6.1 | 1.6 | 18 | 0.5 | 243 |
| 10-33W-10DAA | Well | 470 | 15.0 | 7.7 | 1.12 | 18 | 44 | 13 | 33 | 5.7 | 0.9 | 228 | 0 | 26 | 12 | 1.0 | 16 | 283 | |
| 10-33W-10ADD | Pit | 485 | 21.0 | 8.6 | 1.06 | 20 | 46 | 14 | 32 | 7.9 | 0.8 | 224 | 1.9 | 29 | 14 | 1.0 | 18 | 0.5 | 278 |
| 11-27W-8DAA | Well | 430 | 15.0 | 7.8 | 0.37 | 35 | 56 | 14 | 12 | 4.9 | 1.1 | 237 | 0 | 16 | 7.3 | 0.9 | 9.3 | 0.1 | 264 |
| 11-27W-8DAA | Pit | 340 | 35.0 | 9.2 | 0.48 | 26 | 32 | 14 | 13 | 14 | 0.7 | 124 | 23 | 21 | 14 | 0.8 | 0.3 | 0.1 | 223 |
| *PO4 values below 0.1 ppm not reported. | |||||||||||||||||||
Appreciable increases in NO3 values are not observed in the tailpit waters, nor is a marked build-up of Ca, Mg, Na, SO4, or Cl levels noted. The similarities in the specific conductances and SAR values between well and tail-water pit pairs of data suggest that re-use of the water from any of these pits is a practical option insofar as the inorganic constituents of the water are concerned. All eight wells in this group appear to be associated with 40 feet or more of saturated thickness and none of them is situated in an alluvial valley system. The re-use of water from tail-water pits in areas associated with soils of alluvial valleys where saline soils, shallow groundwater tables, and poorer groundwater quality may exist requires further evaluation. Additional studies related to the build-up of organics, i.e., pesticides and herbicides, in these tail-pit waters would be desirable for total evaluation of their re-use potential. However, data from the present study area do suggest that re-use of tail water in areas other than alluvial valley systems may be feasible in many instances and should be considered as a means of extending the utility of the groundwater resource.
The services of personnel from the USGS offices in Lawrence and Garden City, Kansas, and T. McClain, KGS, in the collection of water samples; A. Diaz, USGS, D. Whittemore, M. Cortese, and H.N. Le, KGS, in the operation of the field laboratory facilities; and the cooperation of W. Bossert, Manager of Groundwater Management District No. 4, are gratefully acknowledged. The authors are also indebted to Dr. J. Tangeman, President, Colby Community College, and Dr. M. Pickerill, Chemistry Department, Colby Community College, for providing field laboratory facilities. The graphics for this publication were prepared by Patricia Renjifo.
Angell, R.C., Soil survey of Rawlins County, Kansas: U.S. Department of Agriculture, in press.
Angell, R.C., Call, C.M., and Linnell, L.D., 1978, Soil survey of Gove County, Kansas: U.S. Department of Agriculture, 71 p.
Angell, R.C., Yonberg, C.F., Bell, E.L., and Hagihara, J.S., 1973, Soil survey of Sherman County, Kansas: U.S. Department of Agriculture, 47 p.
Barker, W.L., Soil survey of Thomas County, Kansas: U.S. Department of Agriculture, in press.
Bayne, C.K., 1956, Geology and ground-water resources of Sheridan County, Kansas: Kansas Geological Survey, Bulletin 116, 94 p. [available online]
Bayne, C.K. and Ward, J.R., 1969, Saturated thickness and specific yield of Cenozoic deposits of Kansas: Kansas Geological Survey, Map M-5.
Bell, E.L., Call, C.M., Hagihara, J.S., and Linnell, L.D., 1964, Soil survey of Logan County, Kansas: U.S. Department of Agriculture, Series 1959, No. 35, 78p.
Elias, M.K., 1931, The geology of Wallace County, Kansas: Kansas Geological Survey, Bulletin 18, 254 p. [available online]
Frye, J.C., 1945, Geology and ground-water resources of Thomas County, Kansas: Kansas Geological Survey, Bulletin 59, 110 p. [available online]
Hathaway, L.R., Magnuson, M.L., Carr, B.L., Galle, O.K., and Waugh, T.C., 1975, Chemical quality of irrigation waters in west-central Kansas: Kansas Geological Survey, Chemical Quality Series 2, 45 p.
Hathaway, L.R., Carr, B.L., Galle, O.K., Magnuson, M.L., Waugh, T.C., and Dickey, H.P., 1977, Chemical quality of irrigation waters in Hamilton, Kearny, Finney, and northern Gray counties: Kansas Geological Survey, Chemical Quality Series 4, 33 p.
Hathaway, L.R., Carr, B.L., Flanagan, M.A., Galle, O.K., Waugh, T.C., and Dickey, H.P., 1978a, Chemical quality of irrigation waters in southwestern Kansas: Kansas Geological Survey, Chemical Quality Series 6, 35 p.
Hathaway, L.R., Galle, O.K., Waugh, T.C., and Dickey, H.P., 1978b, Chemical quality of irrigation waters in Ford County and the Great Bend Prairie of Kansas: Kansas Geological Survey, Chemical Quality Series 7, 41 p.
Hodson, W.G., 1963, Geology and ground-water resources of Wallace County, Kansas: Kansas Geological Survey, Bulletin 161, 108 p. [available online]
Hodson, W.G., 1969, Geology and ground-water resources of Decatur County, Kansas: Kansas Geological Survey, Bulletin 196, 41 p. [available online]
Hodson, W.G. and Wahl, K.D., 1960, Geology and ground-water resources of Gove County, Kansas: Kansas Geological Survey, Bulletin 145, 126 p. [available online]
Johnson, C.R., 1958, Geology and ground-water resources of Logan County, Kansas: Kansas Geological Survey, Bulletin 129, 175 p. [available online]
Pearl, R.H., Roberts, R.S., Keene, K.M., and McClain, T.J., 1972, Water resources of northwestern Kansas: U.S. Geological Survey, Hydrologic Investigations Atlas HA-429.
Prescott, G.C., Jr., 1953a, Geology and ground-water resources of Cheyenne County, Kansas: Kansas Geological Survey, Bulletin 100, 106 p. [available online]
Prescott, G.C., Jr., 1953b, Geology and ground-water resources of Sherman County, Kansas: Kansas Geological Survey, Bulletin 105, 130 p. [available online]
Prescott, G.C., Jr., 1955, Geology and ground-water resources of Graham County, Kansas: Kansas Geological Survey, Bulletin 110, 98 p. [available online]
Stiff, H.A., 1951, The interpretation of chemical water analysis by means of patterns: Journal of Petroleum Technology, v. 3, n. 10, p. 15-17.
U.S. Salinity Laboratory Staff, 1954, Diagnosis and improvement of saline and alkali soils: U.S. Department of Agriculture Handbook 60, 160 p.
Walters, K.L., 1956, Geology and ground-water resources of Rawlins County, Kansas: Kansas Geological Survey, Bulletin 117, 100 p. [available online]
Kansas Geological Survey
Placed on web Nov. 22, 2010; originally published in August 1979.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS8/index.html