
Kansas Geological Survey, Chemical Quality Series 12, originally published in 1991
Next Page--Results and Discussion

Originally published in 1991 as Kansas Geological Survey Chemical Quality Series 12. This publication is also available as an Acrobat PDF file (2 MB).
Drinking-water standards promulgated by the U.S. Environmental Protection Agency (EPA) underscore the importance of detailed water-quality data for sources used in conjunction with public water supplies. It has been recognized that reaction of the chlorine (used as a disinfectant) with organic matter present in surface waters can lead to formation of trihalomethanes (THMs) in concentrations which may exceed the 100 µg/L maximum contaminant level (MCL) established by the EPA. However, relatively little consideration has been given to ground-water sources with regard to the organic carbon content of the waters, the potential for THM formation, or the presence of ammonium ion (NH4+), which both consumes chlorine and serves to reduce THM formation. The successful operation of water-treatment plants for public supplies is dependent upon many factors, such as operator skill and the condition of the equipment, but of similar importance is a detailed knowledge of water quality for the sources being employed.
A survey of Kansas ground waters to determine their concentrations of total organic carbon (TOC) and trihalomethane-formation potential (TFP) was conducted in the spring of 1986. Wells were carefully selected, based on well logs made during construction, to represent particular geologic intervals. Thirty-four samples were collected from public water supply wells and 16 samples were collected from private water wells. Samples from 11 alluvial aquifers, four unconsolidated aquifers in Quaternary and Tertiary formations, and four consolidated aquifers in Cretaceous, Permian, Pennsylvanian, and Cambrian-Ordovician rocks were taken.
The mean and median TOC concentrations were 1.03 ± 0.76 and 0.84 mg/L, while the mean and median TFP concentrations were 46.7 ± 39.5 and 30.6 µg/L, respectively. The mean TFP yield was 0.242 ± 0.07 µmol per mg of TOC, and the TFP concentration in micromoles per liter was very strongly correlated (r = 0.953) with TOC. Only 8% of the samples had a TFP concentration exceeding the present MCL for THMs of 100 µg/L, but 56% exceeded 25 µg/L and 90% exceeded 10 µg/L, suggesting that many Kansas water-supply systems using ground water might have difficulty meeting a substantially lower THM standard.
The average instantaneous THM (ITHM) concentration was only 6.95 µg/L, while the average terminal (TTHM) concentration was 35.6 µg/L. Hence, only a small fraction of the THM concentration to which consumers might be exposed is formed prior to distribution. For the 21 TTHM samples having a free chlorine residual at the end of the incubation period, TTHM was strongly correlated with both TOC (r = 0.819) and TFP (r = 0.926), suggesting that either of these might be a good surrogate measure for TTHM.
TOC (and TFP) appeared to be unrelated to aquifer (well screen) depth, but both were clearly much higher in the alluvial aquifers, all of which were located at relatively shallow depths. TOC also appeared to be unrelated to the inorganic constituents present in the samples, with the exception of a subset of samples from alluvial aquifers having high concentrations of NH4+ (>0.1 mg/L) and Fe + Mn (>1.0 mg/L). For these samples, TOC was strongly correlated with both NH4+ (r = 0.676) and Fe + Mn (r = 0.991). These relationships merit further investigation, since all of the constituents involved pose problems for water-treatment plants.
In Kansas, efforts to control THMs in public water supplies from groundwater sources should focus primarily on alluvial aquifers, especially those having high concentrations of TOC, NH4+, Fe, and Mn. TOC and TFP may be useful surrogates for TTHM and could be used as a basis for exemptions from monitoring requirements. Use of combined chlorine appears to be the simplest and most effective means of limiting THM formation, but the necessary precautions must be taken to ensure that the microbial quality of the drinking-water supply is not compromised.
Since the discovery of trihalomethanes (THMs) in drinking water by Rook (1974), concern has existed over the presence and possible carcinogenicity of these compounds in public water supplies. THMs are volatile organic compounds with a basic chemical structure of methane in which three hydrogen atoms have been replaced with halogen atoms, usually chlorine (Cl) or bromine (Br). These and other halogenated byproducts are formed as the result of the reaction of chlorine, used for disinfection, with the naturally occurring organic material present to some extent in virtually every water supply.
On November 29, 1979, the U.S. Environmental Protection Agency (EPA) promulgated an amendment to the National Interim Primary Drinking Water Regulations limiting the concentration of THMs in drinking water to 100 micrograms per liter (µg/L) for public water supplies serving over 10,000 persons (Federal Register, 1979). Consideration is presently being given to lowering of the standard, perhaps to as low as 10 µg/L, in the future; new standards for a variety of disinfection byproducts are due to be released in the near future.
Although much attention has been given to the control of THMs in surface-water supplies, ground waters in most areas of the United States, including Kansas, have not been extensively studied in this respect. Surface waters, including rivers and lakes, generally contain more organic matter than ground waters, and are therefore more likely to produce high THM concentrations. However, certain ground-water sources have been found to produce THM concentrations above the maximum contaminant level (MCL) of 100 µg/L (Symons et al., 1975). The successful operation of water-treatment plants for public supplies is dependent upon many factors, such as operator skill and the condition of the equipment, but of similar importance is a detailed knowledge of water quality for the sources being employed. Ground waters also may contain significant concentrations of ammonium (NH4+), sulfide, iron (Fe), and manganese (Mn), all of which interfere with the production of a free chlorine residual. Ammonium also can be beneficial because it reacts with chlorine to form monochloramine. In sufficient concentrations, monochloramine is an adequate disinfectant for most ground-water supplies, but it does not form THMs and it greatly reduces formation of other halogenated byproducts.
Because the total organic carbon (TOC) concentrations in uncontaminated Kansas ground waters were largely unknown and because the potential for Kansas ground waters to form THMs had not been systematically investigated, the major goal of this study was to provide and to evaluate this information. Other objectives of the study were 1) to statistically examine the geological and geochemical characteristics of Kansas aquifers in relation to TOC concentrations and THM formation potential (TFP) in order to establish important associations; 2) to identify problem aquifers or problem areas in the state; and 3) to provide information that would be helpful in assessing the impact on Kansas of a lower THM standard.
In his 1974 study, Rook found a good correlation between THM formation and color. The coloration in the surface waters he studied was due to naturally occurring humic substances (humic and fulvic acids). Rook proceeded to prove that humic substances could be precursors by injecting peat into water samples, chlorinating them, and obtaining positive results upon analysis for THMs. Since that time many investigators have examined the factors influencing the formation of THMs and other halogenated byproducts, including temperature, pH, ammonium, bromide, and the concentrations of chlorine and precursor material (TOC).
The rate and extent of THM formation are generally found to increase with increasing temperature, pH, TOC, chlorine, and bromide. The same is true for other halogenated byproducts except that most increase with a decrease in pH. Monochloramine, formed by the reaction of chlorine with ammonium, does not form THMs at low dosages under laboratory conditions; however, small amounts of THMs are likely to be formed upon chlorination of treated-water supplies because of the manner in which the chlorine is added. THM concentrations also can be reduced by avoiding prechlorination, avoiding higher concentrations of chlorine than are necessary for good disinfection, and removing precursor materials prior to chlorination. For more detailed information, the reader may consult Symons et al. (1975, 1981), Randtke (1984), Amy et al. (1984), and numerous other published works on this subject.
Leenheer et al. (1974) examined the concentration of naturally occurring dissolved organic carbon (DOC) at 100 uncontaminated sites dispersed over 27 states. A variety of aquifer materials were represented, including Pleistocene deposits (sand and gravel), Cretaceous rock, Mississippian rock (sandstone), river alluvium, limestone, crystalline rock, dolomite, and basalt. DOC values ranged from < 0.1 to 15.0 mg/L; the median value was 0.7 mg/L and the mean was 1.2 mg/L. The same median value was found for sandstone, limestone, and sand and gravel aquifers. Crystalline rock aquifers had lower DOC concentrations, ranging from 0.4 to 0.5 mg/L. DOC concentrations appeared to be related to those of the source water. For example, a site in Miami, Florida, apparently received its high DOC concentrations from infiltration of surface waters high in DOC. Increasing DOC was directly correlated with increasing specific conductance and alkalinity; however, no correlation was found with pH and well depth.
In the National Organics Reconnaissance Survey for Halogenated Organics (NORS), water samples were collected from 80 public water supplies and analyzed for TOC and six organic chemicals, including the four principal THMs (Symons et al., 1975). The waters sampled included ground waters, lakes, streams, and reservoirs. THM formation was found to be strongly correlated with TOC, which ranged from < 0.05 to 12.2 mg/L, with a median of 1.5 mg/L. Raw-water samples were classified into six divisions according to TOC concentration. The 16 ground-water sources sampled were found to have a lower average THM concentration than surface-water sources except in the upper division (TOC >5 mg/L). This reflected the average TOC concentrations from the different sources: 1.85 ± 2.79 mg/L for the 16 ground-water samples, 3.33 ± 2.02 mg/L for the 26 lake and reservoir samples, and 3.98 ± 3.23 mg/L for the 39 river samples. River-water sources had the highest average THM concentration in four of the six divisions. Twenty-four of 39 river-water samples were in the upper three divisions (TOC > 3 µg/L), compared to only three of 16 ground waters and 10 of 25 impounded waters. TOC concentrations were not correlated with UV absorption or fluorescence.
Junk et al. (1980) investigated vertical, areal, and temporal differences in DOC, nitrate, and pesticide concentrations of surface waters of the Platte River and adjacent ground-water regimes. Ground-water sites were chosen in irrigated bottomlands, near-pristine areas affected by river seepage, and inland terrace deposits. Shallow and deep wells were constructed to determine vertical stratification. DOC concentrations in shallow ground water from near-pristine areas ranged from 1.4 to 3.3 mg/L with an average of 2.3 mg/L, and DOC concentrations in shallow bottomland wells downgradient from irrigated cropland ranged from 3.1 to 4.8 mg/L. In 34 of 35 cases, DOC concentrations decreased with depth. River DOC concentrations were higher than those of the adjacent ground water regardless of season. DOC levels in ground waters were highest in September and levels in river waters were highest in April.
Junk et al. (1980) concluded that the most probable mechanism of DOC removal with increasing depth was adsorption on saturated aquifer sediments and that the source of DOC was primarily from overlying soils. Processes such as the dissolution of the summer's accumulation of decaying organic matter on the river banks and bottom sediments in September, combined with the fluvial seepage contribution of DOC (not removed at the water-sediment interface), were proposed as mechanisms for the observed seasonal differences in the maximum DOC concentrations of river water and adjacent ground water.
Oliver and Thurman (1984) studied the TFP associated with the fulvic- and humic-acid fractions of various aquatic humic materials. They found that, in general, ground-water fulvic acids had the lowest TFPs, with surface-water fulvic acids having greater TFPs, and marsh/ bog fulvic acids having the largest TFPs. Since ground water contains the oldest organic material and marsh/bog water the youngest, they reasoned that structural changes that occur during the maturation process must lead to a lowering of THM potential. In comparing fulvic acids with humic acids, they found that 18-52% more THMs were produced by humic acids from the same source. They concluded that the molecular size of humic organics is related to THM yield, and, as the size of the organic material increases, so does the THM yield. They also found a strong correlation between color and TFP.
In 1981 and 1982, the EPA conducted a survey of public ground-water supplies with 466 randomly selected and 479 selected by State agencies (Westrick et al., 1984). The sampling sites were divided into sites serving fewer than 10,000 persons and those serving more than 10,000 persons. It was found that THMs occurred more frequently in the larger systems; however, this was probably due to a higher percentage of the larger systems chlorinating their water. Median values of the individual THMs were generally low, ranging from 1.2 to 5.1 µg/L, but the maximum values were 430 µg/L for chloroform, 110 µg/L for bromodichloromethane, 63 µg/L for dibromochloromethane, 4.2 µg/L for dichloroiodomethane, and 110 µg/L for bromoform. Chloroform and bromodichloromethane had their highest median and maximum concentrations in the state-selected sites serving more than 10,000 persons.
In January of 1984, seven wells were sampled in a glacial buried-valley aquifer system in northeastern Kansas (Denne et al., 1984). The TOC levels ranged from 0.9 to 2.4 mg/L with a mean concentration of 1.4 mg/L. Two of the samples were found to have THM levels of 81 and 61 µg/L when quenched seven days after collection. In another study of the same glacial buried valley system, Denne et al. (1987) found an average DOC of 2.65 mg/L and an average TFP of 123 µg/L. DOC decreased with depth to the tan/gray contact (where the color of the sediments changed from tan to gray), but increased with depth below the tan/gray contact. DOC and TFP were strongly correlated with Fe, Mn, and NH4+.
O'Conner and Chaffee (1985) investigated areas in Kansas where poor well construction, poor well-plugging procedures, and high well densities might affect water quality and TOC levels. Time-series sampling of 10 wells in the Lincolnville area of Marion County, Kansas, was conducted over a 1-yr period. The wells were screened in Permian-age aquifer units, and TOC levels were observed to range from 0.74 to 6.12 mg/L in May and 1.25 to 5.80 mg/L in August. The average TOC concentrations for this study were 3.04 and 3.26 mg/L for the two sampling periods.
Anne Melia assisted extensively in the analysis of samples for TOC and THM formation potential. Information helpful in contacting public water utilities was obtained from the Kansas Department of Health and Environment. Peter A. Macfarlane generously helped to supply well logs and to collect samples from the Cambrian-Ordovician aquifers. The use of Kansas Geological Survey (KGS) vehicles for sample collection, KGS monies for travel, and a General Research Fund Allocation (3070-XX-0038) from the University of Kansas made this study possible.
Sampling sites were selected to include each of the major aquifer systems in Kansas. Multiple sampling sites were selected for aquifers extending over a large geographic area so that the spatial variability of the water quality in such aquifers might be included.
Water wells were chosen after consulting water-well records, filed by county, at the Kansas Geological Survey (KGS). Sites were selected from these records so that water-well construction materials, screened intervals, geologic logs, and construction methods could be examined and documented. Geologic logs and construction information for wells tapping the Arbuckle aquifer were obtained from a KGS working file because there were no recent water-well records (1974-present) for this aquifer in the main well-log file. Because of the great depths (and accompanying high construction costs) of these wells, few have been drilled in recent years. The oldest well deriving water from the Arbuckle aquifer system sampled in this study was drilled in 1964, and the others were drilled between 1972 and 1979.
Table 1 gives a listing of the sampling sites along with aquifer types and well-construction methods and materials. Each well was chosen to reflect a particular geologic interval or aquifer. The screened interval was examined to determine the aquifer's representation. Wells constructed in such a way that they might also draw water from aquifers other than the aquifer of interest were eliminated as possible sampling sites. As can be seen, fairly detailed information was available for 48 of the 50 wells chosen.
Table 1--Sampling sites, aquifers, and well construction.
| No. | County | Legal Location |
Geologic Source |
Well Type1 |
Casing Material2 |
Total Depth (ft) |
Grouted Interval (ft) |
Screened Interval3 (ft) |
Screened Material4 |
Water Level (ft) |
Est. Yield gpm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Douglas | 12S20E17ABA | Kansas R. Alluv. |
PWS | STL | 78 | 0-20 | 58-78 | MCS,GR,B | 22 | 350 |
| 2 | Buchanan (MO) |
55N37W19CCA (in MO) |
Missouri R. Alluv. |
IND | STL | 80 | 60-80 | MCS,GR | |||
| 3 | Leavenworth | 9S23E08DAA | Missouri R. Alluv. |
PWS | STL | 69 | 0-20 | 49-69 | CMS,GR | 13 | 2000 |
| 4 | Franklin | 18S18E06ACC | Pennsylv. Tonganoxie |
PWS | PLAS | 210 | 3-51 | 141-200 | SS | 99 | 19 |
| 5 | Douglas | 14S17E36DDD | Pennsylv. Tonganoxie |
PWS | STL | 500 | 0-420 | OH420-500 | SS,SH | 348 | 30 |
| 6 | Pottawatomie | 10S12E15ABB | Kansas R. Alluv. |
PWS | PLAS | 47 | 0-20 | 26-47 | MCGR | 21 | 1200 |
| 7 | Geary | 11S05E35B | Republican R. Alluv. |
PWS | STL | 65 | 0-20 | 42-62 | MCS,GR | 17 | 1500 |
| 8 | Clay | 8S03E07BAA | Republican R. Alluv. |
PWS | STL | 55 | 0-20 | 38-53 | CS,GR,FS | 22 | 700 |
| 9 | Republic | 3S04W17DAD | Republican R. Alluv. |
PWS | AS-CE | 42 | 0-20 | 29-42 | GR | 18 | 400 |
| 10 | Ottawa | 11S04W01CBB | Solomon R. Alluv. |
PR | PLAS | 59 | 3-13 | 56-59 | FCS,GR | 25 | 50 |
| 11 | Saline | 16S03W25DAB | Smoky Hill R. Alluv. |
PWS | STL | 53 | 5-25 | 43-53 | MCS | 25 | 90 |
| 12 | Marion | 18S01E33DDD | Cretaceous Dakota |
PWS | STL | 68 | 5-20 | 58-68 | FMSS | 25 | 125 |
| 13 | McPherson | 20S04W01DDD | Equus Beds | PWS | STL | 215 | 0-20 | 138-178 | MCS,GR | 71 | 2500 |
| 194-204 | MCS,GR | ||||||||||
| 14 | Harvey | 24S01W06CBC | Equus Beds | PWS | STL | 150 | 1-20 | 123-148 | FCS | 23 | 600 |
| 15 | Sedgwick | 28S03W01DDD | Equus Beds | PR | PLAS | 45 | 3-14 | 35-45 | MCS | 25 | |
| 16 | Cowley | 35S04E01AA | Arkansas R. Alluv. |
PWS | STL | 30 | 0-12 | 18-30 | MCS | 10 | 300 |
| 17 | Reno | 22S07W10CAA | Arkansas R. Alluv. |
PWS | STL | 53 | 0-20 | 38-53 | MCS,GR | 13 | 800 |
| 18 | Washington | 1S01E07AAA | Cretaceous Dakota |
PWS | PLAS | 285 | 0-20 | 265-285 | SS | 195 | 100 |
| 19 | Marshall | 4S10E17AB | Glacial | PR | PLAS | 185 | 0-20 | 175-185 | GR,SH | 53 | 90 |
| 20 | Doniphan | 1S19E09DBD | Missouri R. Alluv. |
PWS | PLAS | 73 | 4-22 | 68-72 | S,GR,B | 13 | 75 |
| 21 | Brown | 3S16E01DAC | Glacial | PR | PLAS | 58 | 0-10 | 38-58 | S,GR,SH | 20 | 15 |
| 22 | Jackson | 6S16E21BCB | Glacial | PR | PLAS | 145 | 0-10 | 132-142 | CS,MGR | 90 | 50 |
| 23 | Atchison | 6S18E27DAD | Glacial | PR | PLAS | 200 | 0-15 | 190-200 | CS,FGR | 80 | 100 |
| 24 | Thomas | 8S34W12DBA | Ogallala | PWS | STL | 261 | 0-20 | 200-220 | FS,MGR | 134 | |
| 225-235 | S,GR | ||||||||||
| 241-261 | FS,GR | ||||||||||
| 25 | Wichita | 18S37W14DD | Ogallala | PWS | STL | 172 | 0-20 | 154-172 | MCS,GR,CL | 118 | 260 |
| 26 | Seward | 32S33W27ABC | Cimarron R. Alluv. |
PR | PLAS | 100 | 0-10 | 60-100 | MCS | 25 | 50 |
| 27 | Haskell | 30S34W13DAB | Ogallala | PWS | STL | 430 | 0-20 | 329-379 | FMCS,GR | 261 | 2000 |
| 401-426 | FMCS | ||||||||||
| 28 | Edwards | 24S 16W20DD | Big Bend | PWS | PLAS | 100 | 0-20 | 70-100 | S,GR,CL | 21 | 500 |
| 29 | Rush | 18S17W03BCC | Cretaceous Dakota |
PWS | PLAS | 100 | 5-25 | 60-100 | SS,LS | 46 | 50 |
| 30 | Rush | 18S17W22DA | Walnut Ck Alluv. |
PWS | STL | 58 | 0-20 | 48-58 | S,GR | 33 | 200 |
| 31 | Ness | 20S23W24ADD | Pawnee R. Alluv. |
PR | PLAS | 65 | 4-15 | 55-65 | S,SH | 41 | 80 |
| 32 | Ellis | 15S18W30D | Smoky Hill R. Alluv. |
PWS | PLAS | 47 | 0-20 | 37-47 | FMS,GR,CGR | 9 | 300 |
| 33 | Meade | 35S29W13BBC | Cimarron R. Alluv. |
PR | PLAS | 42 | 0-10 | 36-42 | MS | 7 | 25 |
| 34 | Meade | 35S29W10CCB | Pleist.? | PR | (nolog) | ||||||
| 35 | Kingman | 30S08W05ABD | Permian Harper Silts |
PR | PLAS | 74 | 5-15 | 62-74 | SH | 55 | 15 |
| 36 | Cherokee | 33S25E18DAA | Cam-Ord | PWS | STL | 900 | OH575-900 | 145 | |||
| 37 | Cherokee | 34S25E13CCC | Cam-Ord | PWS | STL | 1260 | OH514-1260 | ||||
| 38 | Crawford | 30S25E28DDA | Cam-Ord | PWS | STL | 1050 | 0-550 | OH550-1050 | 217 | ||
| 39 | Crawford | 30S24E02DDD | Cam-Ord | PWS | STL | 1113 | 0-723 | OH723-1113 | 274 | 1000 | |
| 40 | Osborne | 6S11W28ACD | Solomon R. Alluv. |
PWS | PLAS | 68 | 0-34 | 42-68 | S | 34 | 800 |
| 41 | Lincoln | 12S06W115ABD | Saline R. Alluv. |
PWS | (nolog) | ||||||
| 42 | Lincoln | 12S06W15BCD | Saline R. Alluv. |
PWS | PLAS | 55 | 0-22 | 47-55 | CS,GR,SH | 18 | 200 |
| 43 | Dickinson | 13S03E17AB | Smoky Hill R. Alluv. |
PWS | STL | 58 | 7-20 | 43-58 | MCS,GR | 11 | 2000 |
| 44 | Marion | 17S04E12CCC | Permian Nolans |
PR | PLAS | 93 | 0-10 | 65-93 | LS,SH | 65 | 55 |
| 45 | Marion | 22S03E04AAB | Permian Wellington |
PWS | PLAS | 60 | 0-20 | 23-53 | LS,SH | 14 | 100 |
| 46 | Reno | 24S09W16ACB | Big Bend | PR | PLAS | 72 | 0-10 | 52-72 | CL,S,GR | 33 | |
| 47 | Lyon | 19S13E29CCC | Neosho R. Alluv. |
PR | PLAS | 29 | 4-10 | 11-29 | CL,SLT, GR,SH |
10 | 25 |
| 48 | Leaven- worth |
12S20E15ADA | Pennsylv. Tonganoxie |
PR | PLAS | 160 | 0-10 | 120-160 | SS | 80 | 50 |
| 49 | Labette | 31S21E16BDD | Neosho R. Alluv. |
PWS | STL | 33 | 0-20 | 26-31 | MCS,GR | 24 | 20 |
| 50 | Crawford | 30S25E28DDA | Cam-Ord | PWS | STL | 1050 | 0-550 | OH550-1050 | 217 | 2250 | |
| 1 PWS = Public Water Supply; PR = Private; IND = Industrial 2 PLAS = Plastic; STL = Steel; AS-CE = Asbestos-Cement 3 OH = Open Hole 4 B = Boulders; C = Coarse; CL = Clay; F = Fine; GR = Gravel; LS Limestone; M = Medium; S = Sand; SH = Shale; SLT = Silt; SS = Sandstone |
|||||||||||
Public water-supply wells were chosen, wherever possible, because of their generally high quality of construction and the relevance of this study to chlorinated-water supplies. In the selection of private wells, irrigation wells were rejected and domestic wells were preferred, although stock wells were used in a few cases. Well yields were stipulated to be 20 gallons per minute (gpm) or greater.
Any known or possible areas of contamination were avoided, since the objective of this study was to examine the characteristics of naturally occurring ground water. Contamination sources would include oil-field brines, farm chemicals, animal wastes, mineral wastes from mining operations, and nearness to poorly constructed wells. Wells were rejected if they were found to be near industries, grain elevators, oil wells, feedlots, or areas with a high density of water wells, especially those of older construction.
The possible leaching of chlorinated organic substances from well casing and construction materials has recently been pointed out by Gibb and Barcelona (1984). Polyvinyl chlorine (PVC) pipes and casing, as well as sealing cements used in well construction, can leach significant amounts of chlorinated organics. Iron oxides from aged steel casing may be a source of adsorption losses of trace metals or organic compounds. Sampling procedures were designed to minimize these effects, and table I documents the construction materials of the individual wells.
The geochemical character of freshwater aquifers in Kansas varies widely across the state. Alluvial and unconsolidated aquifers are mainly composed of silt, sand, and gravel, and their wells are generally less than 200 ft (60 m) in depth. The consolidated aquifers are composed of sandstone, limestone, or sandy dolomite, and the wells tapping them can be in excess of 1,000 ft (300 m) in depth in areas of southeastern Kansas. Climatic and geographic changes within the state also affect the recharge and discharge relationships of the aquifers.
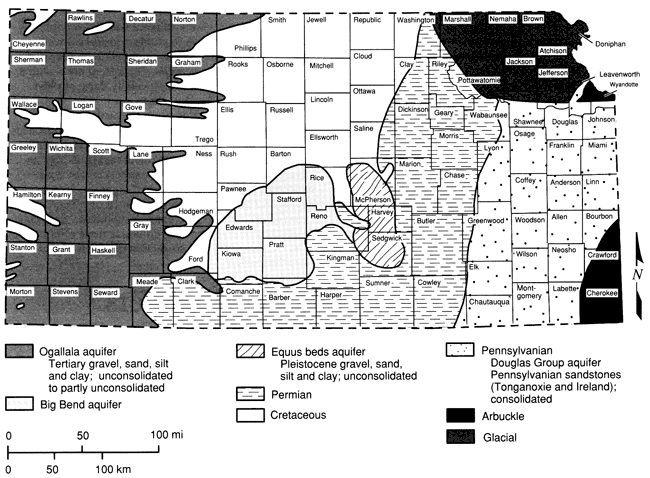
Figure 1 shows the principal drainageways of the state, as well as the locations of the 50 sampling sites employed in this study. The major alluvial aquifers are associated with river systems in the northeastern, eastern, and north-central regions of the state. Major unconsolidated aquifers include the Ogallala in western Kansas, the Big Bend and Equus Beds aquifers in central and south-central Kansas, and the glacial buried-valley deposits in northeastern Kansas. Several consolidated aquifers serve as important sources of potable water. The Dakota Sandstone aquifer, which is present in the Cretaceous geologic sequence, is used extensively in some areas. Permian aquifers are used by small communities and farms in central and south-central Kansas where suitable supplies can be found. In northeastern Kansas, where alluvial and glacial deposits are not present, Pennsylvanian sandstone aquifers are used to supply small communities and farms. The large freshwater supplies in the Arbuckle (Cambrian-Ordovician) aquifer are used as the sole source of water by communities in southeastern Kansas. A generalized map depicting the nonalluvial aquifers of Kansas is shown in fig. 2.
Figure 1--Sampling site locations (see table 1 for well descriptions; base map from Steeples and Buchanan, 1983).
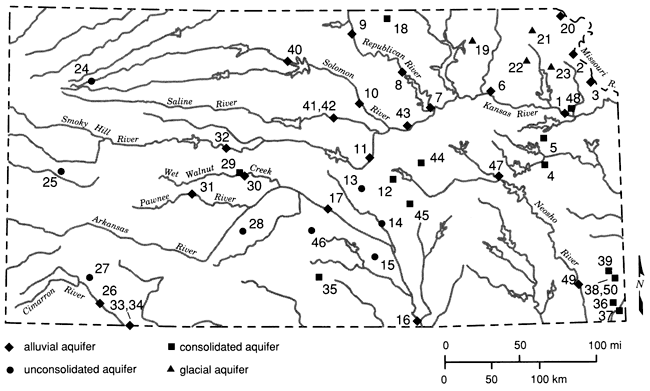
Figure 2--Generalized domains of non-alluvial aquifer materials.
The 50 sites selected in the present study include 23 wells in 11 different alluvial aquifer systems, four wells in glacial buried-valley aquifers, three wells in the Ogallala aquifer, three wells in the Equus Beds aquifer, two wells in the Big Bend aquifer, three wells in the Dakota aquifer of Cretaceous age, three wells.in Permian-age aquifers, three wells in Pennsylvanian-age aquifers, five wells in Cambrian-Ordovician-age aquifer units, and one well believed completed in material of Pleistocene age (in Meade County). Thirty-four of the sites are public-supply wells, 15 are private domestic wells, and one is an industrial well. Samples were collected at all 50 sites, but lack of well logs at sites 34 and 41 and analytical problems associated with the high sulfide level at site 38 restricted use of data from these three sites in some of the statistical analyses performed.
The major alluvial aquifers of Kansas are predominantly in the central and eastern parts of the state. The Smoky Hill, Republican, and Solomon rivers join to form the Kansas River and flow in a general eastward direction (fig. 1). The Kansas River continues eastward into the Missouri River, which forms the northeastern state border. The principal alluvial system in the southern region of the state is associated with the Arkansas River, which enters Kansas from Colorado, continues through central Kansas (where it takes a southerly direction), and leaves the state in Cowley County. The Neosho River travels in a general southward direction in the eastern third of Kansas.
Alluvial-aquifer materials consist of sand, gravel, silt, and clay. Generally, the aquifer grades from coarser materials, such as sand and gravel, at the basal section, to finer materials in the upper portions. Alluvial-deposit thicknesses range from a few feet to around 100 ft (30 m). The major water-bearing formation and the formation which is usually screened in a well is the basal sand and gravel.
Alluvial deposits are formed as a river erodes surrounding deposits and as suspended particles settle to the river bed. Terraces are formed as the river cuts into previously deposited alluvial materials. The alluvial ground water exists in equilibrium with the river water and the surrounding ground water. The alluvium can receive recharge from the river or can supply water to the river, depending on the hydrogeologic conditions.
The Missouri River is the largest river in the state and cuts through an area of Pleistocene glacial deposits. Three sets of samples (sites 2, 3, and 20) were taken from wells located in the Missouri River alluvium. Site 20 in Doniphan County has a reported yield of 75 gpm and is screened in 4 ft (1 m) of aquifer material consisting of gravel, gray sand, and boulders. Site 3 in Leavenworth County has a maximum yield of 2,000 gpm. This well has 20 ft (6 m) of screened aquifer materials consisting of medium- and coarse-grained sand with traces of gravel. The third site, site 2, is a well located in southwestern Buchanan County, Missouri. This well is screened in about 20 ft (6 m) of aquifer material with gravel present.
The Kansas River is fed by the Solomon, Republican, Saline, and Smoky Hill rivers, which originate in western Kansas. The river flows eastward from Junction City to Kansas City where it joins the Missouri River. The Kansas River alluvial deposits grade upward from locally derived flat limestone pebbles and boulders on the bedrock surface to fine sand, silt, and silty clay in the upper part (Fader, 1974). The river cuts through Permian- and Pennsylvanian-age formations and Pleistocene glacial deposits. Two wells (sites 1 and 6) located in the Kansas River alluvium were sampled. At site 1 in northeastern Douglas County, the well is located in Newman Terrace deposits. It is screened in 20 ft (6 m) of medium- to coarse-grained sand, gravel, and rough boulders. At site 6 in Pottawatomie County, the well is screened in 21 ft (6.5 m) of medium to coarse brown gravel. The bedrock at site 1 is shale of Pennsylvanian age, whereas at site 6 a shale of Permian age forms the bedrock unit.
The Republican River enters the state in north-central Kansas and flows southeastward to join the Kansas River at Junction City. It crosses the Smoky Hills area of Kansas, which is characterized by outcropping rock of the Cretaceous System. In the Junction City area, Permian-age rocks form the Flint Hills physiographic region. South of Clay Center and north of Junction City, the Republican River flows into Milford Reservoir. Three sets of samples (sites 7, 8, and 9) were taken from wells located in the Republican River alluvium. The log for the well at site 9 in Republic County indicates 13 ft (4 m) of screened alluvial gravel overlying bedrock of the Cretaceous System (Greenhorn) and Carlile formations. The well at site 8 in Clay County is screened in 15 ft (4.5 m) of fine to coarse sand and gravel underlain by rock of Permian age (Sumner). The screened interval at site 7 has 20 ft (6 m) of medium to coarse sand and gravel and is underlain by Permian-age (Gearyan) bedrock.
The Solomon River originates in northwestern Kansas and flows in a general eastern and slightly southern direction until it reaches Junction City where it joins the Kansas River. Kirwin and Webster reservoirs are located along the north and south forks, respectively, of the Solomon River. The Solomon River alluvium is 44-65 ft (13.5-19.5 m) in thickness and is made up of peat, clay, sandy silt, and sand in the upper portion and coarse sand and gravel in the basal section (Latta, 1949). Two samples (sites 10 and 40) were taken from wells tapping the Solomon River alluvium. The well at site 40 in Osborne County is located on the North Fork about 6 mi (10 km) above the point where the two forks join. It is screened in 26 ft (7.9 m) of sand and is underlain by a Cretaceous-age gray shale at a depth of 68 ft (21 m). At site 10 in Ottawa County, the well is screened in 3 ft (0.9 m) of fine to coarse sand and gravel overlying bedrock of Cretaceous age.
The Saline River originates in western Kansas and forms Wilson Lake Reservoir located in Russell County. It then flows eastward where it joins the Smoky Hill River west of Salina. The Saline River alluvium is 20-92 ft (628 m) in thickness and is composed of sand and gravel overlain by clay and silt with lenses of peat. Limestone, sandstone, and shale fragments are abundant in the coarse gravel deposits (Latta, 1949). Two sets of samples (sites 41 and 42) were taken from wells in the Saline River alluvium in Lincoln County. A record of the well log could not be found for site 41, which is an old well. The well that produced the samples for site 42 is screened in 8 ft (2.4 m) of sand and gravel, which is underlain by Cretaceous-age (Dakota) blue shale.
The Smoky Hill River extends from western Kansas eastward to Bridgeport where it goes northward to Salina and joins the Saline and Solomon rivers. Cedar Bluff Reservoir and Kanopolis Lake are contained along its length. The Smoky Hill River alluvium has a thickness of 30-90 ft (9-27 m). The upper 8-45 ft (2.4-13.4 m) is composed of silt, sandy silt, and fine sand and is underlain by poorly sorted sand and gravel (Latta, 1949). Wells at three sites (11, 32, and 43) are situated in the Smoky Hill River alluvium. At site 11 in Saline County, the well is screened in 10 ft (3.0 m) of medium to coarse sand which is underlain by Permian-age (Sumner) gray shale. In Ellis County, the well at site 32 is screened in 10 ft (3.0 m) of fine to medium sand and gravel with coarse gravel underlain by Cretaceous-age (Greenhorn) black shale. The well at site 43 in Dickinson County is screened in 15 ft (4.6 m) of medium to coarse sand and gravel underlain by Permian-age (Gearyan) red shale.
The Arkansas River cuts across the western two-thirds of Kansas, entering southwest Kansas from Colorado. East of Dodge City it continues in a northeastern direction, and at Great Bend it veers southeastward and leaves the state near Arkansas City. The Arkansas River alluvial deposits are composed of limestone, chert, and arkosic gravel and sands intermixed with differing amounts of silt and clay (Bayne, 1962). Only two sets of water samples, sites 16 and 17, were taken from wells in the Arkansas River alluvium. Logs were not available for most of the wells in the western part of the river's length. In these areas, other aquifers generally are used for drinking water because better qualities and quantities of water may be obtained from the Ogallala, Big Bend, and Dakota aquifers. The well at site 17 in Reno County is screened in 15 ft (4.6 m) of medium to coarse sand and gravel. In this area the Arkansas River cuts through Quaternary- (Big Bend), Cretaceous-, and Permian-age sediments. In Cowley County at site 16, the well is screened in 12 ft (3.7 m) of medium to coarse sand underlain by Permian-age (Gearyan) shale.
The Pawnee River and Wet Walnut Creek are tributaries to the Arkansas River in western Kansas. A set of water samples was taken from the Pawnee River alluvium at site 31 in Ness County. This well is screened in 10 ft (3.0 m) of sand underlain by Cretaceous (Carlile or Greenhorn) black shale. Water samples from the Wet Walnut Creek alluvium were obtained from the well at site 30 in Rush County. The screened interval of this well is set in 10 ft (3.0 m) of sand and gravel.
The Cimarron River originates in extreme southwestern Kansas and flows southeastward into Oklahoma. Samples were collected from two sites (26 and 33) having wells into the Cimarron River alluvial aquifer. At site 26 in Seward County, the well is screened in 40 ft (12 m) of medium to coarse sand. In the region of this site, the river cuts through Ogallala-, Pleistocene-, Cretaceous-, and Permian-age sediments. In Meade County at site 33, the well is screened in 6 ft (1.8 m) of medium sand.
The Neosho River extends from Morris County southeastward to Cherokee County where it bends southward into Oklahoma. Council Grove Lake and John Redmond Reservoir are located along its length. Samples were collected from two sites (47 and 49) where wells derive water from the Neosho River alluvial aquifer. The well at site 49 in Labette County is screened in 5 ft (1.5 m) of medium to coarse sand and gravel underlain by Pennsylvanian-age (Marmaton or Cherokee) bedrock. At site 47 the well is screened in 18 ft (5.5 m) of clay, silt, gravel, and shale, and is underlain by Pennsylvanian (Wabaunsee Group) bedrock.
The Ogallala aquifer is the principal water supply in the High Plains region. High-yielding irrigation wells tap this aquifer throughout western Kansas. The Ogallala Formation originated predominantly from deposits by streams flowing eastward from the Rocky Mountains area during Tertiary time (Moore, 1940). The thickness of the Ogallala deposits varies; thicknesses of less than 200 ft (60 m) are typical north of the Arkansas River (Moore, 1940).
Sand is the most common material in the Ogallala and is mainly composed of quartz with some feldspar and other minerals. Beds of gravel usually contain sand and silt, and beds of sand and gravel may be cemented by calcium carbonate. These cemented beds of coarse material are referred to as "mortar beds" (Prescott et al., 1954). The texture of the Ogallala Formation is not uniform, and gradations occur within short distances. The coarser materials are generally in the lower part of the formation where there are lenses and sinuous beds of gravel (Moore, 1940). Calcium carbonate occurs as stringers, nodules, and caliche, and there are many colors of silt including gray, red-brown, tan, buff, and white (Prescott et al., 1954). Recharge into the Ogallala is mainly from precipitation. Wells at three sites (24, 25, and 27) derive water from the Ogallala aquifer. At site 24 in Thomas County, the well is screened in fine sand and medium gravel and has a total depth of 261 ft (79 m). In Wichita County at site 25, the well is screened in 18 ft (5.5 m) of medium to coarse sand and gravel and is 172 ft (52.4 m) in total depth. The well at site 27 in Haskell County has a depth of 430 ft (130 m) and is screened in 75 ft (23 m) of sand with traces of gravel.
The well at site 34 in Meade County is believed to be completed in Pliocene-Pleistocene-age materials, but no well log was found for this site.
The Equus beds (sometimes referred to as the McPherson Formation) originated in Pleistocene time and cover a broad area between the Smoky Hill and Arkansas River valleys. This aquifer is a principal source of ground water in the south-central area of Kansas, although some areas have had oil-brine contamination (Williams and Lohman, 1949).
The Equus beds are composed of streamborne material deposited by a Pleistocene river that flowed southward from the present Smoky Hill River valley joining the Arkansas River above Wichita (Moore, 1940). The early Pleistocene stream deposits are composed of coarse-grained sand and gravel. As the McPherson Valley became filled after the diversion of the major stream, silt, clay, and fine sand were more prevalent in the Equus beds deposits (Williams and Lohman, 1949). The sand and gravel were derived from weathered shale of Cretaceous and Permian age and from reworking of eolian silt probably transported from the southwest. The Equus beds are from 0 to 290 ft (0-88 m) in thickness (Williams and Lohman, 1949). Three sites (13, 14, and 15) are located in the region of the Equus Beds aquifer. The well at site 13 in McPherson County is screened in 50 ft (15 m) of sand and gravel, with a total depth of 215 ft (65.5 m). In Harvey County the well at site 14 is screened in 25 ft (7.5 m) of fine to coarse sand. Its total depth is 150 ft (45 m). At site 15 in Sedgwick County, the well has a total depth of 45 ft (13 m) and is screened in 10 ft (3 m) of medium to coarse sand.
A major ground-water source in south-central Kansas contains thick deposits of silt, sand, and gravel that overlie Cretaceous bedrock. This aquifer is commonly referred to as the Big Bend aquifer and may encompass several formations of the Pleistocene series. The sediments represent stream-laid debris from the Rocky Mountains deposited during the Pleistocene epoch (McLaughlin, 1949). Pleistocene sand dunes overlie these sediments south of the Arkansas River, although water levels are usually below the sand-dune thicknesses. Basal gravels from these eastward-flowing streams consist of granite, caliche, and material derived from Permian- and Cretaceous-age rocks. Thicknesses of these deposits can reach 300 ft (91 m; Bayne, 1956). Most of the public and private water supplies in the aquifer area are obtained from the Big Bend aquifer, although saline ground waters are present in the Big Bend aquifer at depth in the eastern half of the Big Bend area, south of the Arkansas River (Bayne, 1956).
Two wells (sites 28 and 46) completed in the Big Bend aquifer were sampled. The well at site 46 in Reno County has a total depth of 72 ft (22 m) and is screened in 20 ft (6 m) of sand and gravel. At site 28 in Edwards County, the well has a total depth of 100 ft (30 m) to shale bedrock and is screened in 30 ft (9 m) of sand and gravel.
Glacial sediments of Pleistocene age overlie Paleozoic bedrock in a large area of northeastern Kansas. The basal sands and gravels in these glaciofluvial deposits are an important ground-water source in this area. These coarse-grained beds are mainly located in a buried-valley aquifer system. Glacial buried valleys were formed as glacial ice or its meltwater deposited sediments in Pleistocene age or older stream valleys. The valley sediments can be up to 400 ft (120 m) in thickness and 3 mi (5 km) in width (Denne et al., 1984).
Glacial sediments predominantly are composed of gravelly, silty, sandy clays (glacial till) which can be brown, tan, or blue-gray in color. Generally, the brown and tan clay is present in the upper section and has a thickness of about 40 ft (12 m). The gray and blue-gray glacial clays are present in the lower section and are usually much thicker in the buried valleys. Lenses or beds containing varying amounts of sand and gravel may be present throughout the glacial-sediment thickness. The gravels are composed of limestone, chert, igneous, and metamorphic fragments (Ward, 1974). A major glacial buried-valley system extends from southeastern Marshall County through Nemaha, Jackson, and Atchison counties in Kansas.
Water samples were collected from three wells (sites 19, 22, and 23) associated with the major buried-valley system. Site 19 in Marshall County has 182 ft (55 m) of glaciofluvial sediments, and the well is screened in 10 ft (3 m) of gravel. The well at site 22 in Jackson County is constructed in 145 ft (44.2 m) of glacial-related sediments and is screened in 10 ft (3 m) of coarse sand and medium and pea gravel. Further east along the course of the buried valley in Atchison County, the well at site 23 is situated in 200 ft (60 m) of glacial material and screened in 10 ft (3 m) of coarse sand and pea gravel. A fourth well which taps a glacial aquifer, site 21 in Brown County, also was sampled. At this site the glacial sediments are only 45 ft (14 m) thick and are not part of the major buried valley to the south. The well is screened in 7 ft (2 m) of sand and gravel and 13 ft (4.0 m) of the underlying shale bedrock.
The Dakota Formation of the Lower Cretaceous System is composed of sandstone (some conglomeratic) separated by layers of siltstone, mudstone, shale, and clay (Leonard et al., 1983). The sandstone is iron rich, and saltwater occurs in parts of this formation (Moore, 1940). It has a wide outcrop area in the north-central region of Kansas (fig. 2). Early workers referred to this aquifer as a classical artesian system, receiving recharge at the higher outcrops along the Rocky Mountains and Black Hills and transmitting water into areas of lower head eastward. Subsequent investigations revealed that the aquifer has more hydrologic complexity (Helgesen et al., 1982). Recharge to the Dakota aquifer in sampling areas of this study is from precipitation on outcrop areas or precipitation through overlying permeable formations such as the Ogallala and Pleistocene deposits. The thickness of the Dakota aquifer can range up to 580 ft (176 m; Kume and Spinozola, 1985).
The sandstone of the Dakota Formation is a widely used aquifer unit in the outcrop areas of Kansas as well as in some areas of western Kansas where it is buried deeply in the subsurface. Yields in irrigation wells can be up to 2,200 gpm (Kume and Spinozola, 1985). Three sets of water samples (sites 12, 18, and 29) were taken from wells screened in the Dakota Sandstone aquifer of the Cretaceous System. The well at site 18 is 285 ft (86.9 m) deep and is screened in 20 ft (6 m) of sandstone. This well is located in Washington County near the Nebraska border in a Dakota Formation outcrop area. At site 12 in Marion County, the well is situated in an outcrop island of the Dakota Formation. The well has a depth of 68 ft (21 m) and is screened in 10 ft (3 m) of tan, fine- to medium-grained sandstone. The well at site 29 in Rush County is 100 ft (30 m) deep and screened in 40 ft (12 m) of loose sandstone and limestone. This well is found in a small area of the Cretaceous System adjacent to the Wet Walnut Creek alluvium.
Two wells (sites 44 and 45) were sampled which have screened sections in Lower Permian rocks. The Lower Permian Series comprises more than 1,900 ft (580 m) of evaporite-bearing siltstones, sandstones, and shales in the upper portion and a little less than 800 ft (240 m) of alternating limestone, shale, and minor amounts of gypsum in the lower part (Zeller, 1968). Ground waters in Permian-age deposits vary widely in quality and quantity depending on the location and aquifer material. In Marion County, the Winfield Limestone, the Nolans Limestone, and the Wellington Formation are known to be aquifers. The Winfield Limestone and the Nolans Limestone are part of the Chase Group, which is about 335 ft (102 m) in thickness. The Chase Group consists of limestones alternating with shales which are often red and green in color. The Winfield Limestone is about 25 ft (7.6 m) thick and consists mainly of cherty limestone and contains a massive fossiliferous limestone where cavernous weathering is characteristic (Zeller, 1968). Above this formation lies the Odell Shale, which is chiefly red and green shale with some gray and yellow shale. The Nolans Limestone contains two limestone members separated by a shale member and lies above the Odell. The lower limestone is yellowish brown and is about 4 ft (1 m) in thickness (Zeller, 1968). The upper limestone is yellowish tan and is dolomitic with a thickness of 6-10 ft (2-3 m). The Nolans Limestone is 22-40 ft (6.5-12 m) in thickness.
The Wellington Formation is predominantly shale with limestone, dolomite, siltstone, gypsum, and anhydrite. The shale is gray to greenish gray with some red, maroon, and purple shale. The limestone is light colored and argillaceous (Zeller, 1968). The Wellington is part of the Sumner Group and is several hundred feet thick in Marion County, where it also crops out in the southern region. Thin shale beds alternating with beds of white, pink, or gray gypsum can be as great as 20 ft (6 m) in thickness (Byrne, 1959). The limestone of the Wellington weathers blocky and cavernous to porous (Byrne, 1959).
At site 44 in northeastern Marion County, the well is constructed to a total depth of 93 ft (28 m) and is screened in 28 ft (8.5 m) of limestone and shale which are probably part of the Nolans Limestone (O'Connor, 1987). The well at site 45 in the southern part of Marion County is 60 ft (18 m) deep and screened in 30 ft (9 m) of limestone and shale of the Wellington Formation.
One set of samples, site 35 in Kingman County, was taken from a well which is screened in the "red beds" section of the Nippewalla group in the Permian System. These rocks are exposed in south-central Kansas, and water is believed to occur only in the weathered part of the formation (Lane, 1960). The well at this site in southwestern Kingman County is 74 ft (23 m) deep and is screened in 12 ft (3.7 m) of shale in the Harper Siltstone.
Rocks of the Pennsylvanian System crop out in the eastern quarter of the state. Sandstones of the Douglas Group provide potable water to small public and private users in areas of Leavenworth, Douglas, and Franklin counties. The Tonganoxie Sandstone Member of the Stranger Formation occupies an erosional river valley cut into older rocks of the Stranger and Stanton formations. The valley is 14-20 mi (22-32 km) wide and trends southwestward (O'Connor, 1960). The Tonganoxie Sandstone Member is made up of conglomerate, sandstone, shale, and coal and can be as great as 120 ft (37 m) in thickness. The sandstone is light to dark gray and contains fine to very fine, angular to subangular quartz and is up to 70 ft (21 m) in thickness (O'Connor, 1960).
The Ireland Sandstone Member of the Lawrence Shale is an important sandstone aquifer occupying a west-southwest-trending erosional valley in southern Douglas and parts of Franklin counties (O'Connor, 1960). The valley is 0.5 mi (0.8 km) wide, and the Ireland Sandstone can reach a thickness of 115 ft (35.0 m). The sandstone is similar to the Tonganoxie except that it is coarser. It is light gray where it is clean and medium to dark gray where carbonaceous material is more abundant. The sandstone contains a small percentage of mica, pyrite, and clay minerals and weathers tan or yellow brown (O'Connor, 1960).
Recharge to both sandstone aquifers is mainly through precipitation in the outcrop areas, and the water becomes more mineralized farther from the recharge area. There is recharge to the Tonganoxie Sandstone from the Ireland Sandstone where they are interconnected. Discharge occurs from the Tonganoxie into alluvial deposits of the Wakarusa and Kansas River valleys (O'Connor, 1960). Wells in Douglas Group sandstones yield 5-100 gpm.
Three sets of water samples (sites 4, 5, and 48) were taken from wells in the Douglas Group sandstones. The well at site 4 in Franklin County is screened in 59 ft (18 m) of white sandstone and has a depth of 210 ft (63.5 m). In Douglas County at site 5, the well has a total depth of 500 ft (152 m), including 80 ft (24 m) of uncased open hole at its base. The aquifer material at this location consists of sandstone and shale. The well at site 48 in southwestern Leavenworth County is 160 ft (48.5 m) deep and is screened in 40 ft (12 m) of sandstone.
The Arbuckle aquifer refers to the lower Paleozoic units of Cambrian-Ordovician age located in southeast Kansas and adjoining areas of Oklahoma, Arkansas, and Missouri. The Mississippian and Cambrian-Ordovician aquifers are separated by confining layers of shale and dense dolomite except in a few areas (Macfarlane et al., 1981).
Freshwater wells in the Arbuckle aquifer are on the order of 1,000 ft (300 m) in depth and are usually completed as open bore holes. The first freshwater wells were drilled in the 1800's and were used for milling lead-zinc ores which were being mined in the area. At the present time, the freshwater of the Arbuckle is widely used for public supplies and industry (Macfarlane et al., 1981). Recharge into the Cambrian-Ordovician aquifer is from the outcrop area in the Ozark region of Missouri, and the general flow of the water is westward. The Mississippian and Cambrian-Ordovician aquifers produce oil west of Crawford and Cherokee counties, and water in these units increases in salinity in a westward direction. The presence of a water-quality transition zone in the aquifer is demonstrated by increasing amounts of sodium, chloride, and hydrogen sulfide (Macfarlane et al., 1981).
The bedrock in southeastern Kansas consists of sedimentary rocks ranging in age from Middle Pennsylvanian to Late Cambrian and rests unconformably on the Precambrian surface. These sedimentary rocks range in thickness from 1,200 to 2,800 ft (360-850 m) and are composed of limestone, dolomite, sandstone, and shale. The Cambrian-Ordovician section contains many different formations, among which are the Cotter and Roubidoux and the Gasconade Dolomite. These formations are considered to encompass the major permeable zones of the aquifer system (Macfarlane et al., 1981). The Cotter formation is composed of cherty, silty dolomite with lenses of sandstone and has a thickness ranging from 0 to 300 ft (0-91 m). A particular layer of sandstone from 5 to 10 ft (1.5-3 m) in thickness has been informally named the "Swan Creek." The Roubidoux Formation is composed of white sandstone; gray, medium-grained, sandy dolomite; and chert dolomite. It is generally around 140 ft (42 m) in thickness. The Gasconade Dolomite is primarily vuggy, cherty dolomite with a basal section composed of sandstone or sandy dolomite which is named the Gunter Sandstone Member.
Five sets of water samples (sites 36, 37, 38, 39, and 50) were taken from wells deriving water from the Cambrian-Ordovician aquifer system. Sites 38 and 50 are in close geographic proximity in southeastern Crawford County. These two wells have 500 ft (152 m) of open bore hole drilled into the Cotter and Jefferson City dolomites, the Roubidoux Formation, and the Gasconade Dolomite. At site 39, about 6.5 mi (10 km) northwest of sites 38 and 50 in Crawford County, the well has a total depth of 1,113 ft (339 m) and 390 ft (119 m) of open bore hole in the Lower Ordovician section including the Cotter, Jefferson City, Roubidoux, and Gasconade. Site 36 in east-central Cherokee County has a well with a total depth of 900 ft (274 m) and 325 ft (99 m) of open hole which includes the Jefferson City Dolomite and the Roubidoux Formation. The well at site 37 in southeastern Cherokee County has a total depth of 1,260 ft (384 m) and 746 ft (227 m) of open hole in the Cotter, Jefferson City, Roubidoux, Gasconade, and a small section of the Eminence.
After permission to sample a well was granted, raw water samples were taken from the outlet closest to the well. The well was allowed to pump for a period considered sufficient to obtain formation water. Since the water in the casing itself may be chemically altered, at least one casing volume of water was pumped before sampling. The water temperature was observed until it stabilized, serving as an indication of the "freshness" of the water. Generally, public-supply wells did not need a long pumping period because their pumping rates were much higher and they had often been operating for long periods of time before the sample was taken. Most public-supply systems had a raw-water outlet where a sample could be taken before treatment. Other systems had to turn off their chlorination or softening processes before untreated raw water could be obtained. In a few cases, chlorine was still detected in the raw-water sample, which was probably the result of backflow in the system or valve leakage from the chlorinator.
At each site three sample bottles were filled with the raw water: one 500-mL polyethylene bottle for determination of pH, specific conductance, and major anions and cations; one 250-mL polyethylene bottle acidified with 2 mL of redistilled 6 N hydrochloric acid and filled to 200 mL for analysis of trace metals, nitrate, and ammonium; and one 250-mL glass bottle (with a TeflonTM-lined cap) for the determination of TOC and TFP.
Finished water samples also were collected from public-supply wells so that instantaneous and terminal THM concentrations at the normal chlorination levels could be determined. Samples were usually taken at the well-house in small systems where the only treatment was chlorination. In larger systems, finished water was taken from inside the water plant and was usually a combination of water from several different wells taking water from a single aquifer. The finished water was collected in two 50-mL glass serum bottles (one containing about 0.5 g of powdered sodium sulfite). Each bottle was filled to overflowing so that a convex meniscus was formed at the top. A PTFE-lined septum was then inserted in an aluminum seal and carefully placed over the bottle and crimped into place. The sample treated with sodium sulfite and the unaltered sample were used to determine instantaneous and terminal THM concentrations, respectively.
Water temperature was determined at the time of water sampling. Field measurements were made immediately after the water was sampled. Portable pH (Model 607, Fisher Scientific Co., St. Louis, MO) and conductivity (Lectro-MHO Meter Model MC-1, Mark 4, Lab-Line Instr. Co., Melrose Park, IL) meters were used to determine pH and specific conductivity. Hydrogen sulfide (H2S) was determined using a hydrogen sulfide kit (CHEMet, CHEMetrics Inc., Calverton, VA) employing a colorimetric method with a detection limit of 0.1 mg/L. Free and total residual chlorine concentrations in the finished water samples were measured with a colorimetric comparator kit (Hellige Inc., No. 605-A, Garden City, NY) employing DPD tablets.
Most of the samples were collected between March 7 and April 11, 1986, but samples from sites 48 through 50 were collected from June 5 to July 17, 1986. Following collection, all samples, raw and finished, were numbered by site, stored on ice and transported to Lawrence for analysis. The glass bottles were taken to the Environmental Health Laboratory housed in Learned Hall at the University of Kansas for analysis of TOC, THM, and TFP. The polyethylene bottles were taken to the Analytical Services Laboratory of the Kansas Geological Survey housed in Moore Hall. All samples were refrigerated while awaiting analysis.
Although most of the samples were fairly clear, three (5, 20, and 49) contained dark-colored suspended solids and were filtered through a glass-fiber filter (934 AH) to remove particulate organic matter prior to determination of TOC and TFP. Table 2 shows TOC concentrations determined for six samples under various suspended solids conditions. Samples 5 and 20 contained high concentrations of particulate organic carbon. Sample 5 had fine dark solids high in TOC. The TOC concentrations of the samples containing yellowish-orange suspended solids (samples 2, 3, 10, and 11) were similar for filtered and unfiltered portions. Because it was thought that the TOC associated with the solids in these samples had probably been absorbed from solution after oxidation and precipitation of iron, these samples were not filtered prior to TFP analysis.
Table 2--TOC concentrations of filtered and unfiltered turbid samples.
| Sample No. |
TOC, mg/L | ||
|---|---|---|---|
| Whole Mixed |
Settled Decanted |
Filtered | |
| 2 | 3.31* | 2.59 | ND** |
| 3 | 2.56* | 2.09 | ND |
| 5 | 11.45 | 1.45 | 0.48* |
| 10 | 1.03* | 0.92 | ND |
| 11 | 1.90* | 1.66 | ND |
| 20 | 7.43 | 2.40 | 2.84* |
| *This sample was chlorinated to determine TFP. **Not determined. |
|||
TOC was determined using a Dohrman DC-80 TOC Analyzer (Xertex Corp., Santa Clara, CA), according to the persulfate-ultraviolet oxidation method described in Standard Methods (1985). Samples were acidified to pH < 2, purged with nitrogen to remove CO2 and shaken well prior to injection. TOC concentrations were determined for all raw-water samples and 26 of the 31 finished-water samples. The system blank of 0.055 mg/L, determined on 11 replicates of fluid drawn from the reactor inside the TOC analyzer, was subtracted from all of the measured values of TOC. Analytical precision was ±2% for concentrations greater than 1.0 mg/L.
After the TOC concentration of a raw-water sample was determined, TFP analysis was performed. Samples were adjusted to pH 8.2, divided into a series of 61-mL bottles dosed with free chlorine in 5-mg/L increments, and incubated heact-space free for 96 hours. The sample in the bottle having the lowest free chlorine residual in excess of 0.2 mg/L, determined using the DPD titrimetric procedure described in Standard Methods (1985), was then quenched with sodium sulfite and extracted with pentane. The THM concentrations in the extracts were determined using liquid-liquid extraction and gas chromatography (Varian Model 2400, Varian Corp., Palo Alto, CA). Chlorinated blank samples produced less than 5 mg/L of THMs, and analysis of independent quality-control samples always produced results within ±3% of the stated value. More detailed descriptions of the analytical procedure for TFP are given by Randtke et al. (1987) and Denne et al. (1987).
After the odor of chlorine was noticed in one rawwater sample (8), subsequent raw-water samples were tested for free chlorine, with three out of the 13 samples tested showing detectable amounts. In these samples, it is probable that a small portion of the THMs escaped during sample collection and analysis.
Samples analyzed to determine the THM concentration at the time of sampling (instantaneous THMs) were dechlorinated at the time of collection, immediately placed on ice and then refrigerated at 4°C (39°F) in the laboratory for up to two days until they could be extracted. They were then extracted at room temperature. For all but three samples, the remainder of the sample was then acidified, purged, and analyzed for TOC. Samples analyzed to determine the THM concentration in the finished water after a four-day (96-hr) incubation period (terminal THM) were incubated at 25°C (77°F), extracted, and analyzed for THMs. TOC was not determined for these samples.
A portion of each unacidified sample (collected in a 500-mL polyethylene bottle) was allowed to come to room temperature in the laboratory. Specific conductance measurements were then made using a Lab-Line Lectro Mho-Meter, Mark IV unit. These values were used later as guides in the determination of dissolved-concentration levels of major cation and anion constituents of the water samples.
Measurements of sample pH and alkalinity, herein expressed as mg/L bicarbonate (HCO-), were obtained using a Fisher Titrimeter 11 Titration System. The alkalinity was derived from the volume of 0.02 N H2SO4 necessary to bring a room-temperature 50-mL aliquot of sample to pH 4.5 according to the procedure described in Standard Methods (1985).
A Technicon Auto Analyzer 11 continuous-flow system was used in the analysis of sulfate (SO4-2), chloride (Cl-), nitrate (NO3-) and ammonium (NH4+). SO4-2 and Cl- were determined on room-temperature aliquots of unacidified samples, using the methylthymol blue and ferric thiocyanate methods, respectively. NO3- and NH4+ were determined, using the cadmium reduction-diazotiza-tion and indophenol methods, respectively, on room-temperature aliquots of acid-preserved samples neutralized to pH 6 prior to analysis. These automated procedures were based on chemistries developed for the Auto Analyzer 11 system and supplied by Technicon Industrial Systems.
Concentrations of major [calcium (Ca), magnesium (Mg), and sodium (Na)] and minor [potassium (K) and strontium (Sr)] metallic constituents and selected trace metals [barium (Ba), iron (Fe), and manganese (Mn)] were determined using a Jarrell-Ash Model 975 Plasma Atomcomp inductively coupled argon-plasma optical-emission spectrophotometer (ICP). Room-temperature aliquots of unacidified samples were used in the determinations of Ca, Mg, Na, K, and Sr, whereas room-temperature aliquots of the acid-preserved samples were employed for the determinations of Ba, Fe, and Mn.
Next Page--Results and Discussion
Kansas Geological Survey
Placed on web Nov. 6, 2012; originally published in 1991.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/CQS12/index.html