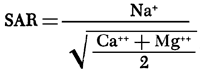
where the ionic concentrations are expressed in milliequivalents per liter.
Absorption: The process by which a substance is taken into and included within another substance, i. e., intake of water by soil, or intake of gases, water, nutrients, or other substances by plants.
Acre-foot: The volume of water equivalent to an area 1 acre and a depth of I foot.
Adsorption: The increased concentration of molecules or ions at a surface, including exchangeable cations and anions on soil particles.
Base runoff (base flow): Sustained or fair-weather runoff. In most streams, base runoff is composed largely of ground-water effluent.
Bulk density: The ratio of the mass of water-free soil to its bulk volume. Bulk density is expressed in pounds per cubic foot or grams per cubic centimeter and is sometimes referred to as "apparent density." When expressed in grams per cubic centimeter, bulk density is numerically equal to apparent specific gravity or volume weight.
Bypass water: Water diverted for irrigation, but returned to the river or source of supply without being applied to the agricultural land. See Waste.
Cation exchange: The interchange of a cation in solution with another cation on a surface-active material.
Cation exchange capacity: The total quantity of cations that a soil can adsorb by cation exchange, usually expressed as milliequivalents per 100 grams. Measured values of cation exchange capacity depend somewhat on the method used for the determination.
Coefficient of correlation: A statistic used in linear correlation that provides a measure of the proportion of variation in one variable that is associated with variation in another variable.
Cubic feet per second (cfs): A unit expressing rates of discharge.
Cfs-day: The volume of water represented by a flow of 1 cubic foot per second for 24 hours.
Chemical discharge (load): The weight of dissolved chemical constituents passing a stream section in a unit time.
Concentration: The quantity of dissolved material in a unit volume or weight of water. In this report, concentration is expressed in milligrams per liter, parts per million, equivalents per million, specific conductance in micromhos per centimeter at 25°C, and tons per acre-foot.
Concentration ratio: Ratio of the specific conductance or concentration of an ion in a sample of water to the specific conductance or concentration of the corresponding ion in the irrigation water.
Consumptive use: Use of water that is changed from the liquid to the gaseous state by evaporation or transpiration and is thereby lost from the soil-plant ecosystem. See Evapotranspiration.
Conveyance losses (L): Water lost from the canal and laterals by seepage and evaporation.
Deep percolation losses: The part of the irrigation water applied to the land that percolates below the crop root zone, and is not subject to consumptive use by the agricultural crops.
Degradation of water quality: A deterioration in water quality due to increased concentration of any substance classified as a pollutant.
Dilution: The reduction of concentration in water by the addition of water having a lesser concentration of a constituent.
Direct runoff: The runoff entering stream channels promptly after rainfall or snowmelt.
Drainage water: Surface and subsurface water coming from irrigated areas, which may be commingled with precipitation, surface runoff, and groundwater flow from nonirrigated lands.
Effective rainfall: Rainfall during the growing season for each crop.
Efficiency of irrigation: The fraction of the water diverted from a river or other source that is consumed by the crop, expressed as percent. See Consumptive use. Often applied to whole irrigation systems and takes account of conveyance losses.
Equivalent weight: The weight in grams of an ion or compound that combines with or replaces 1 gram of hydrogen. The atomic weight or formula weight divided by its valence.
Evapotranspiration: The consumptive use of water from the soil. Water lost as vapor from a given area of soil through the combined processes of evaporation from the soil surface and transpiration from plants.
Exchangeable cation: A cation that is adsorbed on the exchange complex, and that is capable of exchange with other cations.
Exchangeable sodium percentage: The degree of saturation of the soil exchange complex with sodium. It may be calculated by the formula:
E S P = [(Exchangeable sodium, in milliequivalents per 100 grams of soil / Cation exchange capacity, in milliequivalents per 100 grams of soil) X 100]
Farm delivery (I): Quantity of water delivered to farms for irrigation. Equal to irrigation water delivered to the district less conveyance losses and bypass water.
Hardness: The water property attributable to the presence of alkaline earths, mainly calcium and magnesium.
Infiltration: The downward entry of water into soil.
Irrigation reach: Reach of the Smoky Hill River adjacent to the main part of the irrigation district between stations at river miles 350.0 and 338.0.
Irrigation requirement: The quantity of water exclusive of precipitation required for crop production. This includes surface evaporation and other economically unavoidable wastes. It is usually expressed in depth (volume per unit area) for a given time. (American Society of Agricultural Engineers and American Society of Civil Engineers. )
Irrigation return flow: Any water diverted for irrigation purposes that finds its way back into a source of supply (stream or ground-water basin). This includes bypass water, deep percolation losses, tail-water runoff and seepage.
Leaching: The process of removal of soluble material by the passage of water through soil.
Milliequivalents per liter (me/l): A unit for expressing the concentration of chemical constituents in terms of the interacting values of the electrically charged particles, or ions, in solution.
Milligram per liter (mg/l): A unit for expressing the concentration of dissolved chemical constituents by weight, as milligrams of constituents per liter of solution.
Moisture percentage: 1. Dry-weight basis. The weight of water per 100 units of material dried to constant weight at a standard temperature. 2. Depth basis. The equivalent depth of free water per 100 units of depth of soil. Numerically this value approximates the volume of water per 100 units of volume of soil.
Net drainage: Inflow, excluding canal and lateral waste, to the reach of the Smoky Hill River between Cedar Bluff Fish Hatchery and the station near Schoenchen during periods of stable streamflow.
Net gain (or loss): Increase (or decrease) in water or chemical discharge in each reach of the river during a seepage-salinity survey.
Net input (N): Quantity of irrigation water delivered to the district less waste (W). The algebraic sum of deliveries to farms (I) and losses (L) from the canal and laterals.
Net seepage gain (or loss): Net gain minus tributary inflow to each reach of the river during a seepage-salinity survey. Net seepage gains represent unmeasured inflow, normally effluent ground water, directly to the main stem. Net seepage losses represent unmeasured losses or withdrawals from the channel or stream.
Nutrients: Compounds of nitrogen, phosphorus, potassium, and other elements essential for plant growth.
Percent sodium: The ratio, expressed in percentage, of the sodium ion to the sum of the positively charged ions (calcium, magnesium, sodium, and potassium); all ions, in milliequivalents per liter.
Perennial stream: A stream that flows continuously.
Permeability: The specific property governing the rate or capacity of a porous medium to transmit fluids under standard conditions.
Pesticides: Chemical compounds used for the control of undesirable plants, animals, or insects. The term includes insecticides, weed killers, rodent poisons, fungicides, and growth regulators.
pH: The negative logarithm of the concentration of hydrogen ions, in moles per liter. It is lower than 7 in acid solutions and higher than 7 in basic solutions.
Pollutants: Substances that may become dissolved, suspended, absorbed, or otherwise contained in water, that impair its usefulness.
Pollution: The presence of any substance (organic, inorganic, biological, thermal, or radiological) in water at intensity levels that tend to impair, degrade, or adversely affect its quality or usefulness for a specific purpose.
Salinity: The dissolved-mineral content or total concentration of solids in solution.
Salt balance: The quantity of salt entering an area by way of the irrigation water as compared with the quantity of salts removed from the area by return water.
Salts: Dissolved mineral matter or soluble inorganic salts.
Saturated soil paste: A particular mixture of soil and water. At saturation the soil paste glistens as it reflects light, flows slightly when the container is tipped, and the paste slides freely and cleanly from a spatula for all soils except those with high clay content.
Saturation extract: The solution extracted from a soil at saturation percentage.
Saturation percentage: The moisture content of a saturated soil paste, expressed on a dry-weight basis.
Seepage gains: Unmeasured inflow to the river mainly from small tributaries and from nearby areas.
Seepage losses: That water which is lost from the conveyance channels of rivers or other water-supply systems.
Sodium-adsorption ratio: A ratio for soil extracts and irrigation waters used to express the relative activity of sodium ions in exchange reactions with soil.
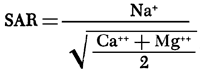
where the ionic concentrations are expressed in milliequivalents per liter.
Soil extract: The solution separated from a soil suspension or a soil at a particular moisture content.
Soluble sodium percentage: A term used in connection with irrigation waters and soil extracts to indicate the proportion of sodium ions in solution in relation to the total cation concentration. It may be calculated by the formula:
SSP = [(Soluble sodium concentration, in milliequivalents per liter / Total cation concentration, in milliequivalents per liter) X 100]
Specific conductance: A measure of the ability of a water to conduct an electrical current expressed in micromhos per centimeter at 25°C.
Standard deviation: A statistic used to measure the dispersion of a set of values around their mean.
Tail-water runoff: Irrigation water that runs off the surface of irrigated fields. Sometimes referred to as waste water or surface return flow.
Waste (W): Water discharged from the end of canals and laterals to maintain flow through the system. See Bypass water.
Water table: The upper boundary for ground water. The upper surface of the locus of points at which the pressure in the ground water is equal to atmospheric pressure.
Water year: The 12-month period, October 1 through September 30, designated by the calendar year in which it ends.
The Cedar Bluff Irrigation District diverts water from the Smoky Hill River to irrigate about 6,000 acres (2,400 hectares). Although drainage from irrigated land replenishes ground water in transient storage and augments low flow in the river, the drainage adversely affects the chemical quality of the related water. Specific conductance of the irrigation water increased progressively from 800 to 980 micromhos per centimeter at 25°C between 1964 and 1971. The percent composition of this calcium-sulfate type water remained relatively constant.
Infiltration of the irrigation water, which contained additional salts leached from the soil, increased the concentration and changed the ionic composition of the ground water. Generally, calcium and sulfate became the predominant ions. Concentrations of calcium, magnesium, and sulfate present in the irrigation water were augmented by the natural content in the soil and the aquifer. Chemical analyses of soil samples indicated that significant amounts of sodium and chloride were leached from naturally accumulated salts in the soil. Increased concentrations of nitrate probably resulted from applying water in excess of that required by the fertilized crop.
Data from seepage-salinity surveys indicate that water discharge of the Smoky Hill River in the irrigation reach increased progressively from about 1.5 to 10 cubic feet (0.04 to 0.28 cubic meters) per second between 1964 and 1971. Specific conductance in the reach increased from 880 to 900 micromhos per centimeter at 25°C in 1964 and from 1,070 to 1,230 micromhos per centimeter at 25°C in 1971. The concentration ratios for specific conductance increased from 1.01 in 1964 to 1.15 in 1971. Concentrations of sodium and chloride increased downstream, but the concentration of sulfate decreased to values lower than that of the irrigation water. Although net gains in chemical discharge increased from 1964 to 1971, the rate of annual increase diminished from year to year.
The use of water for irrigation in the district and the increase of drainage to low flow have not caused serious degradation of chemical quality of the Smoky Hill River.
Irrigation is the largest use of water in Kansas. In 1970, about 1,500,000 acres (610,000 ha) were under irrigation and additional acreage is being added each year. Most is in the semiarid western part of the state. Application of irrigation water in a semiarid region accelerates natural leaching processes. Soluble salts carried by the irrigation water and those originally present in the soil or ground water normally appear in the increased drainage from the irrigated area. Relatively pure water is removed from the groundwater reservoir by plants through evaporation and transpiration, thereby raising the concentration of residual salts. These salts, possibly augmented by excess fertilizers, pesticides, and sediment, can render water in the receiving streams or ground-water reservoirs unsuitable for agricultural re-use and for municipal and many industrial purposes.
During the recurrent periods of drought to which Kansas is subject, the addition of saline drainage from irrigated land to water containing naturally high concentrations of dissolved solids could cause severe degradation of quality in water supplies. Conversely, drainage from the irrigated land could benefit depleted ground-water reservoirs and sustain perennial streamflow where natural streamflow is ephemeral. However, little definitive information about the effects of irrigation on the chemical quality of related water resources in Kansas is available. Most of the previous work relating irrigation to water quality in the State described the effects of the chemistry of the irrigation water on soils and crop yields. Published results of investigations of the effects of return flow on streams in other states provides useful guidelines for anticipating changes, but the results are not necessarily applicable everywhere.
Irrigaation will continue to be a substantial factor affecting the water-resources development and the economic health and growth of Kansas. Most of the irrigation is with ground water for which the rate of withdrawal exceeds natural recharge; therefore, surface-water sources may become more important in the future. Development of surface water for irrigation has been confined chiefly to six U.S. Bureau of Reclamation projects in the Kansas River basin in the north and west-central part of the State. In 1966, about 70,000 acres (28,000 ha) were irrigated with water from project reservoirs, all of which lie upstream from major centers of population that depend mainly on the river and its tributaries for a water supply. Development of additional acreage is contemplated. Quantitative information describing the effects of irrigation on water quality is needed to facilitate planning for equitable distribution of the limited supply of water and to promote the most efficient use of water for irrigation with a minimum degradation of quality in related water supplies.
In 1964, the Kansas Water Resources Board and other concerned State and Federal agencies considered preliminary proposals for a detailed interagency investigation of the hydrologic system of a typical irrigation project in Kansas. The newly organized Cedar Bluff Irrigation District in west-central Kansas (fig. 1) was selected for the study, but funding was inadequate to support the extensive staffing and instrumentation necessary for the proposed comprehensive study. To satisfy the urgent need for information, and to collect data that could constitute the framework for more detailed studies, the less ambitious pilot investigation described herein was begun in 1965 as a cooperative effort by the Environmental Health Services (now Division of Environment) of the Kansas Department of Health and Environment, the Kansas Geological Survey, and the U.S. Geological Survey. Data collection for the project was concluded in December 1971.
Figure 1--Index maps showing location of report area.
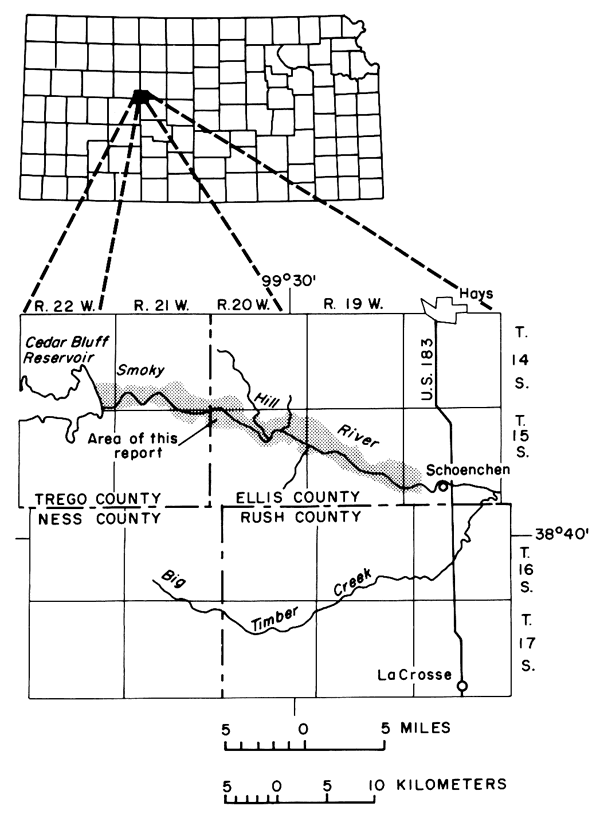
The Cedar Bluff Irrigation District was selected for the study because the natural equilibrium of the semiarid hydrologic environment had only recently been upset by irrigation that began in 1963. No critical pollution problems were known to exist, but degradation in the chemical quality of low streamflow of the adjacent Smoky Hill River by drainage from the irrigation district could adversely affect the municipal water supply for the city of Hays which is obtained from wells in the valley alluvium about four miles downstream from the study area. Pertinent background information describing the geology and water resources is available from published reports by Hodson (1965) and Leonard and Berry (1961) and unpublished data in the files of State and Federal agencies. Useful data also were collected on a routine basis from existing observation wells and recording gages by personnel of the U.S. Bureau of Reclamation and the irrigation district as part of the operation of the Cedar Bluff Dam and district.
The Cedar Bluff study is unique in Kansas because it began soon after the first irrigation season and before return How became a significant component of streamflow adjacent to the district. Data representing the preirrigation base line are scarce, but progressive changes in water quality caused by irrigation could be separated from natural variations as the district converted from range land and dryland farming to intensive cultivation under irrigation. Increased crop diversification, livestock production, and use of chemicals are peripheral results of irrigation that may complicate the water-quality picture. However, the effects on water quality of cultural factors other than irrigated agriculture appeared to be relatively insignificant or amenable to measurement. The irrigation water from Cedar Bluff Reservoir and runoff through or from the sparsely populated drainage area of the district seem to be relatively unaffected by municipal or industrial effluent. Ground water withdrawals in the district are generally small and widely scattered.
The principal objective of the study was to evaluate the progressive effects of irrigation with water from Cedar Bluff Reservoir on the chemical quality of ground water and surface water in and adjacent to the newly established irrigation district. A secondary objective was to relate the changes in the chemical quality of water to natural and manmade factors in the environment to a sufficient degree that the relations would be useful for water-resources evaluation and planning. Development of a precise hydrologic model for use as a planning tool is beyond the scope of this investigation, but most of the data collected are applicable to that type of endeavor.
A wide variety of data was collected and analyzed by different methods to evaluate their application to the potential problem of quality deterioration resulting from irrigation. If the results serve as guidelines for selection of the type and minimum quantity of data needed for similar or related investigations of the movement of inorganic pollutants or of artificial recharge under somewhat similar geohydrologic conditions, an implied objective of the study has been attained.
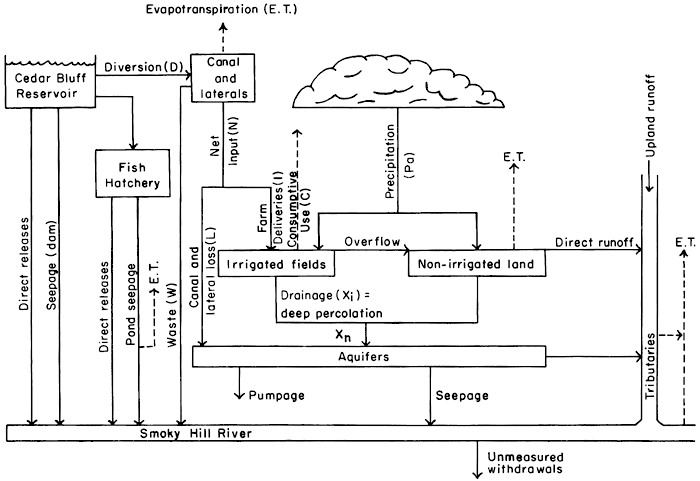
A large volume and wide variety of basic data were collected at the sites shown in plate 1 between 1964 and 1971 to describe semiquantitatively the system shown schematically in figure 2. The data include measurements of precipitation, water levels and water discharge; lithologic and gamma-ray logs of observation wells and test holes; chemical analyses of ground water, surface water, and soil; analyses for pesticides in ground and surface water; and selected particle-size analyses of soil. Except where noted in the text, water analyses were performed in the Division of Environment laboratory, Kansas Department of Health and Environment. The methods of analysis generally were those recommended in "Standard Methods for the Examination of Water, Sewage, and Industrial Wastes" (American Public Health Association and others, 1971). Definitions of technical terms, as used in this report, are summarized in the glossary. Some were from publications of the U.S. Department of Agriculture and the Federal Water Pollution Control Administration (now Environmental Protection Agency). Extensive use of existing digital-computer programs (Lowell, et al., 1970) facilitated compilation and analysis of the varied and voluminous data.
Figure 2--Diagram of the local hydrologic system.
Only the most significant part of the basic data is reproduced herein, and that part mostly in graphical form. Data have been released in the U.S. Geological Survey annual series "Water Resources Data for Kansas, Part 2. Water Quality Records," in interpretative progress reports (Leonard 1969a, b, 1970), and in a compilation (Leonard and Stoltenberg, 1972) intended for limited distribution to researchers engaged in similar or related studies. Information relating to the operation of the irrigation district and Cedar Bluff Reservoir was drawn freely from published and unpublished reports of preliminary investigations, work plans, and operations provided by the U.S. Bureau of Reclamation, McCook, Nebr. and by Mr. Robert Schamel, Manager, Cedar Bluff Irrigation District. Observation-well information not included in published reports are on file at the U.S. Geological Survey, Lawrence, Kans.
The data are used to describe the geohydrologic setting in sufficient detail to relate the physical and chemical properties of the system to the quantity and quality of water passing through it. Input of water and salts is estimated from the measured quantity and quality of irrigation water, the quantity and distribution of rainfall, and estimates of the quantity of water returned to the atmosphere by crops.
Recorded changes in the altitude and configuration of the water table and in the concentrations of the major ions in ground water in and adjacent to the irrigated area reflect the arrival, distribution, lateral migration, and discharge of water in and from the ground-water reservoir. Detailed data are used to relate corresponding changes to the geology, distribution of water, and changes in the chemistry of the soil profile in three experimental plots.
Data from sixteen seepage-salinity surveys describe the quantity and quality of ground-water discharge from the district and its effect on low streamflow of the Smoky Hill River. Additional data collected at more frequent intervals at four stations adjacent to the district describe streamflow over a wider range of conditions. Estimates of the quantity and quality of drainage from the district based on the results of the salinity surveys were compared with estimates based on continuous records and with estimates based on irrigation requirements and distribution of the irrigation water.
English units of measurements used in this report are given in equivalent metric units (in parentheses) using the following conversion factors:
| English units | Multiply by | Metric units |
|---|---|---|
| inch (in) | 2.54 | centimeter (cm) |
| foot (ft) | .3048 | meter (m) |
| mile (mi) | 1.609 | kilometer (km) |
| acre | .4047 | hectare (ha) |
| 4.047 x 10-3 | square kilometer (km2) | |
| square mile (mi2) | 2.590 | square kilometer (km) |
| gallon (gal) | 3.785 | liter (l) |
| cubic foot (ft3) | .02832 | cubic meter (m3) |
| cfs-day (ft2/s-day) | 2447 | cubic meter (m3) |
| acre-foot (acre-ft) | 1233 | cubic meter (m3) |
| 1.233 X 10-3 | cubic hectometer (hm3) | |
| cubic feet per second (ft3/s) |
.02832 | cubic meters per second (m3/s) |
| gallons per minute (gpm) | .06309 | liters per second (l/s) |
Wells and soil-sampling sites are numbered according to the Bureau of Land Management's system of land subdivision. The first set of digits of the location number indicates the township south of the north border of Kansas, which is the 40th parallel; the second set, the range west of the sixth principal meridian; and the third set, the section in which the well is situated. The first letter denotes the quarter section, or 160-acre tract; the second, the quarter-quarter section, or 40-acre tract; and the third (when shown), the quarter-quarter-quarter section, or 10-acre tract. The quarter sections, quarter-quarter sections, etc., are designated A, B, C, and D in a counterclockwise direction beginning in the northeast quarter section. For example, well 14-21W-27DAD is in the SE NE SE sec. 27, T. 14 S., R. 21 W. (fig. 3).
Figure 3--Numbering system for wells and soil-sampling sites.
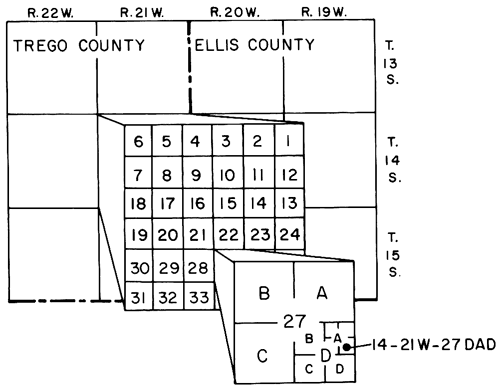
If more than one well is in the same tract, consecutive numbers beginning with 2 are added to the letters in the order in which the wells were installed. To simplify the description and graphical illustration of experimental plots, the closely spaced wells therein are designated by the last letter and number in consecutive order beginning with 1.
[Note: The classification and nomenclature of rock units used in this report are those of the Kansas Geological Survey and differ somewhat from those used by the U.S. Geological Survey.]
Cedar Bluff Irrigation District No. 6 is north of the Smoky Hill River and east of Cedar Bluff Dam, which is about 22 miles (35 km) southwest of Hays in west-central Kansas. About 80 percent of the irrigable area in the district is on a high terrace at an average elevation of about 80 feet (24 m) above the river. In the study area, the terrace ranges from 1 to 2 miles (1.6 to 3.2 km) in width, and slopes gently to the east and south. Most of the remainder of the district is within the flood plain of the Smoky Hill River or on low discontinuous terraces adjacent to it.
The principal natural drains are stream valleys that transect the terrace at intervals of 1 to 1.5 miles (1.6 to 2.4 km). Most of the streams are intermittent, but some streams have become perennial since irrigation began. Small undrained depressions are common. The permeability of the unconsolidated sediments over most of the area is adequate to permit infiltration and deep percolation of rainfall and irrigation water.
Relatively impermeable limestone and shale form the irregular sides and bottom of the container in which unconsolidated sediments (collectively termed valley-fill deposits) were laid down by the Smoky Hill River and its tributaries. The older valley-fill deposits, mapped as undifferentiated Pleistocene, underlie the prominent terraces on which most of the irrigated acreage is located. Younger valley-fill deposits, mapped as Wisconsinan deposits and Recent alluvium, underlie the low terraces and the flood plain of the river. Variation in texture, composition, and distribution of the sediments and the configuration of the bedrock surface affect the rate and direction of flow as well as the quality of water passing through the unconsolidated sediments.
The generalized surficial geology of the area, the general configuration of the bedrock surface, and the lithologic sections, shown on plate 2, are based on information from published reports, on unpublished logs of wells, maps, and reports by the U.S. Bureau of Reclamation, and on data from wells and test holes augered during the present investigation. Land-surface elevations were obtained from detailed topographic maps of the area at a scale 1 inch (2.5 cm) equals 400 feet (120 m) made by the Bureau of Reclamation before the district was developed and from traverses made to locate wells installed during this investigation.
Differences in the quality of lithologic samples from jetted, augered, and drilled holes, differing descriptions of the material penetrated by original and replacement wells at the same site, and differences in textural classification between drillers' logs and mechanical analyses of samples were commonly found during analysis of the data. Information from closely spaced wells augered in experimental plots indicates that the lithologic characteristics of the unconsolidated sediments are quite variable and that relief on the bedrock surface is greater than shown; therefore the geologic picture presented herein probably is oversimplified. Resolution of the minor discrepancies in the presentation of geologic and hydrologic data taken from other sources was beyond the scope of this project, and of little significance in the ultimate conclusions.
The consolidated rocks that underlie the valley and crop out on the uplands are of Cretaceous age including, in ascending order, the Greenhorn Limestone, the Carlile Shale, and the Niobrara Chalk. Individual units have not been differentiated on the geologic map. The rocks are generally impervious and do not yield significant amounts of water to wells except where highly weathered. The Greenhorn and Carlile function chiefly as a lower boundary for ground-water circulation; however, the formations contain soluble minerals that are a source of ions in the ground water.
Only the upper part of the Greenhorn Limestone, which consists of alternating beds of chalky limestone and shale, crops out and underlies part of the irrigated area. The Greenhorn is a source of calcium and carbonate for ground water in the overlying unconsolidated sediments.
The Carlile Shale, which underlies most of the irrigated area, is about 300 feet (91 m) thick. The lower part (Fairport Chalk Member) consists of alternating beds of chalky limestone and shale similar to the underlying Greenhorn Limestone. The upper part (Blue Hill Shale Member) is about 175 feet (53 m) thick. It consists of blue-gray shale containing crystals of selenite (CaSO4 · 2H2O); plates, layers, and flakes of iron oxides; and large calcareous concretions. The sulfate in the selenite may have been derived from oxidation of pyrite. Leachate (distilled water) from a sample of the shale was an acid water that contained more than 1,100 mg/l (milligrams per liter) of sulfate, nearly 300 mg/l of calcium, and 4 mg/l of chloride. The pH of the solution was 1.0. Relatively high concentrations in the sample of the nutrients; potassium (44 mg/l), nitrate (104 mg/l), and phosphate (22 mg/l) were found.
Deep weathering of the Blue Hill Shale Member of the Carlile Shale to residual clay precluded precise definition of the bedrock surface in some wells. However, the effect of the relatively impermeable buff to yellow clay on the movement of ground water is probably similar to the effect of the unaltered shale.
The Fort Hays Limestone Member of the Niobrara Chalk consists of massive chalk beds separated by thin chalky shale partings that locally yield small amounts of water to wells in the uplands north and south of the study area. Other than contributing calcium carbonate to upland runoff carried by the natural drains that traverse the area, the Fort Hays Limestone has little effect on water quality in the study area. Dissolution of fragments of the Cretaceous limestones in some stream deposits maintains high concentrations of calcium and bicarbonate in the included waters.
Deposits of Pleistocene age in high terrace positions fill and overlap channels eroded in a pediment on the Cretaceous bedrock. Previous investigators subdivided the Pleistocene deposits into five formations. In ascending order, these are the Grand Island, Sappa, Crete, Loveland, and Peoria Formations. The Grand Island and Crete Formations consist chiefly of arkosic sand and gravel. They are separated by the Sappa Formation, which consists chiefly of silt and sandy clay. The Grand Island and Sappa are reportedly (Leonard and Berry, 1961; Hodson, 1965) restricted to a narrow meandering ancestral channel of the Smoky Hill River north of the present channel (See section A-A', plate 2). The available subsurface information is inadequate to define the limits of the channel, if indeed a single channel exists.
The high terraces are mapped herein as undifferentiated Pleistocene deposits on the geologic map. These deposits generally coincide with the Crete and Loveland Formations mapped by Leonard and Berry (1961). In Trego County, the boundaries are nearly similar to those mapped by Hodson (1965) as terrace deposits of the Grand Island, Sappa, and Crete Formations and loess classified as the Loveland and Peoria Formations. Most of the sediment described as terrace deposits by Hodson would be included in the Crete Formation, which apparently grade upward into yellow and buff silt and sandy silt classified as Loveland by Leonard and Berry (1961). Part of the loess was deposited in or reworked by moving water. Where the terraces have not been dissected, loess and colluvial deposits obscure the upper contact with the bedrock, which is located mainly on a topographic discontinuity.
Soils developed on the silt (either Crete or Loveland) are mainly silty clay with high water-holding capacity and adequate internal drainage for good yields under irrigation. The loess deposits normally are calcareous and contain lenses and nodules of caliche (calcium carbonate), which is readily dissolved by water containing carbon dioxide to yield calcium and bicarbonate ions.
Wide lateral and vertical variability in texture and composition characterize the fluvial deposits; however, the sediments underlying the high terraces consist basically of a section of sand or sand and gravel overlain by silt and clay. Lenses of silt and clay in the coarse-grained section and of sand and gravel in the overlying section are common. In the eastern part of the area, the lower coarse-grained section contains less gravel. Most of the section probably represents the Crete and Loveland Formations as described above, but data generally are inadequate to justify placement of the formational contacts.
Relief on the bedrock surface that affected the nature and distribution of the sediments at the time of deposition, now affects to a large extent the thickness of the saturated section, the direction of movement of ground water, and the points of maximum discharge into the Smoky Hill River. Ground-water movement in the reservoir beneath the terrace, as shown in plate 1, is southward where surface or subsurface channels drain water toward the river. Where bedrock ridges retard flow toward the river, ground water moves eastward in channels beneath the terrace. Additional drilling to define the configuration of the aquifers and pumping tests to evaluate their hydraulic properties is necessary for precise mathematical modeling of the system; but collection of the data was beyond the scope of the investigation.
As illustrated in plate 2, the present channel of the Smoky Hill River occupies only a small part of the valley eroded in the bedrock during late Pleistocene time. There is little or no surface expression of the ancestral. channel beneath the modern flood plain and low terraces. However, the ancestral and modern channels generally are parallel. Limited test drilling adjacent to the river penetrated about 60 feet (18 m) of coarse sand and gravel that generally is overlain by 10 to 20 feet (3 to 6 m) of gray silt or sandy silt. Lesser thicknesses of generally fine-grained alluvium fills the channels of tributary streams that traverse the terrace.
Most of the alluvium is lithologically similar to the fluvial deposits from which it was largely derived. Some of the coarse-grained sediment classified as early Pleistocene in test holes north of the flood plain may be younger alluvium that fills deeply incised buried tributaries. Abundant fragments of limestone and shale in the streambed of the Smoky Hill River attest to relatively recent and continuing erosion of the nearby Cretaceous bedrock. As illustrated by the map showing the general configuration of the water table (pl. 1), virtually all excess water from the irrigation district will pass through or over these generally permeable deposits. Several irrigation wells installed in the alluvium between the river and the irrigated land since 1965 are capable of sustained yields of from 800 to 1,000 gpm (50 to 63 l/s).
In western Kansas, more than 95 percent of the precipitation on the land surface is evaporated from the soil or is transpired by plants. Seepage of part of the sporadic upland runoff carried by natural drains that traverse the study area and infiltration and percolation of rainfall on the cultivated area during abnormally wet years probably contributes a small amount of recharge to the ground-water reservoir. However, consumptive use of water by natural and cultivated vegetation exceeds normal rainfall during the growing season. Where the moisture deficiency was overcome by irrigation, more water became available to percolate below the root zone into the groundwater reservoir.
Official records of precipitation at Cedar Bluff Dam, Ellis, and Hays (National Weather Service) were supplemented by daily observations at five project stations equipped with wedge-shaped plastic rain gages (fig. 2). Records were kept by volunteer observers including: Mrs. S. Irwin (rain gage 1); Mr. R. Schamel (rain gage 2); Mrs. F. Dinkel (rain gage 3); Mr. J. T. Wanamaker (rain gage 4); and Mr. B. Younger (rain gage 5). Average annual precipitation is less than 23 inches (58 cm), about 75 percent of which normally occurs during the growing season April through September. Deficiencies in annual rainfall at Hays, about 8 miles (13 km) northeast of the district, in 1964, 1966, 1968, and 1970 far exceeded the recorded excess during the alternate years (table 1). Without the additions of water from irrigation , the period of study might have been characterized by a declining water table and diminished base flow in the Smoky Hill River adjacent to the district.
Table 1--Annual precipitation at stations near Cedar Bluff Irrigation District, 1963-71. [U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Environmental Data Service, Climatological data, Kansas, Annual Summaries 1963-71.]
| Year | Precipitation at indicated stations, in inches1 | Departure from normal at Hays |
||
|---|---|---|---|---|
| Cedar Bluff Dam | Ellis | Hays | ||
| 1963 | 18.79 | 19.32 | 22.17 | -0.70 |
| 1964 | 18.54 | 16.72 | 19.76 | -3.11 |
| 1965 | 23.85 | 35.95 | 24.49 | 1.62 |
| 1966 | 12.75 | 16.78 | 17.14 | -5.73 |
| 1967 | 18.74 | 22.12 | 23.64 | .77 |
| 1968 | 13.83 | 20.181 | 18.83 | -4.04 |
| 1969 | 24.10 | 27.49 | 25.12 | 2.25 |
| 1970 | 15.78 | 18.51 | 18.23 | -4.64 |
| 1971 | 23.04 | 26.45 | 23.75 | .88 |
| 1 Inches X 2.54 equals centimeters. 2 Estimated. |
||||
During 1963-71 the annual precipitation at Hays generally exceeded the annual precipitation at Cedar Bluff Dam by several inches. A large part of the rainfall occurred during intense localized convective storms of short duration; therefore, recorded daily values at one station do not necessarily apply to the entire district. However, the apparent increase in precipitation from west to east was also observed at the project stations. The values for annual precipitation used in calculations for this report represent areally weighted averages of values recorded at the dam and at project stations. During 1964-71, annual rainfall ranged from about 17 to 29 inches (43 to 74 cm); the mean was about 22 inches (56 cm).
The quantity of dissolved solids in rainfall is negligible; therefore, increased rainfall normally causes dilution of ground and surface water. During periods of high runoff, calcium and bicarbonate are transported into and through the district from limestone areas to the north, and salts that have accumulated near the land surface throughout the drainage area during drier periods are flushed to the river. The quantity of accumulated salts carried from the district by storm runoff is virtually impossible to measure; therefore, no attempt has been made to describe a total salt balance for the irrigation district.
Data from previous investigations and from adjacent nonirrigated areas during this investigation indicate that the quantity and chemical quality of ground water beneath the district before irrigation varied within a relatively narrow range in response to variations in precipitation. Although the rate of application of irrigation water generally varied inversely with precipitation, the introduction into the system of large volumes of water containing significant quantities of dissolved solids caused progressive changes in the quantity and quality of the ground water that were distinct from those caused by variations in rainfall.
The quantity and rate of application of irrigation water affects the quality of related water supplies as well as the crop response in the irrigated area. For example, a rising water table, local waterlogging of soils, or acceleration of ground-water discharge may indicate excessive applications of water. Reduction of diversions would save water and reduce the volume of potentially pollutant return flow. Conversely, reduction of the quantity of applied water could cause undesirable diminution of sustained low flow in the river, degradation of ground-water quality, increased salinity in stock ponds and other impoundments receiving drainage from the district, and an unfavorable salt balance in the soil.
The acreage under irrigation expanded from 2,226 acres (900 ha) in 1963 to 5,897 acres (2,400 ha) in 1971. Diversions to the irrigation canal ranged from 7,146 acre-feet (8.8 hm3) in 1963 to 17,725 acre-feet (21.9 hm3) in 1968 (fig. 4 and table 2). The net input (N) of water delivered to the district is equal to the total diversion from the reservoir (D) minus waste (W) (fig. 2). Waste is water discharged from the end of the canals and laterals to maintain flow throughout the system. Presumably, the waste returns to the river virtually undiminished in quantity and unchanged in quality, but a small part is consumed by plants in the natural wasteways.
Figure 4--Acres irrigated, canal diversions, and average distribution of water per irrigated acre, 1963-71.
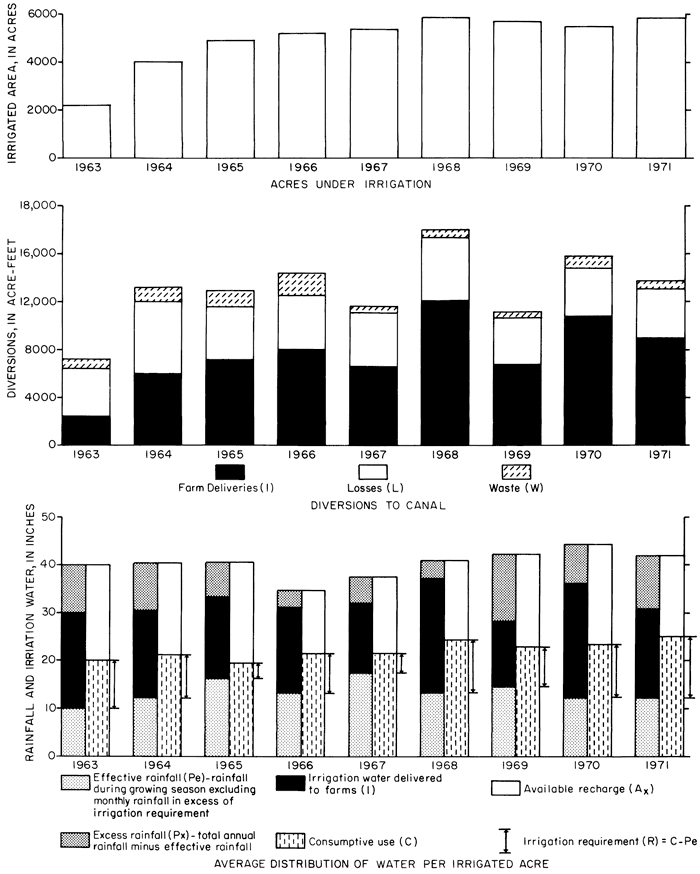
Table 2--Distribution and consumption of water in and adjacent to Cedar Bluff Irrigation District, 1963-71.
| Symbol | Identification | Units | Calendar year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1963 | 1964 | 1965 | 1966 | 1967 | 1568 | 1969 | 1970 | 1971 | |||
| Acres irrigated1 | acres | 2,226 | 4,017 | 4,910 | 5,314 | 5,417 | 5,795 | 5,742 | 5,423 | 5,897 | |
| D | Diversion to canal | acre-feet | 7,146 | 12,821 | 12,700 | 14,273 | 11,562 | 17,725 | 11,016 | 15,796 | 13,686 |
| W | Waste2 | acre-feet | 633 | 964 | 1,191 | 1,800 | 592 | 629 | 525 | 748 | 507 |
| N | Net input (D-W) | acre-feet | 6,513 | 11,857 | 11,509 | 12,473 | 10,970 | 17,096 | 10,491 | 15,048 | 13,179 |
| L | Losses' | acre-feet | 4,038 | 5,712 | 4,554 | 4,564 | 4,424 | 5,420 | 3,969 | 4,281 | 4,244 |
| I | Deliveries to farms | acre-feet | 2,475 | 6,145 | 6,955 | 7,909 | 6,546 | 11,676 | 6,522 | 10,767 | 8,935 |
| Pa | Annual rainfall on irrigated area3 |
inches | 22.5 | 22.2 | 23.8 | 17.0 | 23.4 | 16.8 | 28.5 | 20.6 | 23.9 |
| acre-feet | 4,174 | 7,431 | 9,723 | 7,536 | 10,572 | 8,127 | 13,656 | 9,309 | 11,745 | ||
| Pe | Effective rainfall on irrigated area4 |
inches | 12.2 | 16.6 | 13.2 | 17.7 | 13.2 | 14.7 | 12.6 | 12.9 | |
| acre-feet | 4,095 | 6,786 | 5,836 | 7,972 | 6,368 | 7,057 | 5,666 | 6,321 | |||
| C | Consumptive use in irrigated area |
inches | 21.1 | 19.5 | 21.7 | 21.5 | 24.8 | 23.0 | 23.6 | 25.4 | |
| acre-feet | 7,062 | 7,963 | 9,614 | 9,692 | 11,997 | 11,019 | 10,655 | 12,490 | |||
| R | Irrigation requirement, R = C - Pe |
inches | 8.9 | 2.9 | 8.5 | 3.8 | 11.6 | 8.3 | 11.0 | 12.6 | |
| acre-feet | 2,967 | 1,177 | 3,778 | 1,720 | 5,629 | 3,962 | 4,989 | 6,169 | |||
| F | Irrigation efficiency, F = R/D x 100 |
percent | 23.1 | 9.3 | 26.5 | 14.9 | 31.8 | 36.0 | 36.1 | 45.1 | |
| Xn | Excess input, Xn = N - R | acre-feet | 8,890 | 10,332 | 8,695 | 9,250 | 11,467 | 6,529 | 10,059 | 7,010 | |
| Xi | Excess farm deliveries, Xi = I - R |
acre-feet | 3,178 | 5,778 | 4,131 | 4,826 | 6,047 | 2,560 | 5,778 | 2,766 | |
| Ax | Excess water from irrigated land,5Ax = Pa + I - C |
acre-feet | 6,514 | 8,715 | 5,831 | 7,426 | 7,806 | 9,159 | 9,421 | 8,190 | |
| CR | Estimated concentration ratio, CR = N/Xn |
1.33 | 1.11 | 1.43 | 1.19 | 1.49 | 1.61 | 1.50 | 1.88 | ||
| En | Predicted specific conductance of annual excess input, En= EiN/Xn, Ei, specific conductance of irrigation water |
micromhos per centimeter at 25°C |
1,040 | 905 | 1,150 | 971 | 1,280 | 1,420 | 1,360 | 1,850 | |
| Ex | Predicted specific conductance of cumulative excess input, Ex = ΣEiNn/ΣXn |
micromhos per centimeter at 25°C |
1,040 | 966 | 1,020 | 1,010 | 1,080 | 1,120 | 1,150 | 1,220 | |
| 1Monthly water distribution records, U.S. Bureau of Reclamation (written commun.). Acres X 0.4047 equal hectares. 2 Canal and lateral. 3 1966-69, average of rain gages; 1963-65, 1970, 1.3 X rainfall at Cedar Bluff Dam; 1971, average of rainfall at dam and rain gage No. 5. Inches X 2.54 equals centimeters. 4 The part of monthly rainfall during growing season that was equal to or less than consumptive use for each crop. 5 Excess rainfall (Pa - Pe) + excess irrigation water (I-R) = Pa + I - C. |
|||||||||||
Net input (N) consists of deliveries to farms (1) and losses (L) from the canal and laterals by seepage and evaporation. Evaporative losses from the canal appear to be negligible. Evaporation and transpiration of part of the irrigation water leaves the remainder more highly concentrated. Only slight changes were observed in the specific conductance and concentrations of the ions along the canal during hot weather of the irrigation season; therefore, losses are attributed mainly to seepage.
To estimate the quantity of excess water available for recharge, the average consumptive use (C) of water in the irrigated area was calculated by a slight modification of the method of Blaney and Criddle (1950). The method is based on the tested premise that the consumptive use of water by any crop is determined by temperature, available water, length of the growing season, and of daylight. Monthly consumptive use of water by each crop can be expressed as
U = K f
where U is the monthly consumptive use; K is an experimentally determined coefficient for each crop based on tank and plot studies, and f is the product of the monthly mean temperature and the percent daylight hours that occur in that month. Acreage devoted to each crop was obtained from the annual crop summaries issued by the U.S. Bureau of Reclamation; monthly mean temperatures and date of the first and last frost were obtained from records of the National Weather Service for Hays; the percent daylight hours, definition of the growing season, and crop coefficients applicable to Kansas were taken from Hanson and Meyer (1953). The average consumptive use for the irrigated acreage is shown in table 2 and in figure 4.
The irrigation requirement (R) is defined as consumptive use (C) minus the effective rainfall. The effective rainfall for each year is the cumulative monthly effective rainfall for all crops during the growing season. As used in table 2, the monthly effective rainfall for any crop was the rainfall equal to or less than the consumptive use. If all rainfall during the growing season had been included as effective precipitation, the irrigation requirement would have been negative for several months when heavy rainfall during one or two days exceeded the monthly consumptive use. Calculations are based on the assumption that adequate irrigation water was applied to ensure optimum growth during the growing season; therefore, most of the heavy rainfall would run off the moist soil. Observations during and after heavy convective storms support this assumption, although some of the runoff from the irrigated land undoubtedly infiltrated the soil in adjacent nonirrigated land.
Farm deliveries (1) exceeded the irrigation requirements (R) during 1963-71; therefore, adequate water evidently was available for leaching and recharge in the irrigated area during the growing season. Available recharge from the irrigated land probably is more nearly equal to the excess rainfall for the entire year plus the excess farm deliveries. The excess water from irrigated land (Ax) that is available for recharge is calculated in table 2 from annual rainfall on irrigated land (Pa) plus farm deliveries (1) minus consumptive use (C).
If losses from the canal and laterals (L) represent seepage to the ground-water reservoir, the quantity of water available for recharge as a result of all deliveries of water to the irrigation district would be equal to the net input (N) minus the irrigation requirement (R). The difference is defined as excess input (Xn) in table 2. Thus, concentration of the ions in the infiltrated water depends largely upon the chemical quality of the irrigation water.
The concentrations of dissolved solids in the irrigation water from Cedar Bluff Canal generally increased progressively from year to year (table 3). The specific conductance, in micromhos per centimeter at 25°C, increased from about 800 in 1964 to 980 in 1971. For the purposes of this report, waters are described in terms of the most abundant anion and cation, in me/l (milliequivalents per liter). The percent composition of the calcium-sulfate type water remained relatively constant except for a progressive increase in sulfate and a concomitant decrease in bicarbonate. Bicarbonate and sulfate comprised about 93 percent of the anions. Sulfate and dissolved solids were higher than maximum concentrations recommended for drinking water by the Kansas State Board of Health (1973). Low concentrations of nitrogen and phosphorus indicate that eutrophication of the reservoir is not an immediate problem although the water has a distinct greenish cast during the late summer. The paucity of these nutrients may be responsible for a less than desirable rate of propagation and development of some game fish in the reservoir.
Table 3--Average composition of irrigation water from the Cedar Bluff Canal, 1963-71.
| Year | Number of samples |
Dissolved Silica (SiO2) |
Dissolved Calcium (Ca) |
Dissolved Magnesium (Mg) |
Dissolved Sodium (Na) |
Dissolved Potassium (K) |
Bicarbonate (HCO3) |
Dissolved Sulfate (SO4) |
Dissolved Chloride (Cl) |
Dissolved Fluoride (F) |
Dissolved Nitrate (NO3) |
Dissolved Phosphate (PO4) |
Dissolved Boron (B) |
Dissolved solids residue at 180°C |
Hardness (Ca, Mg) |
Sodium adsorption ratio (SAR) |
Specific conductance ratio (micromhos per cm at 25°C) |
pH (units) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, in milligrams per liter | ||||||||||||||||||
| 1963 | *5 | 11.0 | 103 | 22 | 30 | 14 | 163 | 258 | 17 | 0.6 | 0.6 | 0.14 | 0.17 | 546 | 345 | 0.7 | 802 | 7.7 |
| 1964 | 1 | 102 | 17 | 28 | 14 | 146 | 230 | 18 | .6 | .6 | 538 | 324 | .7 | 780 | 7.5 | |||
| 1965 | *5 | 5.3 | 103 | 19 | 30 | 16 | 148 | 266 | 19 | .6 | .25 | 541 | 336 | .7 | 815 | 7.5 | ||
| 1966 | 8 | 8.6 | 107 | 22 | 31 | 16 | 148 | 275 | 20 | .6 | 1.8 | .13 | .17 | 543 | 355 | .7 | 806 | 7.8 |
| 1967 | 6 | 4.1 | 110 | 22 | 35 | 16 | 141 | 296 | 20 | .7 | .8 | .02 | .33 | 587 | 363 | .9 | 823 | 7.7 |
| 1968 | 1 | 4.7 | 105 | 29 | 33 | 18 | 132 | 318 | 20 | .8 | 2.0 | .10 | .19 | 604 | 381 | .7 | 860 | 7.4 |
| 1969 | 5 | 4.8 | 112 | 27 | 35 | 18 | 131 | 328 | 22 | .8 | .3 | .04 | .17 | 630 | 390 | .8 | 886 | 7.8 |
| 1970 | 9 | 5.2 | 120 | 24 | 38 | 18 | 135 | 336 | 22 | .7 | .6 | .10 | .14 | 647 | 398 | .8 | 907 | 7.6 |
| 1971 | 4 | 6.7 | 129 | 24 | 39 | 19 | 124 | 374 | 26 | .7 | 1.1 | <.1 | .18 | 694 | 421 | .8 | 983 | 7.8 |
| Concentration, in milliequivalents per liter | ||||||||||||||||||
| 1963 | 5.12 | 1.78 | 1.30 | 0.37 | 2.67 | 5.37 | 0.49 | 0.04 | 0.01 | |||||||||
| 1964 | 5.09 | 1.39 | 1.20 | .41 | 2.40 | 4.79 | .51 | .03 | .01 | |||||||||
| 1965 | 5.18 | 1.58 | 1.31 | .41 | 2.43 | 5.53 | .54 | .03 | ||||||||||
| 1966 | 5.32 | 1.77 | 1.33 | .40 | 2.42 | 5.72 | .55 | .03 | .03 | |||||||||
| 1967 | 5.50 | 1.81 | 1.52 | .41 | 2.31 | 6.25 | .56 | .04 | .01 | |||||||||
| 1968 | 5.24 | 2.38 | 1.44 | .46 | 2.16 | 6.62 | .56 | .04 | .03 | |||||||||
| 1969 | 5.57 | 2.22 | 1.53 | .46 | 2.15 | 6.83 | .62 | .04 | .01 | |||||||||
| 1970 | 6.00 | 1.98 | 1.66 | .47 | 2.22 | 6.98 | .63 | .04 | .01 | |||||||||
| 1971 | 6.40 | 1.97 | 1.70 | .49 | 2.03 | 7.79 | .73 | .03 | .02 | |||||||||
| Percent total anions and cations | ||||||||||||||||||
| 1963 | 59.7 | 20.9 | 15.1 | 4.3 | 31.1 | 62.6 | 5.3 | 0.5 | 0.1 | |||||||||
| 1964 | 62.9 | 17.2 | 14.8 | 5.1 | 31.1 | 62.1 | 6.6 | .4 | ||||||||||
| 1965 | 61.1 | 18.6 | 15.4 | 4.8 | 28.5 | 64.8 | 6.3 | .4 | .3 | |||||||||
| 1966 | 60.4 | 19.9 | 15.1 | 4.5 | 27.7 | 65.4 | 6.3 | .3 | .3 | |||||||||
| 1967 | 59.5 | 19.6 | 16.4 | 4.6 | 25.5 | 68.2 | 6.1 | .4 | .1 | |||||||||
| 1968 | 58.5 | 25.0 | 15.1 | 4.8 | 23.0 | 70.2 | 6.0 | .4 | .3 | |||||||||
| 1969 | 56.9 | 22.7 | 15.6 | 4.7 | 22.3 | 70.8 | 6.4 | .4 | .1 | |||||||||
| 1970 | 59.3 | 19.6 | 16.4 | 4.6 | 22.5 | 70.6 | 6.4 | .4 | .1 | |||||||||
| 1971 | 60.6 | 18.7 | 16.1 | 4.6 | 19.2 | 73.5 | 6.9 | .3 | .2 | |||||||||
| * Samples at the gage on the Smoky Hill River at Cedar Bluff Dam. | ||||||||||||||||||
According to the classification of the U.S. Department of Agriculture (1954, p. 81), which is based on the specific conductance and sodium-adsorption ratio of the irrigation water (table 3), the water has a low sodium (alkali) hazard and a medium to high salinity hazard for irrigation. According to another classification (Jacobs and Whitney, 1968, p. 17) based on specific conductance and percent soluble sodium, the sodium and salinity hazard for field crops in medium textured soil is low. A moderate amount of leaching is required to prevent accumulation of salt in the soil.
More than 100 observation wells with 1 1/4- or 2-inch (3.2- to 5.1-cm) diameter casings were installed in and adjacent to the irrigation district by the U.S. Bureau of Reclamation, the Kansas Department of Health and Environment, the Kansas Geological Survey, and the U.S. Geological Survey from 1947 to 1971. Water levels were measured and water samples for chemical analysis were collected periodically at selected wells.
Pertinent data for each well, depths to water below land surface datum, and results of chemical analyses of water samples collected from 1964 to 1971 are included in a separate basic data report by Leonard and Stoltenberg (1972). Only the most significant information is portrayed herein.
As shown in plate 1, the water table generally sloped gently toward the south and east and was interrupted by erosional remnants of relatively impermeable bedrock. Maximum and minimum recorded depths to water between 1964 to 1971 show that the water level in most of the wells in and adjacent to the irrigated land fluctuated within a range of from 4 to 10 feet (1.2 to 3.0 m), but generally remained more than 20 feet (6.1 m) below the land surface under the high terrace. The lowest levels generally were recorded near the beginning of irrigation in 1964-65; the highest in 1969 or 1970. Some areas of seepage developed in topographically low areas along natural drains, and where the bedrock was near the surface between the irrigated acreage and the river. However, the water table remained well below the root zone under most of the irrigated land.
Hydrographs of selected observation wells in and adjacent to the district (fig. 5) illustrate the progressive rise of the water table in or down the groundwater gradient from the irrigated area in response to irrigation. Water levels generally were highest in the late summer and early fall as excess rainfall and irrigation water accumulated in the aquifers. As a result of drainage from the aquifer and the paucity of recharge during the winter, water levels normally declined to a minimum in early spring.
Figure 5--Fluctuations of water levels in selected observation wells, 1961-71.
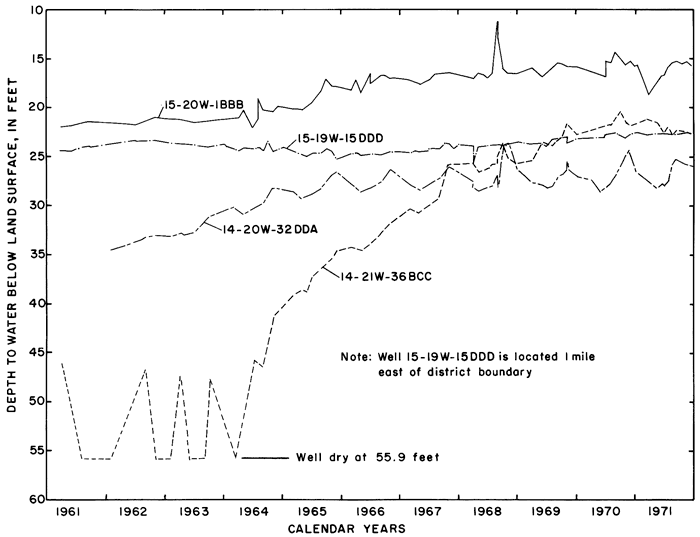
Although most of the wells were screened in lithologically similar sediments, the chemical quality of water differed widely from well to well and changed with time. The concentrations of the ions in successive samples from many of the wells remained constant only after about 1 gallon (3.8 l) for each 10 feet (3.0 m) of standing water was withdrawn and discarded. After 1967, a sampler designed specifically for use in the 1 1/4- or 2-inch (3.2- or 5.1-cm) diameter casings was used instead of conventional bailers. "Mean" or "typical" analyses were inadequate to describe the chemical quality or ground water in the area.
The rising water table in and adjacent to the irrigated land reflected infiltration of excess water. If all of the thousands of tons of dissolved solids delivered to the district in net input (N) were dissolved in the excess water remaining after evapotranspiration (Xn) (table 2), the concentration of a given ion in that water theoretically would be equal to N/Xn times the concentration of the ion in the irrigation water. For example, the concentration ratios (CR) shown in table 2 range from about 1.1 to 1.9. If Ei represents the specific conductance of the irrigation water, the corresponding values of the specific conductance of the excess water (Xn) would range from 905 micromhos per centimeter at 25°C in 1965 to 1848 micromhos per centimeter at 25°C in 1971. The estimated specific conductance (EiI/Xi) of soil water (Xi) in the irrigated fields would be higher.
If the specific conductance of the excess water were determined by mixing the excess water for each year with the excess from preceding years, the specific conductance of the cumulative mixture (Ex) would range from about 966 micromhos per centimeter at 25°C in 1965 to 1,220 micromhos per centimeter at 25°C in 1971. The latter alternative more closely approximates the natural situation and the results of analyses of ground water in the area.
Mixing with natural ground water, selective precipitation of the least soluble salts on the surface and in the soil profile, ion-exchange reactions, and leaching of residual salts from the soil profile can alter the ionic composition of the ground water from the predicted values. To relate variations in the composition of sampled waters to the irrigation water in parts of this report, the specific conductance and concentrations of the ions are expressed as "concentration ratios" to the specific conductance and concentrations of the corresponding ions in the irrigation water. Abnormally high or low ratios for individual ions reveal that processes other than dilution and evapotranspiration affected the composition of the water.
The locally variable chemical composition of natural waters in the area was caused chiefly by soluble minerals in the soil and rock. Calcium, derived chiefly from limestone, caliche, and gypsum is normally the predominant cation. Bicarbonate from limestone and sulfate from gypsum or the oxidation of pyrite in shale are predominant anions. Most of the sodium and chloride seems to have been derived from the soil, however, locally high concentrations of both ions are associated with oil-field or livestock operations. Water in the Dakota Formation, which underlies the area at depth, contains high concentrations of sodium and chloride ions. Although the water generally is confined by overlying shale, there are unconfirmed reports that a few abandoned wells in the Dakota flowed at the surface near the river. The plant nutrients, potassium, nitrogen, and phosphorus, are constituents of the rocks, particularly organic shale, as well as of fertilizers that are used extensively in the irrigated area.
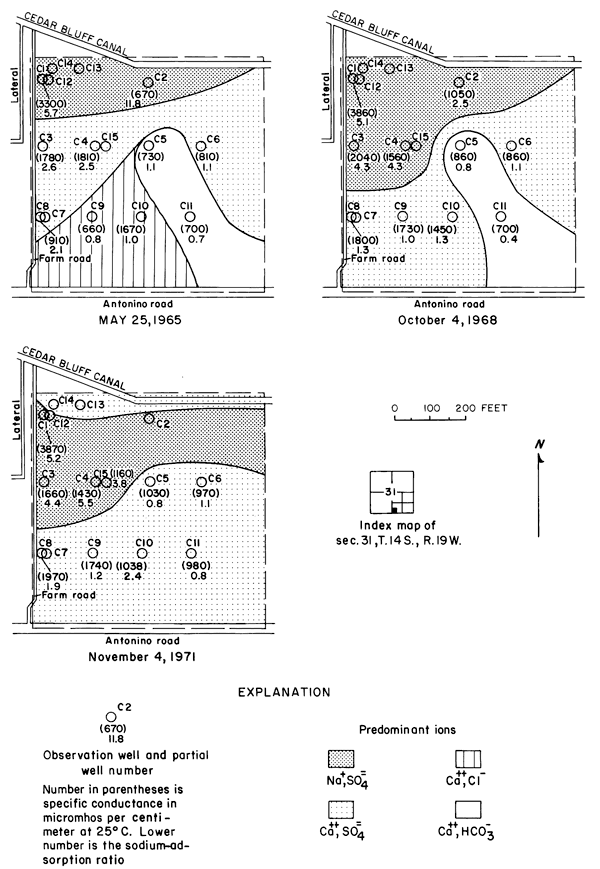
Between 1965 and 1971, the specific conductance in most of the well waters increased and the water type changed as shown in plate 3. In 1965, calcium-bicarbonate type water underlaid most of the eastern part of the area. Sodium-sulfate and sodium-bicarbonate types generally were associated with buried channels in the bedrock (pl. 2). By 1971, nearly all of the well waters were of the calcium-sulfate type, evidently as a result of infiltration of calcium-sulfate type irrigation water. Most of the changes in concentrations were progressive, but the rates of change were most pronounced during 1967-69.
The increases in specific conductance (proportional to the concentration of dissolved solids) in many of the well waters were larger than would be predicted from the estimated consumptive use of irrigation water, and the concentration ratios for the ions varied widely. Evidently, residual salts that had accumulated in the soils and aquifers under semiarid conditions before irrigation began bad been leached to the ground-water reservoir. However, the changes in the distribution of the ions from well to well and in the configuration of the water table as irrigation continued indicate that lateral movement of ground water had significantly affected the chemistry of the well waters. Lack of uniformity in the chemistry of the ground water is related to lack of homogeneity of the subsurface hydrogeologic and chemical environment.
The specific conductance of water is a measure of the total concentration of all of the ionic constituents, however, each of the constituents can behave differently in the hydrologic environment. In the Cedar Bluff area, changes in the distribution and concentration of the chloride ion are more readily related to changes in the hydrologic system than are the changes in the concentrations of the other ions or in the specific conductance.
Under the conditions prevailing in the study area the chloride ion is virtually unaffected by precipitation or ion exchange; therefore, it is a relatively effective tracer. Chloride comprised only about 7 percent of the anions in the irrigation water at a maximum concentration of about 26 mg/l (table 3). The concentrations of chloride in many well waters and in groundwater discharge from the district to tributaries, as shown in figure 6, far exceeded the concentration of chloride in the irrigation water and the concentrations that were predicted from consumptive use.
Figure 6--Map showing concentration of chloride in water from wells and tributaries, Fall 1971, and changes in the concentration of chloride from Fall 1965 to Fall 1971. [A larger version of this figure is available.]
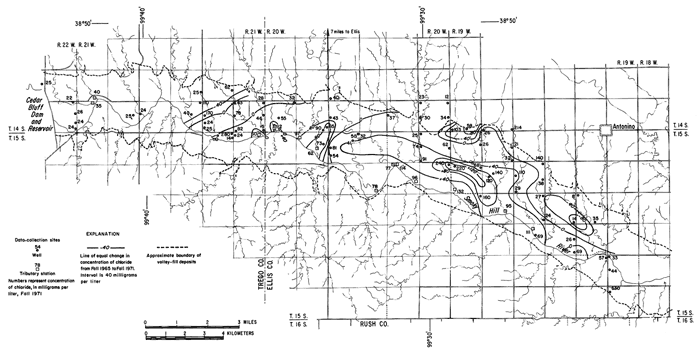
In general, the concentration of chloride increased under the irrigated land except where high concentrations prevailed in 1965. In the western part of the area, locally high concentrations are believed to have been associated with pollution of the shallow aquifers by oil-field brine. For example, a decrease of more than 200 mg/l in the domestic well serving the Cedar Bluff Irrigation District office (15-20W-5AD) in the central part of the area is attributed to dilution and displacement to the south of waters containing brine from improper oil-field operations in the past. Similar changes were noted elsewhere.
The wide distribution of chloride in transient storage suggests a more extensive source than the limited disposal of oil-field brine in the area. The greatest increase in concentration over an appreciable area was on, and north of, an elongated erosional remnant of bedrock that separates the water table under the high terrace from the water table in the alluvium along the river (pl. 2). In part of the area, high concentrations of chloride appear to have been caused by evapotranspiration of water from a shallow water table over the bedrock. The barrier is breached by tributaries that are sustained mainly by chloride-rich drainage from the irrigated land. The nature and configuration of the changes shown in plate 3 and in figure 6 seem to indicate that infiltrated water in excess of crop requirements leached chloride and other accumulated salts from the soil, then mixed with and partially displaced natural water down gradient. More detailed data from three experimental plots support this hypothesis.
In 1964, nine or more observation wells with 2-inch (5.1-cm) galvanized steel casing were installed in each of three experimental plots (pl. 1) that are underlain by differing types and thicknesses of unconsolidated sediment. Brass well-point screens in most of the wells are set near the undulating base of the saturated section at depths ranging from 20 to 70 feet (6 to 21 m). Additional wells were installed in 1967 and 1971 to test for vertical stratification of the ions in the groundwater reservoir. To minimize interference with farming operations the wells were located along the edges of the fields and on berms separating graded benches. Despite the inconvenience, the owners, Mr. Vernon Moore (plot 1) and Mr. Philip Nicholson (plots 2 and 3) and their renters graciously made their fields available and contributed valuable assistance and information.
Plot 1 includes about 10 acres (4.0 ha) of a larger field in the northeastern part of the district, south of and adjacent to the irrigation canal near the upper boundary of the high terrace. Plot 3, about 40 acres 16.2 ha) in area, is about 1 1/2 miles (2.4 km) to the south, down the regional ground-water gradient. Both are located near and east of geologic section C-C' in plate 2. Both were primarily cornfields during the study, although a minor amount of grain sorghum was planted in each during some years. Irrigation water was transferred by siphon to the individual furrows from lateral ditches along the western boundaries. Plot 2 is located near the western edge of the district on a low alluvial terrace near the river. Alfalfa was grown in plot 2 until 1970; grain sorghum was planted in 1971.
Inflow to the fields consists mainly of applied irrigation water and direct precipitation on the land surface, but some ground-water inflow probably migrated laterally from adjacent land. Rainfall was recorded daily for varying periods at stations near the fields. The distribution of water during 1966-71 is summarized in table 4. Annual applications of irrigation water exceeded the irrigation requirement for all three fields.
Table 4--Distribution of rainfall and applied water on experimental plots, 1966-71.
| Water | Plot | 1965 | 1966 | 1967 | 1968 | 1969 | 1970 | 1971 |
|---|---|---|---|---|---|---|---|---|
| Annual rainfall (Pa), in inches1 | 1,3 | 23.8 | 19.4 | 24.3 | 18.6 | 29.3 | 20.6 | 24.6 |
| 2 | 23.82 | 14.4 | 20.8 | 14.8 | 20.0 | 15 .92 | 23 .02 | |
| Effective rainfall (Pe), in inches3 | 1,3 | 16.1 | 15.6 | 21.3 | 14.5 | 20.8 | 11.1 | 14.0 |
| 2 | 18.0 | 11.4 | 17.6 | 12.1 | 16.4 | 12.8 | 11.5 | |
| Consumptive use (C), in inches: corn |
1,3 | 22.1 | 25.3 | 23.4 | 26.3 | 24.4 | 25.2 | 26.7 |
| Consumptive use (C), in inches: alfalfa (1965-70), sorghum (1971) |
2 | 28.0 | 29.1 | 28.5 | 33.1 | 31.9 | 32.3 | 22.8 |
| Irrigation requirement, in inches (R = C - Pe) |
1,3 | 6.0 | 9.7 | 2.1 | 11.8 | 3.6 | 14.1 | 12.7 |
| 2 | 10.0 | 17.7 | 10.9 | 21.0 | 15.5 | 19.5 | 11.3 | |
| Farm deliveries (I), in inches4 | 1 | 18.8 | 13.8 | 4.3 | 21.6 | 8.6 | 16.1 | 14.0 |
| 3 | 24.1 | 16.0 | 12.5 | 17.8 | 13.8 | 19.5 | 15.8 | |
| 2 | 17.4 | 28.7 | 24.5 | 38.5 | 20.6 | 28.0 | 28.0 | |
| Excess farm deliveries (Xi = I - R), in inches |
1 | 12.8 | 4.1 | 2.2 | 9.8 | 5.0 | 2.9 | 1.3 |
| 3 | 18.1 | 6.3 | 10.4 | 6.0 | 10.2 | 5.4 | 3.1 | |
| 2 | 7.4 | 19.0 | 13.6 | 17.5 | 5.1 | 8.5 | 16.7 | |
| Seasonal concentration ratio (CRi -- I/Xi) |
1 | 1.5 | 3.4 | 1.9 | 2.2 | 1.7 | 8.1 | 10.8 |
| 3 | 1.3 | 2.6 | 1.2 | 3.0 | 1.4 | 3.6 | 5.1 | |
| 2 | 2.4 | 1.5 | 1.8 | 2.2 | 4.1 | 3.3 | 5.5 | |
| Annual concentration ratio [CRa -- 1/(Pa + I - C)] |
1 | 0.9 | 1.8 | 0.8 | 1.6 | 0.6 | 1.4 | 1.2 |
| 3 | .9 | 1.6 | .9 | 1.8 | .7 | 1.3 | 1.2 | |
| 2 | 1.3 | 2.0 | 1.5 | 1.9 | 2.4 | 2.4 | 1.0 | |
| 1 Inches X 2.54 equal centimeters. 2 Based on rainfall at Cedar Bluff Dam. 3 Based on rainfall during growing season for each crop in experimental plots. 4 Written communications, R. Schamel, Manager, Cedar Bluff Irrigation District. |
||||||||
Samples were collected for chemical analysis more frequently in the experimental plots than in the remainder of the study area. Soil samples from the fields and from adjacent nonirrigated areas were analyzed to determine if the natural salt content of the soil in the irrigated areas has been sufficiently depleted by leaching to account for the observed changes in the irrigation water. Plots 1 and 3 on the high terrace typify most of the irrigated land in the district; therefore far more data were collected from them than from plot 2.
Unconsolidated fluvial deposits and colluvium overlain by loess increase in thickness from about 10 feet (3 m) at the northwestern corner of the plot to about 80 feet (24 m) near the southeastern corner (fig. 7). Drillers' and gamma-ray logs, as well as particle size analyses of cores and auger cuttings reveal wide vertical and lateral variation in lithology. Silt and clay predominate, but the proportion of sand and gravel in most of the wells increases with depth.
Figure 7--Configuration of bedrock surface and water table in plot 1.
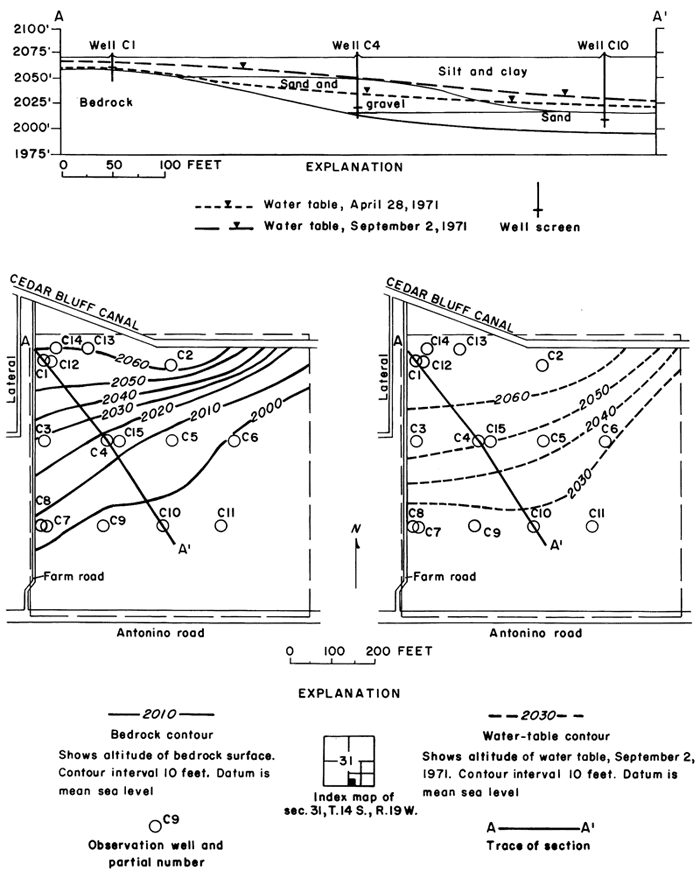
The sampled water entered the observation wells through brass screens set near the bedrock surface in discontinuous lenses of permeable sand and gravel that seem to be the main conduits for lateral migration of ground water. Many of the water samples were obtained by personnel of the Entomology Department of Kansas State University in conjunction with a study of pesticides in the soil (Knutsen and others, 1971). Results of that study show that the movement of chlorinated hydrocarbons was confined to the upper foot of soil.
Inflow to the field consisted mainly of applied irrigation water and direct precipitation on the land surface. Overland runoff from highland areas to the north is intercepted by the main irrigation canal that forms the northern boundary of the field. Seepage from the canal and from a lateral west of the field seemed to have a negligible effect on the configuration of the water table, which rose rapidly in response to infiltration of excess water applied during the irrigation season (fig. 8).
Figure 8--Fluctuations of water levels in observation wells in plot 1, May 1965 to November 1971.
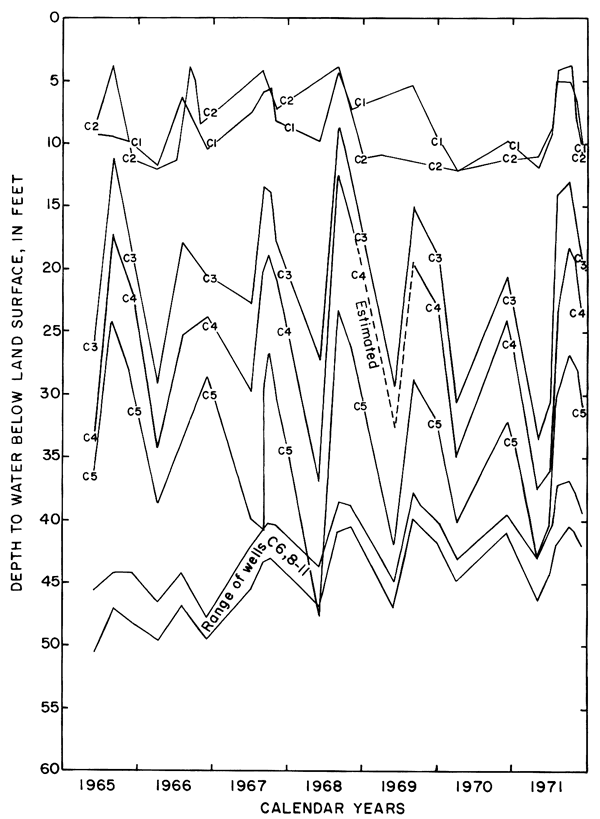
Mainly as a result of the inhomogeneity of the aquifer and the wide variation in depth of well screens, changes in water levels in the wells were neither simultaneous nor of equal magnitude. The southeasterly slope of the water table was maintained during the irrigation season, but water levels fluctuated over a wider range in the central wells than elsewhere. Excess water from irrigation evidently infiltrated the sand and gravel penetrated by wells C3, C4, and C5 more rapidly than the less permeable fine-grained parts of the aquifer penetrated by the other wells. The fluctuations in wells C6, and C8 to C11 show a more subdued seasonal response and a general rise as water moved down gradient under the extensive irrigated area to the south.
Although the wells in plot 1 were only about 200 feet (61 m) apart, the chemical quality of waters differed from well to well. The variations and fluctuations with time, as shown in figure 9, indicate that calcium-sulfate type irrigation water mixed with and displaced other types of ground water to the south and east down gradient. In August 1968, 70 cubic centimeters of 40 percent Rhodamine BA dye were injected to a depth of 18 inches (46 cm) in the soil 10 feet (3.0 m) north of well C1. The first appearance of dye in wells C3, C4, and C5 one year later, and in wells C10 and C11 four months after that confirmed the direction of movement of the infiltrated water.
Figure 9--Specific conductance, sodium-adsorption ratio, and predominant ions in water from wells in plot 1.
In May 1965, sodium was the predominant cation in waters from the relatively shallow wells C1 and C2 in the northwestern part of the field. Sodium evidently had accumulated in the fine-grained sediments penetrated by the two wells where the water table lay near the surface before irrigation began. Salts of sodium are relatively soluble but sodium ions are readily adsorbed by some clay minerals. By 1968, significant quantities of sodium had been leached and displaced by infiltrated water into the permeable sand and gravel penetrated by wells C3 and C4. The persistence of sodium in wells C1 to C4 in 1971 suggests that abundant calcium in the percolating water continued to displace sodium from the fine-grained sediments by ion exchange.
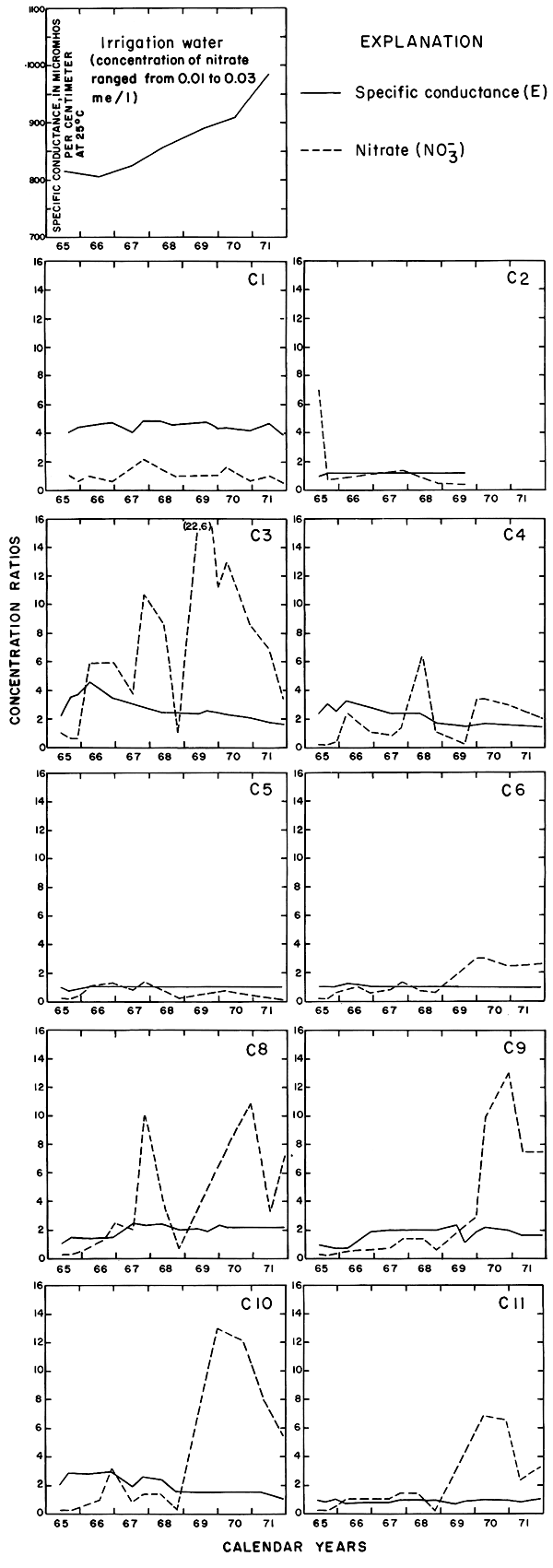
Progressive changes in the specific conductance and concentrations of the major ions in the well waters are shown in terms of concentration ratios in figures 10, 11, and 12. The graphs representing each well are located in the illustrations in the same relative positions as are the wells in the experimental plots. Theoretically the concentration ratios should approach the ratios used for predicting the specific conductance of soil water and recharge (tables 3 and 4) after accumulated residual salts are flushed from the soil by excess water from irrigation. Figure 12 shows that the ratios for the specific conductance of most of the well waters remained relatively constant or decreased to values between one and two after an initial rise during the first 2 years of irrigation. The ratios for most of the ions seem to be approaching that range.
Figure 10--Concentration ratios for cations in water from wells in plot 1, 1965-71.
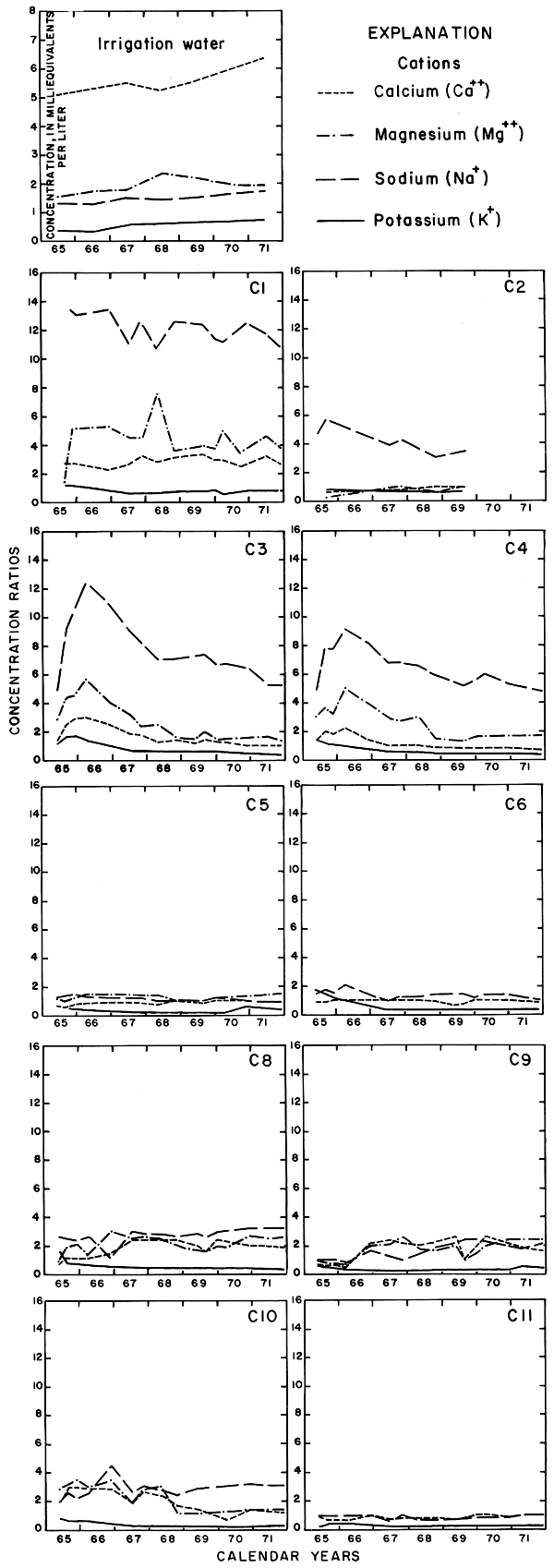
Figure 11--Concentration ratios for selected anions in water from wells in plot 1, 1965-71.
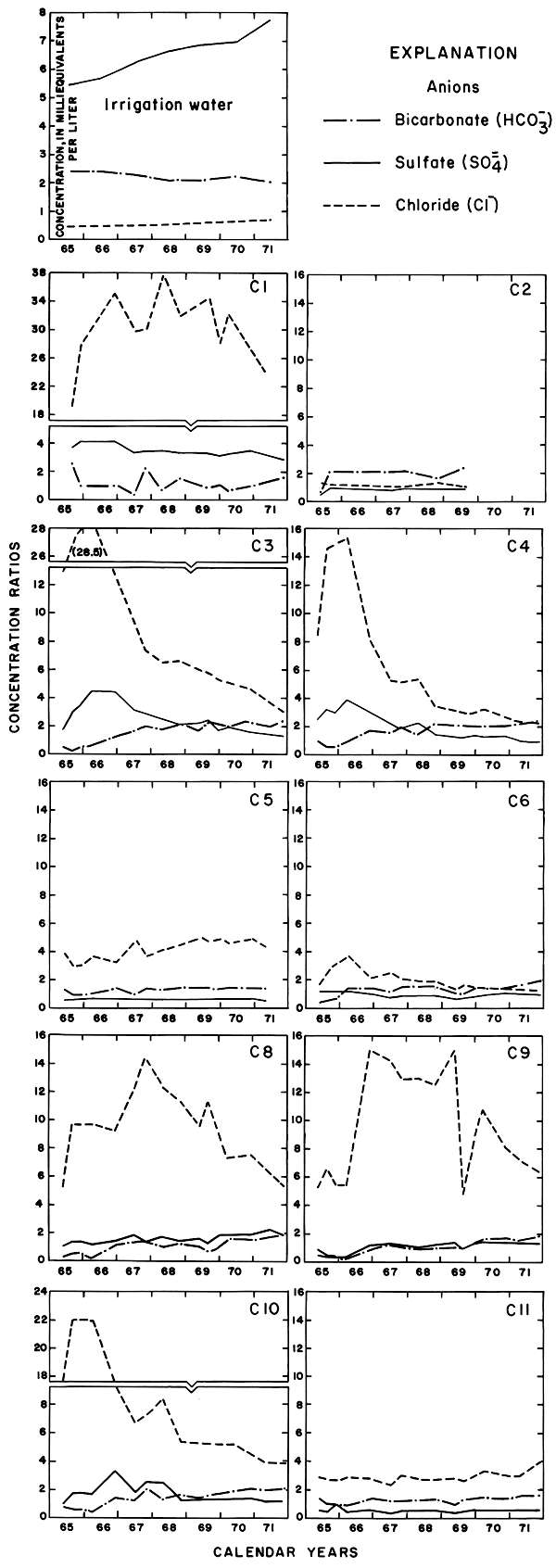
Figure 12--Concentration ratios for the specific conductance and nitrate in water from wells in plot 1, 1965-71.
For most wells, the configurations of the curves for calcium, sulfate, and specific conductance were somewhat similar. Convergence of the curves indicates infiltration of excess water from irrigation, but the concentration ratios for calcium and sulfate were generally equal to or less than the ratios for the other constituents, which were evidently derived from another source. In 1971, for example, the ratio for sulfate in most of the wells was equal to or less than the ratio for specific conductance, but the ratio for bicarbonate increased to two or more. In part, the changes reflect the inverse effect on the ratios of the progressive increase in sulfate at the expense of bicarbonate in the irrigation water. However, the changes are mainly dependent on the availability to percolating water of bicarbonate from caliche and fragments of limestone in the soil and aquifers.
Sodium and chloride were present in water from most wells at higher concentrations than in the irrigation water before irrigation began, but the ratios increased rapidly during the first two or three years of irrigation when the salts leached from the soil reached the aquifer. In 1966, the concentration ratios exceeded 10 for sodium and 30 for chloride. As the supply of salt that accumulated before irrigation began was depleted, progressive dilution in some waters accompanied displacement of the mixture down the groundwater gradient. Greater fluctuations in the concentrations of chloride than of sodium in water from the deeper wells probably represent greater mobility of the anion in the aquifer.
The curves for nitrate and potassium, both plant nutrients and components of fertilizer, differ markedly from the other curves. Small variations in the concentrations of nitrate in the well waters caused wide variations in the ratios because the concentration of nitrate in the irrigation water was less than 2 mg/l. The general increase in the concentration of nitrate in 1969 probably reflects leaching of fertilizer nitrogen carried below the root zone by irrigation water during the preceding dry year. The concentration of potassium in the well waters remained uniformly lower than the concentration of about 18 mg/l in the irrigation water.
According to calculations based on changes in the saturated thickness and in concentrations of chloride in the wells, the average concentration of chloride in the ground water underlying the field decreased and the outflow of chloride exceeded the inflow. The chloride was evidently displaced laterally toward plot 3 and the river.
The subsurface geology of plot 3 is less complex than that of plot 1 (fig. 13) and the effects of irrigation alone are more readily discernible. The screened wells range in depth from about 14 to 60 feet (4 to 18 m) and the saturated zone lies entirely within relatively homogeneous sand and gravel. Although rainfall and crop use for the two plots were about the same, far more water was applied per acre to plot 3 than to plot 1 except during the dry 1968 season (table 4).
Figure 13--Configuration of bedrock surface and water table in plot 3.
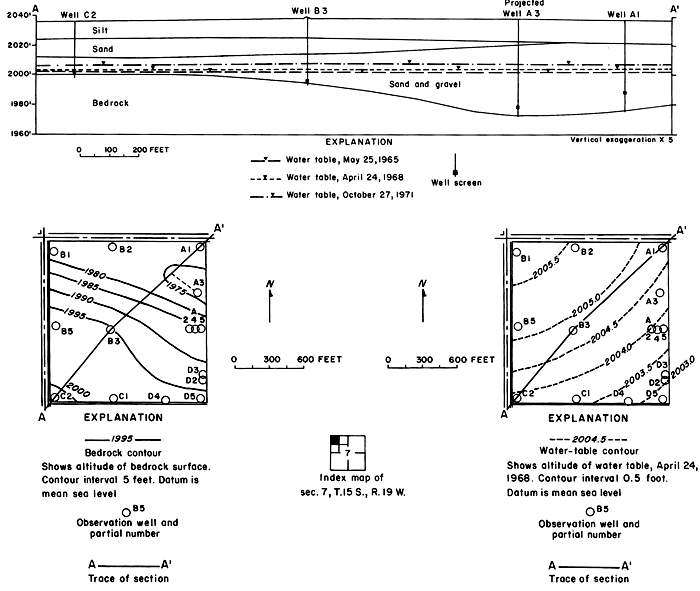
Since 1965, the water table beneath plot 3 has risen about 5 feet (2 m) (fig. 14); the minimum depth to water was 29 feet (9 m) in well A1, which was measured in September 1971. Most of the water-table rise occurred during the first 3 years of irrigation. The configuration of the water table, which normally slopes gently toward the southeast, changed in delayed response to the application of irrigation water. According to continuous records for 1971 from well B3 in the center of the field, the water level declined to about 32 feet (10 m) below land surface by June 5, 1971. Irrigation began on June 9, but the water level remained constant until June 28 when it began to rise. Irrigation of the field was discontinued on August 25, but the water level continued to rise until September 14 to 30.4 feet (9.3 m) after which it again began to decline. Perhaps, coincidentally, the start and finish of irrigation for that year preceded the response at the water table by 19 days.
Figure 14--Fluctuations of water levels in observation wells in plot 3, 1965-71.
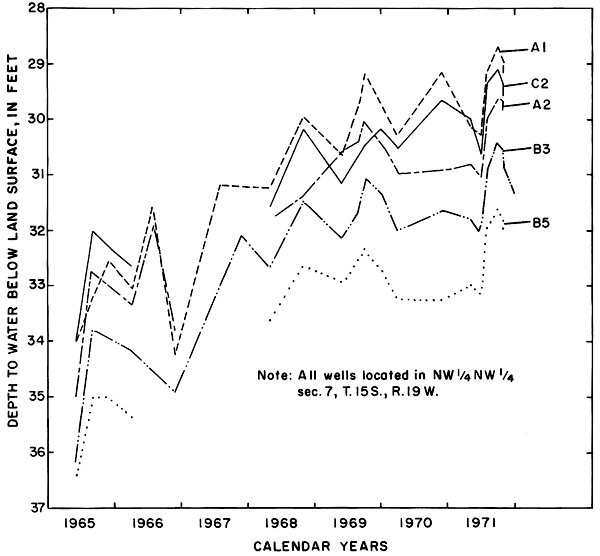
Figure 15--Specific conductance, sodium-adsorption ratio, and predominant ions in water from wells in plot 3.
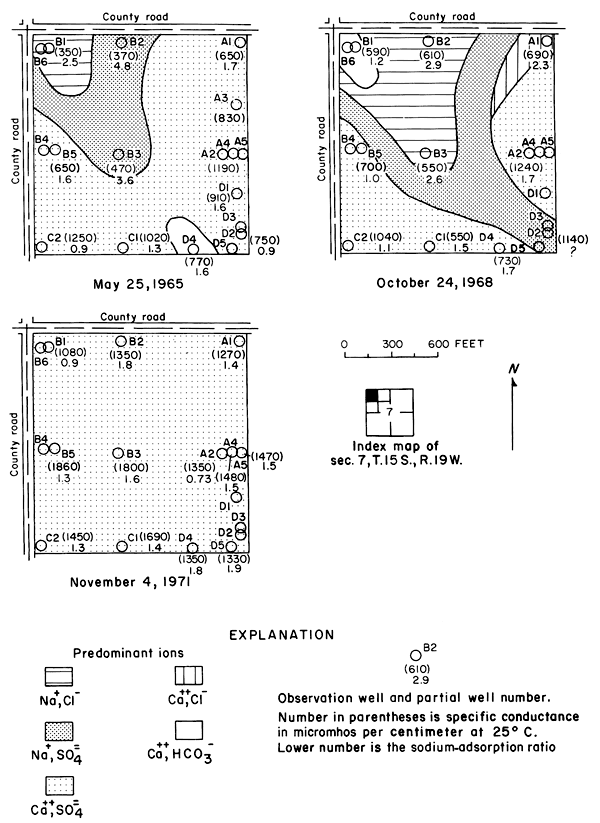
The progressive changes in water type, increases in specific conductance, and reduction of the sodium-adsorption ratios in the well water (fig. 15) reflect more uniform vertical and lateral movement of larger volumes of water through more homogeneous and permeable aquifers than in plot 1. Analyses of samples from closely spaced wells with screens set at different levels imply no significant vertical stratification of the major ions in the saturated zone. The apparent southeastward migration of the water and the trend toward conformity in the composition of the ground water with the calcium-sulfate type irrigation water indicate percolation of excess water to the aquifer and displacement of the altered ground water down gradient.
In virtually all the wells, calcium, expressed as percent of total cations, increased at the expense of sodium, while magnesium remained relatively constant at percentages lower than the irrigation water. Sulfate and chloride, expressed as percent of total anions, varied inversely, with the percent sulfate always less than in the irrigation water and the percent chloride greater. By 1969, all of the waters were of the calcium-sulfate type.
Following the moisture deficiency in 1968, when the predicted annual concentration ratio for specific conductance was 1.8 (table 4), the ratio for specific conductance of most of the well waters remained relatively constant between 1.5 and 2.0. Unlike the ratios in plot 1 (fig. 7), however, the ratios for the ions in plot 3 do not appear to converge toward a common value (figs. 16, 17, and 18). Instead, each of the ratios for the cations remained relatively constant, while the ratios for some of the anions varied more widely. Evidently, the salts in the well waters were not derived solely from salts accumulated in the soil or carried into the plot by the applied irrigation water. The plot is located over the south bank of an extensive buried channel in the Cretaceous bedrock and is down gradient from other irrigated areas (pls. 1 and 2); therefore, changes caused by irrigation in the plot probably are superimposed on changes caused by lateral migration of ground water.
Figure 16--Concentration ratios for cations in water from wells in plot 3, 1965-71.
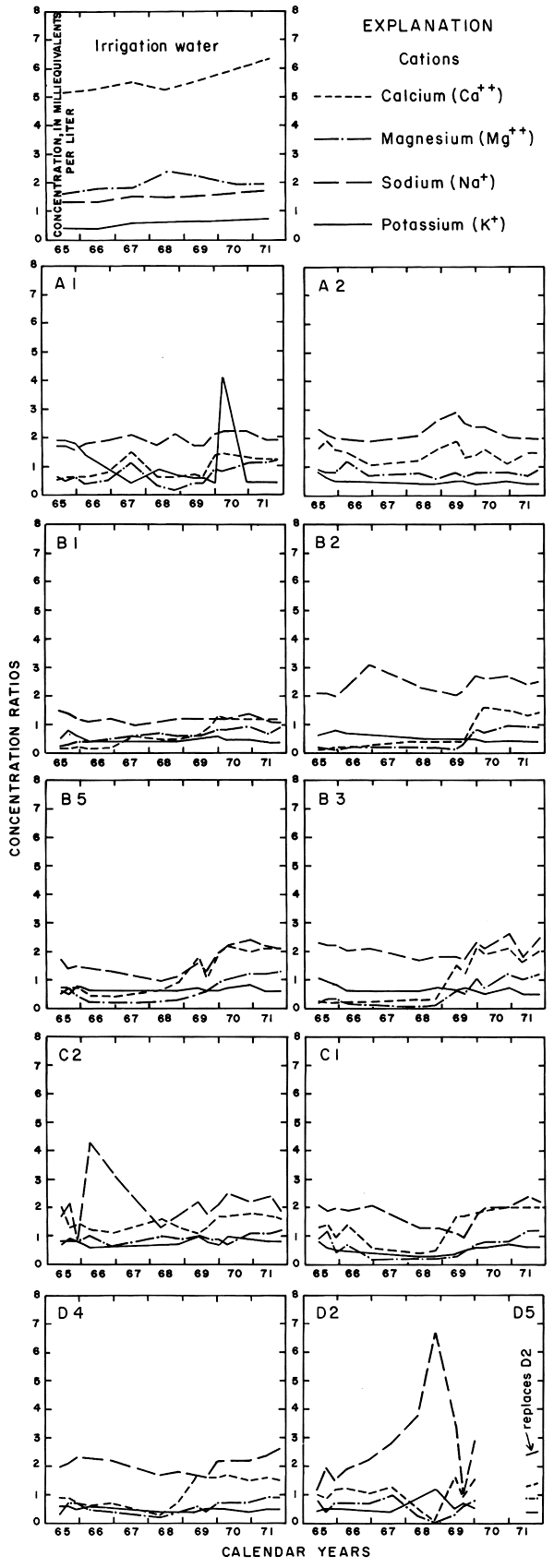
Figure 17--Concentration ratios for selected anions in water from wells in plot 3, 1965-71.
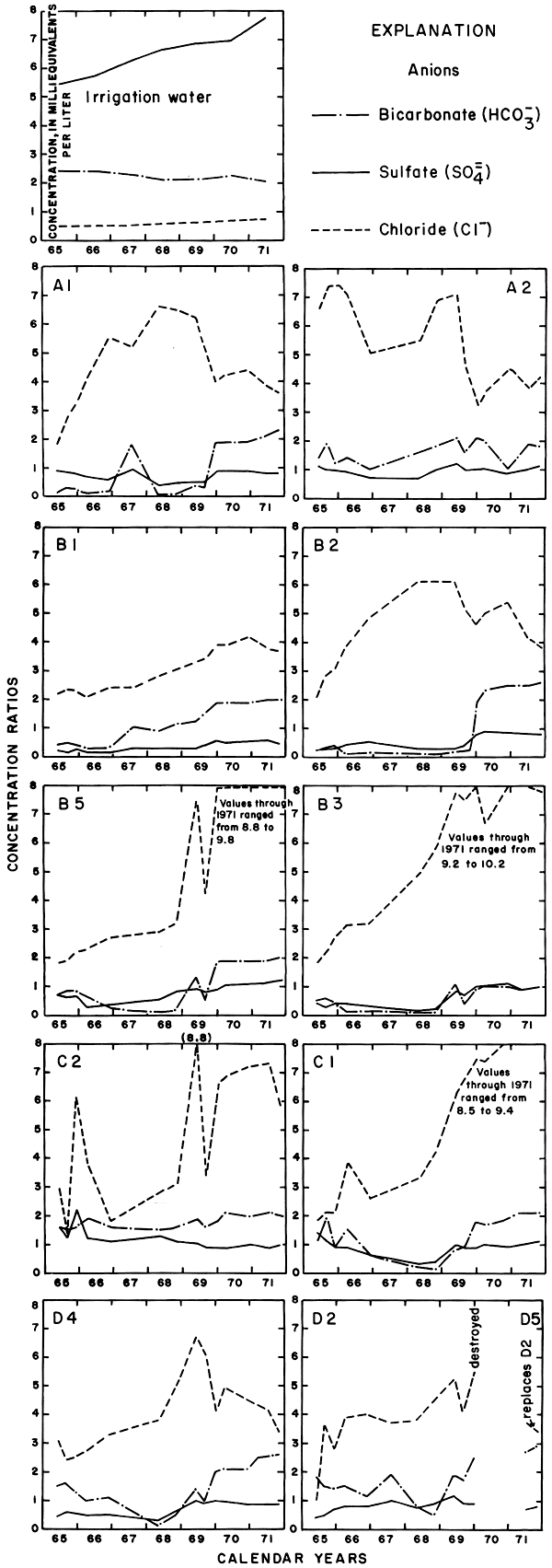
Figure 18--Concentration ratios for the specific conductance and nitrate in water from wells in plot 3, 1965-71.
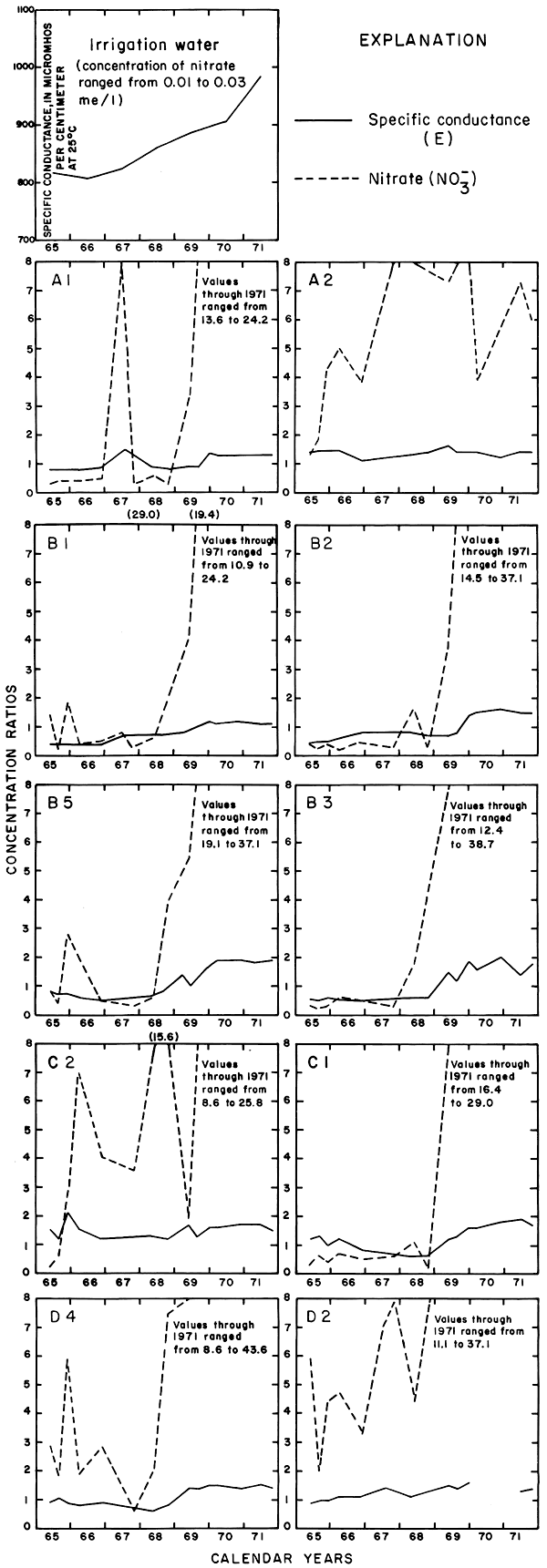
The arrival of large quantities of irrigation water in the aquifers evidently caused the concentrations of calcium and sulfate in most of the well waters to increase rapidly during 1968 and 1969, but concentration ratios less than one for sulfate indicate loss of sulfate to the soil, lateral inflow of ground water containing less sulfate than the irrigation water, or both. During the entire 1965-71 period, the ratio for sodium remained near two. After 1966 the ratio for chloride exceeded two in all samples and reached peaks of more than 10 (more than 260 mg/l) in some of the wells in 1969. The high ratios indicate a source other than the irrigation waters. The decline in the ratios after 1969 may represent depletion by leaching of accumulated salts in the soil. The highest concentrations of chloride were recorded in waters from wells in the southwest part of the plot where the water table is discontinuous on an erosional remnant of bedrock.
Variations in the concentrations of the nutrients, potassium and nitrate, were somewhat similar to those in plot 1. The ratio for potassium generally was less than one, but the ratios for nitrate in nearly all the wells increased rapidly in 1968 to values exceeding eight. Anhydrous ammonia used as fertilizer probably is the main source of the nitrate.
Plot 2 is on the flood plain of the Smoky Hill River and overlies a buried channel in the bedrock (pl. 2 and fig. 19). The lithologic characteristics of the alluvium differ vertically and laterally; therefore, the contact between clay, sandy clay, and sandy silt shown between wells D6 and D7 may be gradational rather than erosional.
Figure 19--Location of water table and configuration of the bedrock surface in plot 2.
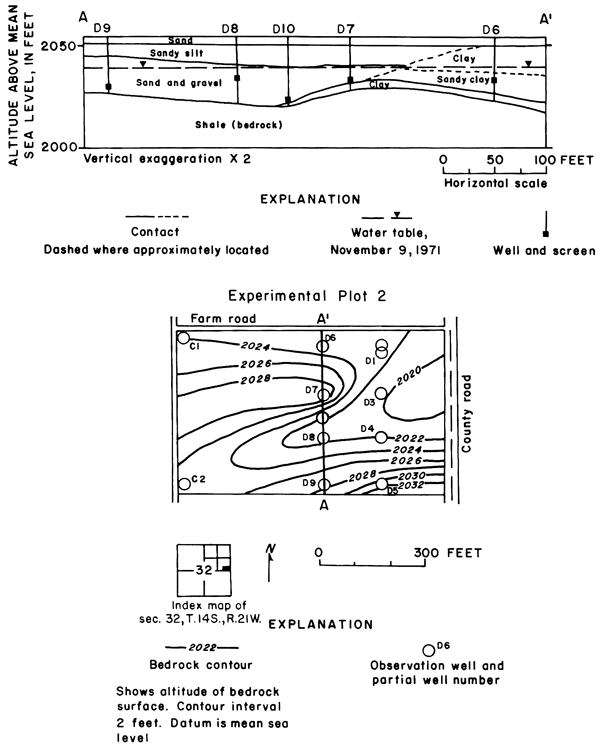
Far more water was applied to the alfalfa (1965-70) and grain sorghum (1971) in plot 2 than was applied to crops in plots 1 and 3 (table 4). Water readily infiltrated the sandy surface soil and was transpired by the deep-rooted alfalfa, but a large excess percolated to the underlying sand and gravel aquifer and moved laterally to the river, which is about 600 feet (180 m) from the southern boundary. Water levels fluctuated within a range of about 2 feet (0.6 m) at a depth of about 16 feet (4.9 m) below the irrigated field.
The main sources of ions in the well waters appear to be salts delivered in the irrigation water and the calcium and bicarbonate ions derived from fragments of limestone in the alluvium. Lesser amounts of salts have accumulated in the sandy soil at plot 2 than at the other sites owing to periodic flushing by river water during floods. Calcium and sulfate were the predominant ions in virtually all samples, although bicarbonate was the predominant anion in several samples containing calcium carbonate that bad precipitated in the well or sample bottle. The concentration ratios for specific conductance generally ranged between 1.2 and 1.6 (figs. 20, 21, and 22), which is lower than was predicted from the consumptive use (table 4). The limited quantity of calcium carbonate available may have affected the ratios in the well waters.
Figure 20--Concentration ratios for cations in water from wells in plot 2, 1965-71.
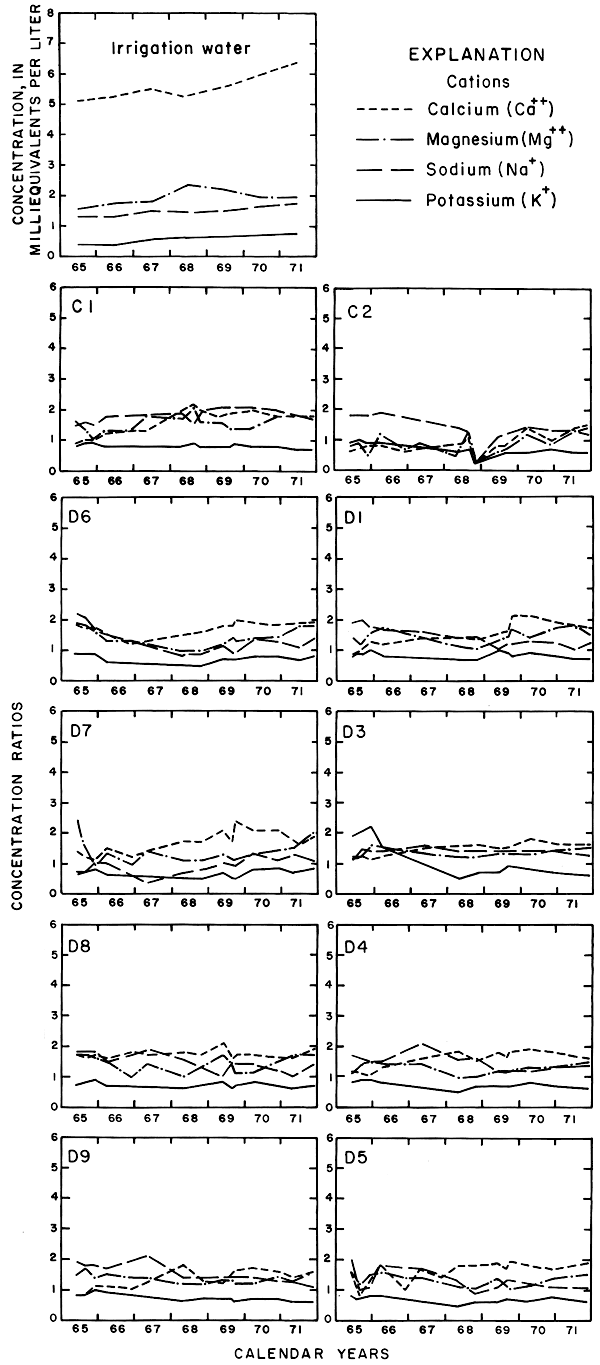
Figure 21--Concentration ratios for selected anions in water from wells in plot 2, 1965-71.
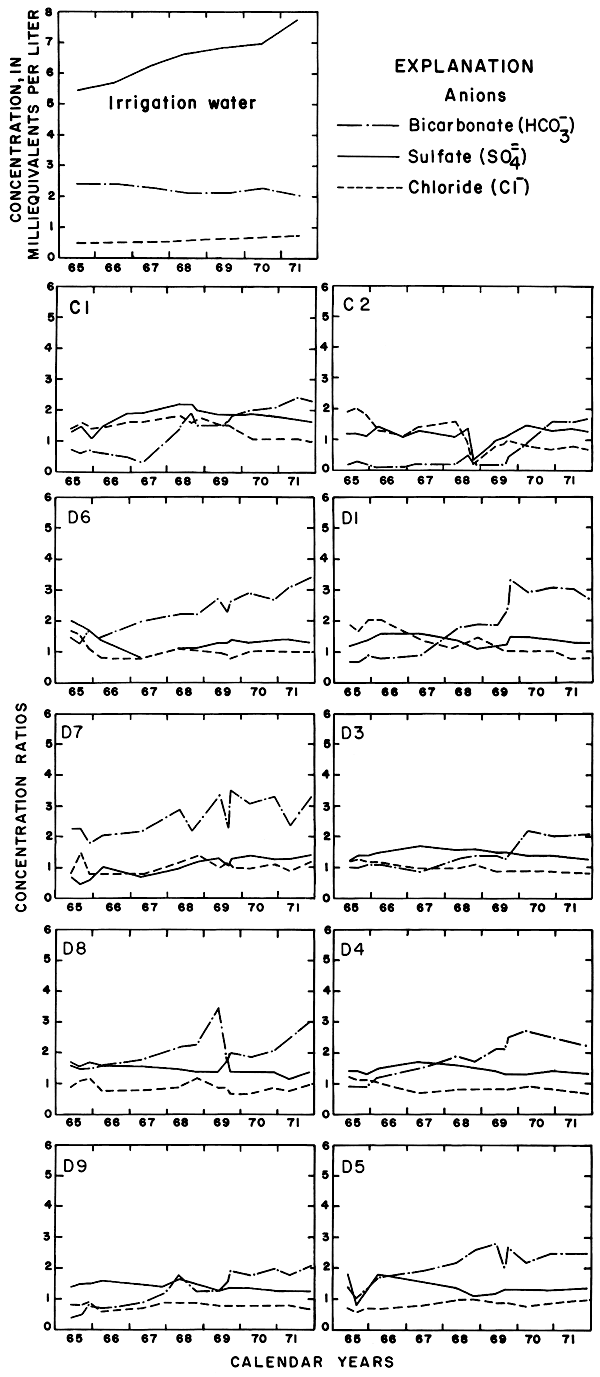
Figure 22--Concentration ratios for the specific conductance and nitrate in water from wells in plot 2, 1965-71.
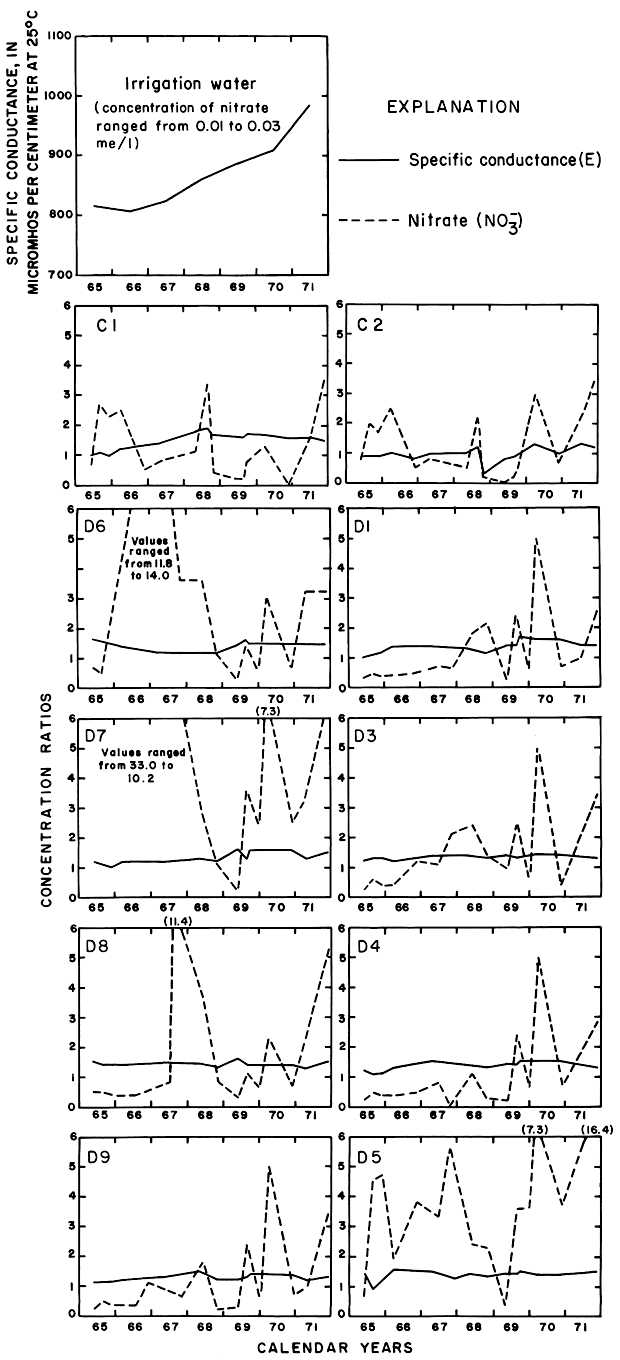
Unlike well waters from the other plots, the concentration ratios for calcium and bicarbonate generally exceeded the ratios for the other major ions. The concentration of bicarbonate increased more rapidly in the well waters than in the irrigation water. The curves describing the ratios for sulfate are similar to the curves for specific conductance, and the ratios or sodium and chloride indicate that the irrigation water was an adequate source of those ions. After 1968, the ratio for chloride from well water was unity or less, indicating dilution of the irrigation water with respect to that ion or underestimation of rainfall.
As shown for the other plots, the concentration ratio for potassium in plot 2 generally was less than one. Potassium from the irrigation water may have been used by the crops. The ratio for nitrate fluctuated widely. Fixation in the soil of atmospheric nitrogen by alfalfa is a well-known phenomenon. The observed wide fluctuations in the concentration of nitrate in the ground water beneath the root zone are not readily evaluated.
To some extent, the difference in the effects of irrigation on the chemical quality of ground water beneath plots 1 and 3 on the high terrace and plot 2 on the flood plain are predictable. In the semiarid environment, soluble salts could accumulate in the sediments on the high terrace until irrigation provided excess water to transport them to the ground-water reservoir. Most of the accumulated salts would have been washed away during erosion and redeposition as the younger alluvium on which plot 2 is located. Periodic inundation and leaching by river water before construction of the dam forestalled significant reaccumulation of soluble salts other than those carried by the river water, which is generally similar in quality to the irrigation water. By analogy, the chemistry of the ground water beneath plot 2 may closely resemble the chemistry of ground water under the irrigated land on the high terrace after prolonged application of irrigation water has leached away previously accumulated salts.
Comparison of the chemistry of the irrigation water and well waters from plots 1 and 3 using graphical methods and a "mix-match" computer program (Lowell, et al., 1971) showed that the well waters were not simple mixtures of the original ground water and infiltrated irrigation water. Excessive concentrations of some of the ions evidently were derived from the soil. In particular, evidence that disproportionately high concentrations of sodium and chloride in well waters could be attributed to leaching of natural salts from the soil by excess irrigation water should allay suspicion that deleterious effects of oil-field and livestock operations were widespread or that sources of the sodium and chloride were perennial.
Soil samples from the experimental plots and from adjacent nonirrigated fields were analyzed to determine if the natural salt content of the soil profile in the irrigated areas had been depleted sufficiently by leaching to account for the observed changes in the chemistry of the well waters. The soils in the experimental plots are typical of a large part of the irrigated acreage; therefore, extrapolation of the results to describe a wider area probably is justified. Drs. O. Bidwell, R. Ellis, H. Jacobs, L. Murphy, and D. Whitney, Kansas State University (oral commun., 1972), contributed valuable information to this phase of the project.
Corresponding soil profiles in each of the experimental plots and adjacent nonirrigated areas were sampled in March 1971. Samples were taken to a depth of 128 inches (325 cm) with a power probe loaned by the Agricultural Experiment Station at Hays. Mr. Carlyle Thompson, Soils Specialist, selected the sampling sites and supervised the sampling operation. At the selected sites, the chemistry of the profile should be unaffected by oil-field or livestock waste or by upward movement of salts from the deep-lying water table. Results of particle-size analyses for each of the profiles are shown on figure 23. The profiles in plots 1 and 3 contain material that is mainly silt and clay sized, and the profiles in irrigated and in adjacent nonirrigated sites are similar. Profiles in the coarse-grained alluvium for the irrigated and nonirrigated sites in plot 2 are dissimilar.
Figure 23--Particle-size distribution in soil profiles in and adjacent to experimental plots.
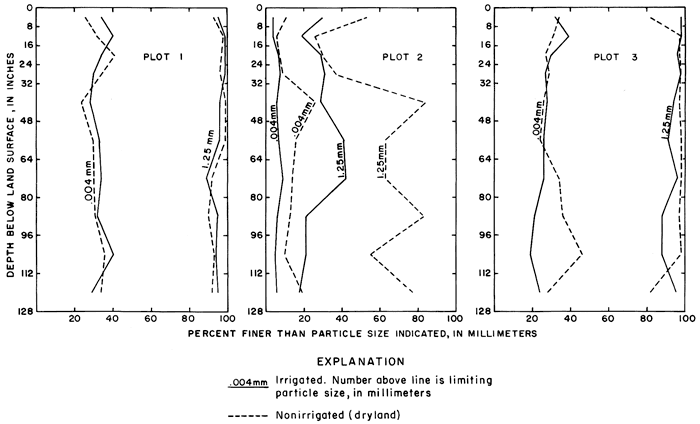
The soils in and adjacent to the experimental plots are classified as listed below according to an unpublished soil map of Ellis County provided by Mr. Bert Soderbloom, Soil Conservation Service, Hays.
| Plot | County | Owner | Soil type in irrigated field |
Soil type in nonirrigated field |
|---|---|---|---|---|
| 1 | Ellis | V. Moore | Harney silt loam (M3B) |
Harney silt loam (M3A) |
| 2 | Trego | P. Nicholson | Alluvium (unclassified) |
Alluvium (unclassified) |
| 3 | Ellis | V. Moore | Harney silt loam (M3A) |
Armo loam (M31D) |
The Harney and Armo are classified as medium-textured soils; the sandy soil in plot 2, which was highly disturbed by leveling, has not been classified.
Soil-saturation extracts of samples were analyzed by the Agronomy Department, Kansas State University. Saturation percentage, soluble (distilled water) and extractable (ammonium acetate) cations, and nitrogen were determined for samples representing 8-inch (20-cm) intervals to a depth of 32 inches (81 cm) and 16-inch (40-cm) intervals to a depth of 128 inches (325 cm). The methods used were those described by M. L. Jackson (1958). The pH, and the concentrations of phosphate, zinc, and copper also were determined for samples representing comparable depth intervals.
Duplicate soil-saturation extracts (distilled water) were analyzed for pH, specific conductance, the major anions, and for boron by the Kansas State Department of Health. The laboratory results are included in the basic data report (Leonard and Stoltenberg, 1972).
Analyses of samples of the 0 to 8-inch (20-cm) "A" soil horizon in plot 3 and an adjacent uncultivated field, as given in table 5, by E. A. Jenne, U.S. Geological Survey, indicate minor enrichment of the trace-element content of the soil by cultivation under irrigation. The methods of analyses were those of the U.S. Geological Survey, 1970. This single pair of samples, which may not be conclusive, were collected mainly to test results from field and laboratory methods.
Table 5--Non-silicate trace element content of samples of the "A" horizon in plot 3 and an adjacent uncultivated field, [Analyses by E. A. Jenne, U.S. Geological Survey, July 1972. Concentrations in micrograms per gram, oven dried at 110 °C.]
| Location | Beryllium (Be) |
Cadmium (Cd) |
Cobalt (Co) |
Copper (Cu) |
Iron (Fe) |
Lead (Pb) |
Manganese (Mn) |
Mercury (Hg) |
Molybdenum (Mo) |
Nickel (Ni) |
Silver (Ag) |
Zinc (Zn) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15-19W-7BBA (irrigated soil) |
0.26 | 0.32 | 4.0 | 2.6 | 9,900 | 9.3 | 360 | 0.07 | 1.2 | 7.2 | 0.04 | 17 |
| 15-19W-7BDA (nonirrigated soil) |
.16 | .22 | 3.3 | 2.9 | 9,300 | 8.0 | 270 | .11 | 1.1 | 6.4 | .13 | 17 |
If the accumulated soluble salts were leached from the soil by the irrigation water, the salt content of the profile and the concentrations of those salts in the saturation extract should be less in the irrigated area than in the nonirrigated area. However, as a result of evapotranspiration, the concentration of the ions in the soil solution in an irrigated area normally exceeds the concentration of the same ions in the irrigation water. To relate variations in the composition of the soil water to the irrigation water, the concentrations of the soluble ions in the saturation extracts (distilled water) are expressed as ratios to concentrations of the corresponding ions in the irrigation water in 1970. The concentration ratios are comparable to those shown in tables 2 and 4 and in figures 10 to 12, 16 to 18, and 20 to 22 describing variations in the concentration ratios of ions in the well waters and irrigation water.
Figure 24--Concentration ratios for specific conductance and soluble ions in saturation extracts of samples from irrigated and nonirrigated soil profiles in and adjacent to experimental plots, March 1971. [A larger version of this figure is available (Acrobat PDF).]
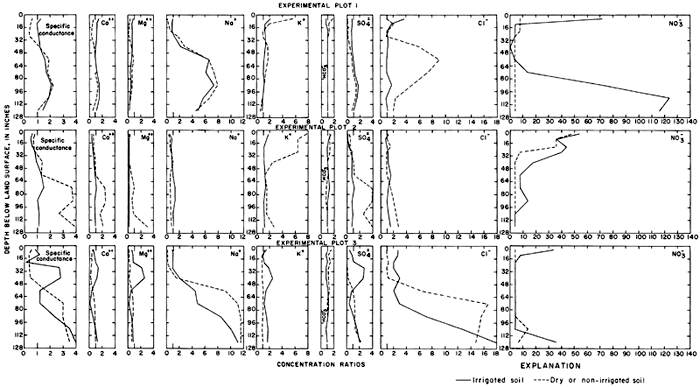
Table 6--Exchangeable cation content and sodium percentages of samples from irrigated and nonirrigated soil profiles in and adjacent to experimental plots. [Cations extracted with I N. ammonium acetate minus soluble cations extracted with distilled water. Cation content given in milliequivalents per 100 grams of soil.]
| Plot number |
Depth interval (inches)1 |
Calcium (Ca) (me/100 g) |
Magnesium (Mg) (me/100 g) |
Sodium (Na) (me/100 g) |
Potassium (K) (me/100 g) |
Exchangeable sodium percentage |
Soluble sodium percentage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | Nonirrigated | Irrigated | ||
| 1 | 0-8 | 14.18 | 16.05 | 3.09 | 4.17 | 0.06 | 0.23 | 2.32 | 1.20 | .33 | 1.04 | 3.6 | 17.0 |
| 8-16 | 15.99 | 20.67 | 3.88 | 6.10 | .09 | .31 | 1.79 | 1.14 | .42 | 1.11 | 11.3 | 19.7 | |
| 16-24 | 19.32 | 21.94 | 5.57 | 5.89 | .28 | .29 | 1.18 | .97 | 1.05 | 1.00 | 31.0 | 19.5 | |
| 24-32 | 19.20 | 21.96 | 5.98 | 5.98 | .41 | .33 | 1.22 | .98 | 1.52 | 1.12 | 53.1 | 22.0 | |
| 32-48 | 21.42 | 21.28 | 5.58 | 5.87 | .81 | .74 | 1.19 | 1.07 | 2.79 | 2.55 | 48.4 | 41.2 | |
| 48-64 | 21.11 | 22.35 | 5.48 | 4.77 | 1.43 | 1.18 | 1.22 | .95 | 4.88 | 4.02 | 64.3 | 68.9 | |
| 64-80 | 21.78 | 23.15 | 4.93 | 4.12 | 1.51 | 1.35 | 1.08 | .81 | 5.16 | 4.58 | 69.8 | 66.2 | |
| 80-96 | 23.59 | 23.80 | 4.55 | 3.70 | 1.50 | 1.28 | .92 | .85 | 4.92 | 4.34 | 66.7 | 64.6 | |
| 96-112 | 23.00 | 22.28 | 3.83 | 3.58 | 1.39 | 1.28 | .84 | .80 | 4.78 | 4.58 | 67.0 | 59.4 | |
| 112-128 | 24.37 | 25.08 | 4.05 | 3.38 | 1.50 | 1.01 | .84 | .68 | 4.86 | 3.35 | 73.1 | 61.8 | |
| 2 | 0-8 | 15.08 | 9.61 | .97 | .64 | .05 | .05 | .61 | .22 | .28 | .43 | 4.6 | 11.4 |
| 8-16 | 11.96 | 9.91 | .48 | .57 | .06 | 0.5 | .61 | .22 | .28 | .43 | 13.0 | 25.6 | |
| 16-24 | 12.80 | 12.69 | .53 | .77 | .06 | .07 | .37 | .24 | .41 | .52 | 9.8 | 19.0 | |
| 24-32 | 13.23 | 12.47 | .48 | .73 | .07 | .07 | .41 | .24 | .48 | .50 | 13.7 | 18.3 | |
| 32-48 | 21.44 | 10.91 | 1.46 | .48 | .18 | .06 | .89 | .20 | .77 | .55 | 15.8 | 19.6 | |
| 48-64 | 18.53 | 12.63 | 1.86 | .44 | .12 | .08 | .53 | .20 | .57 | .57 | 11.2 | 18.5 | |
| 64-80 | 15.57 | 13.85 | 1.19 | .44 | .07 | .06 | .31 | .22 | .40 | .44 | 4.7 | 20.8 | |
| 80-96 | 18.49 | 11.64 | .63 | .32 | .05 | .04 | .33 | .16 | .26 | .37 | 4.2 | 20.9 | |
| 96-112 | 14.51 | 11.78 | 1.07 | .36 | .05 | .06 | .28 | .16 | .33 | .48 | 6.3 | 21.2 | |
| 112-128 | 17.28 | 11.41 | 3.25 | .40 | .08 | .07 | .5o | .16 | .39 | .61 | 6.2 | 23.3 | |
| 3 | 0-8 | 16.10 | 15.55 | 1.71 | 3.70 | .07 | .21 | .90 | 1.11 | .35 | 1.03 | 3.9 | 23.4 |
| 8-16 | 17.82 | 21.39 | 2.12 | 5.16 | .06 | .28 | .72 | .02 | .31 | 1.00 | 10.0 | 20.9 | |
| 16-24 | 21.95 | 23.12 | 3.23 | 4.74 | .10 | .22 | .68 | .94 | .37 | .75 | 7.8 | 16.3 | |
| 24-32 | 18.56 | 21.51 | 4.09 | 5.07 | .13 | .20 | .54 | 1.00 | .57 | .71 | 19.1 | 8.4 | |
| 32-48 | 15.78 | 20.01 | 4.10 | 5.50 | .50 | .37 | .53 | 1.14 | 2.37 | 1.38 | 56.3 | 18.2 | |
| 48-64 | 16.51 | 18.14 | 4.20 | 4.62 | .86 | 1.00 | .57 | 1.00 | 3.90 | 4.04 | 79.1 | 67.7 | |
| 64-80 | 18.09 | 17.68 | 3.85 | 3.77 | 1.38 | 1.47 | .57 | 1.08 | 5.79 | 6.13 | 72.2 | 78.2 | |
| 80-96 | 20.61 | 16.20 | 3.90 | 2.52 | 9.30 | 1.06 | .72 | .70 | 26.9 | 5.20 | 72.8 | 71.7 | |
| 96-112 | 26.55 | 17.31 | 4.42 | 2.46 | 11.34 | 1.05 | .93 | .70 | 26.2 | 4.90 | 71.8 | 64.4 | |
| 112-128 | 20.72 | 18.21 | 2.87 | 2.35 | 7.83 | 1.12 | .76 | .71 | 24.3 | 5.00 | 67.4 | 61.5 | |
| Inches X 2.54 equal centimeters. | |||||||||||||
Variations in the ratios for the soluble ions with depth in each profile are shown in figure 24. Dilution by rainfall, variations in the solubility of the salts, and ion-exchange reactions within the soil complex alter the ionic composition of the soil water, but the major differences between irrigated and nonirrigated profiles probably are attributable to leaching. The concentration ratios for the specific conductance of the saturation extracts from the upper 2 feet (0.6 m) of the irrigated profiles are generally similar to the predicted annual concentration ratios (CRa) shown in table 4.
Variations in the concentrations of calcium and bicarbonate ions are probably governed by the solubility of calcium carbonate in each profile. The tops of calcareous zones of varying thickness were found in the soil profiles at depths from 18 to 35 inches (46 to 89 cm) in and adjacent to plots 1 and 3 and at the land surface in plot 2. Magnesium carbonate is more soluble than calcium carbonate; ratios less than one for magnesium throughout most of the profiles may represent ion exchange for sodium, which forms more soluble salts. Relatively low concentration ratios for specific conductance, sulfate, and other ions near the surface probably represent leaching by rainfall after the irrigation season. The concentration of sulfate in soil profiles in plots 1 and 3 was increased by application of irrigation water.
In plots 1 and 3 the concentration ratios for soluble sodium, chloride, and nitrate ranged more widely than for the other ions. In plot 1, the concentration of soluble sodium was only slightly lower in the irrigated than in the nonirrigated profile, but the concentration of chloride was much lower. In plot 3, high concentrations of sodium and chloride appear to have been displaced downward by the excess water. In plots 1 and 2, excess potassium seems to have been leached from the irrigated profile, but the concentration of potassium in plot 3 is higher in the irrigated than the nonirrigated profile, which could be a result of fertilization.
High ratios for nitrate near the surface of irrigated plots 1 and 3 reflect application of anhydrous ammonia fertilizer before soil sampling. The high ratios at the base of the sections probably represent excess fertilizer that was leached below the root zone.
In this report, the exchangeable cation content is defined as the difference between the extractable and the soluble cation content of each sample of soil. Extractable cations are the total cationic content of that part of a soil sample which was removed by ammonium acetate; soluble cations are those removed from the soil sample by distilled water (table 6). The soluble anions also were removed by distilled water. The exchangeable sodium percentages generally increased with depth and the percentages for the irrigated profiles exceeded the percentages for the dry profiles in the upper part of the section. The results substantiate the prediction by Jacobs and Whitney (1968, p. 5) that the exchangeable sodium percentage of the upper layers would not exceed 10 percent subsequent to irrigation with water of the type applied.
The extractable cation content was reported in terms of milliequivalents per 100 grams of soil (me/100 g). For purposes of comparison, the soluble ion content of the soil (M) in each depth increment was converted to the same units from the saturation percentage (SP), the concentration (C), and a units conversion factor (K) characteristic of each ion.
M (me/100 g) = [C(mg/l) X SP (%) X K(me/mg)] / 1000
The soluble cation content generally represented less than 10 percent of the extractable cation content for each profile.
Differences in content of the exchangeable cations, the soluble cations, and the soluble anions for corresponding depth intervals in the irrigated and nonirrigated profiles are shown in figure 25. Values representing ionic content of nonirrigated profiles were subtracted from corresponding values representing ionic content of irrigated profiles. Positive values probably indicate accumulation of salt and negative values probably indicate leaching of salts by irrigation water if the profiles are equivalent. At depths greater than 48 inches (120 cm), most of the differences are negative or near zero. Calcium, magnesium, and sulfate from the irrigation water evidently augmented the natural accumulation of the ions in the upper part of the section.
Figure 25--Differences in the exchangeable cation content and the soluble cation and anion content of irrigated and nonirrigated soil profiles in and adjacent to experimental plots. [A larger version of this figure is available (Acrobat PDF).]
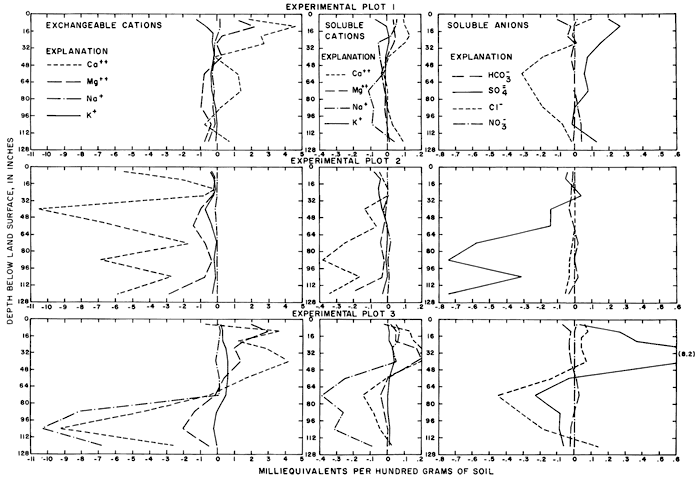
By cumulating the differences for each interval of corresponding profiles, weighted according to the size of the interval, the average net differences (in me/100 g) were calculated for each plot. Negative differences are recorded in table 7 for major ions except calcium in plot 1, magnesium and potassium in plot 3, sulfate in plots 1 and 3, and nitrate for all plots.
Table 7--Net differences in the content of major ions in adjacent irrigated and nonirrigated soil profiles to a depth of 128 inches.
| Plot | Ion type | Content, in milliequivalents per 100 grams dry soil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca++ | Mg++ | Na+ | K+ | HCO3- | SO4-- | Cl- | NO3- | ||
| 1 | Extractable | 1.12 | -0.14 | -0.18 | -0.26 | ||||
| Soluble | .04 | .01 | -.03 | -.01 | -0.01 | 0.07 | -0.13 | 0.02 | |
| Exchangeable | 1.08 | -.15 | -.15 | -.25 | |||||
| 2 | Extractable | -4.89 | -.90 | -.02 | -.30 | ||||
| Soluble | -.17 | -.04 | .00 | -.03 | -.01 | -.35 | -.02 | .00 | |
| Exchangeable | -4.72 | -.86 | -.02 | -.27 | |||||
| 3 | Extractable | -.86 | .24 | -3.25 | .27 | ||||
| Soluble | .05 | .04 | -.14 | .01 | -.01 | 1.24 | -.10 | .00 | |
| Exchangeable | -.91 | .20 | -3.11 | .26 | |||||
Under the tenuous assumption that the negative differences represent the quantity of salts leached from the upper 128 inches (325 cm) of soil in each plot, the amounts by which the concentrations of the ions in the leachate increased can be estimated. Undisturbed soil samples were not available, but a bulk density of 1.45 grams per cubic centimeter is a reasonable estimate for the silt loam sampled in plots 1 and 3. Approximately 13,150 cubic meters of dry soil, about 1.91 X 1010 grams, underlies each acre to a depth of 128 inches (325 cm). The amount of an ion leached from the soil (in grams per acre) is the product of the weight of the soil in grams, the concentration of the ion (in equivalents per gram of soil) and the gram equivalent weight for the ion.
According to table 7, the concentration of soluble chloride in the irrigated profile in plot 1 was 0.13 me/100 g less than in the nonirrigated profile. This is equivalent to a difference of about 900 kilograms per acre, computed as follows:
1.91 X 1010 (grams of soil/acre) X 1.33 X 106 (equivalents/ gram) X 35.46 (grams/ equivalent)
= 9.01 X 105 grams/acre = 900 kilograms/acre
According to table 4, excess water during irrigation seasons 1965-70 was about 3 acre-feet per acre or 3.7 X 106 liters per acre. If the 900 kilograms of chloride were dissolved in the excess water, the concentration of chloride would increase by about 240 mg/l. By similar calculations, based on the difference for soluble sodium, the increase for sodium would be about 40 mg/l. Comparable increases for plot 3 would be about 110 mg/l for both soluble chloride and sodium. If the calculations were based on differences for extractable sodium, the increase in concentration would be about 200 mg/l for plot 1, and more than 2,000 mg/l for plot 3 as a result of anomalously high concentrations of exchangeable sodium reported in the deepest cores from the nonirrigated profile for plot 3.
Figures 12 and 24 and table 7 show that the content of nitrate in the soil and the concentration of nitrate in well waters in plot 1 increased with the application of irrigation water. The apparent additions in the soil, mainly below the root zone, are equivalent to about 178 kilograms per acre of nitrate or 40 kilograms per acre of nitrogen. The increase may indicate excessive application of nitrogen fertilizer or, more likely, applications of water in excess of that required for plant growth.
Peaks in the concentrations of sodium and chloride in the well waters (figs. 12 and 16) after 1 to 3 years of irrigation indicate that the concentrations of those ions in the leachate probably increased rapidly during the first years of irrigation, then decreased as the supply of ions in the soil was depleted. More rigorous quantitative analysis of the relations of changes in the concentrations of the ions in the well waters to changes in the salt content of the soil could probably be made with a relatively small amount of additional data. However, the estimates presented above indicate that leaching of accumulated natural salts and applied fertilizer from the upper part of the soil profile by excess irrigation water, and lateral movement of the displaced ions are the major cause of observed changes in the concentrations of sodium and chloride in the ground water. The effects of the arrival of the chemically altered ground water on low flow of the Smoky Hill River was recorded during a series of seepage-salinity surveys.
Variations and fluctuations in the quantity and quality of streamflow of the Smoky Hill River and tributaries in the reach between Cedar Bluff Dam and station 334.4, which is about 2 miles (3.2 km) downstream from the district, were monitored at the gaging stations shown in plate 1. Because the approximately 6,000 acres (2,400 ha) of irrigated land normally contributed an insignificant part of storm runoff from the 220 square miles (570 km2) of drainage area to the reach, effort was directed mainly to isolating the progressive changes in quantity and quality of low flow caused by irrigation from the wider variations and fluctuations caused by other factors in the hydrologic environment.
Sixteen surveys of a 22-mile (35-km) reach of the Smoky Hill River from the dam to station 334.4 (pl. 1) were made between April 1964 and November 1971 to reveal progressive changes in the quantity and quality of low flow in the main stem, of tributary inflow, and of seepage directly into the main stem as a result of irrigation. The Division of Water Resources of the Kansas State Board of Agriculture made most of the field measurements during the surveys in 1964 to obtain necessary background data before the comprehensive study began. Regulation of releases from the fish hatchery (355.8, pl. 1) by personnel of the U.S. Bureau of Sports Fisheries and Wildlife during most of the surveys facilitated the field work and analysis of the data.
Basic data consisted of current-meter measurements of streamflow at the gaging stations shown in plate I and chemical analysis of water samples taken at the time of measurement. Each survey was completed during a single day when streamflow had stabilized after several weeks without significant rainfall, direct surface runoff, or releases to the river from the dam. All but two of the surveys were made before or after the growing season, when inflow to the stream consisted mainly of effluent ground water from the irrigated area and measured releases from the fish hatchery. Results of the survey in March 1966 were complicated by releases from the fish hatchery and snow melt. Results of the two surveys conducted during the growing season (September 1, 1965; August 31, 1971) were affected by waste, tail-water runoff, unmeasured withdrawals and runoff associated with irrigation outside of the district, and consumptive use by phreatophytes which proliferated along the channel since irrigation began. Significant turbidity, observed only during the survey in August 1971, originated upstream from the district.
Flow in each of the main stem reaches consisted principally of flow entering the upstream end of the reach, tributary inflow, and seepage into the channel. Variations in the quantity and chemical quality of each component, or combination of components were evaluated by use of computer programs (Leonard and Morgan, 1970) developed specifically to facilitate compilation, analysis, and interpretation of the voluminous data. The programs are applicable wherever salinity surveys of low flow can be used for qualitative and quantitative definition of localized sources of chemical pollution.
A progressive increase in the concentration of chloride in a downstream direction in the river adjacent to the irrigation district during successive surveys (fig. 26) reflects accelerated drainage of ground water from the irrigated acreage. Although the concentration of chloride was greater than in the irrigation water it remained well below the maximum of 250 mg/l recommended for drinking water by the Kansas State Board of Health (1973). The rate of increase per river mile was greatest in the reach 350.0 to 338.0 adjacent to the main part of the irrigation district, herein defined as the irrigation reach.
Figure 26--Concentrations of sulfate and chloride in the Smoky Hill River and in irrigation water during seepage-salinity surveys, 1964-71.
The concentrations of the other ions did not increase proportionately. For example, the concentration of sulfate, the most abundant ion in the applied water, generally decreased in a downstream direction from values higher than to values lower than the irrigation water. As was shown in the previous discussions of ground water and soil, some of the sulfate in the applied water evidently was precipitated in the soil or on the land surface, or was removed by plants; thereby depleting the concentration of that constituent in the return flow.
To evaluate the quantity and quality of inflow between successive stations along the main stem, the water and chemical discharge at each of the stations was subtracted from corresponding quantities at the adjacent downstream station. The algebraic difference is the net gain (or loss) of water or chemical discharge in the reach. During the surveys net gains consisted mainly of effluent ground water from the irrigated area north of the river. After the 1965 irrigation season, inflow from right-bank tributaries (south) was a negligible part of total inflow.
The water discharge at stations along the main stem and near the mouths of all known flowing tributaries was reported in cubic feet per second. The chemical discharge, or ionic load (L), of an ionic constituent is defined by the equation
L = KQC
where K is a unit constant, Q is the measured discharge in cubic feet per second (assumed equal to the daily mean discharge), and C is the concentration of the constituent. Load is defined in tons per day when C is in milligrams per liter and K = 0.0027. Load also can be defined in terms of kiloequivalents, or thousands of equivalent weights per day, when C is in milliequivalents per liter and K = 2.446. The latter method of defining L was used for data analysis in this report. Because the sums of the cationic and anionic constituents should be nearly equal, anomalous relations between the ionic loads are readily apparent.
If the water discharge and ionic load at the downstream and upstream stations respectively are Q2, Q1, L2, and L1, then the net gain in the reach is Q2-Q1 for water discharge and L2-L1 for chemical discharge. Negative values represent losses from the channel, changing stage, or erroneous measurements of water discharge or concentration. Differences greater than about 8 percent between the anionic load and cationic load in net gains or losses, in ke/day (kilo equivalents per day), normally indicated erroneous calculations or measurements.
Despite wide and relatively random variation in antecedent rainfall, the magnitude of net gains between successive stations adjacent to the district generally increased as low flow was augmented by effluent ground water and salts from the irrigated land. However, as shown in figure 27, the inflow per mile varied from reach to reach in response to variations in the distribution of irrigation water and localized differences in the geohydrology. For example, high inflow in the reach 340.3 to 338.0 probably represents discharge of effluent ground water from a buried channel that drains a large part of the irrigated area (pl. 2). During the irrigation season, canal waste also augments flow in that reach.
Figure 27--Net gains of water, sodium, sulfate, and chloride during seepage-salinity surveys 1964, 1966, 1968, and 1970, and of concentrations in 1970.
Progressive changes in the magnitude and ionic composition of net gains to the irrigation reach 350.0 to 338.0 are caused mainly by changes in the quantity and chemical quality of drainage from most of the irrigated land. Net gains upstream from the irrigation reach consisted mainly of variable inflow from the fish hatchery and from a seepage area east of the dam. Part of that inflow is now diverted for irrigation of land outside of the district. Net gains downstream from the irrigation reach appear to be relatively unaffected by irrigation of land in the district, although withdrawals from wells adjacent to the channel between stations 338.0 and 335.5 caused minor net losses during several of the surveys. The wells may intercept return flow from the district.
To illustrate the effect of irrigation on flow downstream from the district, exclusive of variable releases from the fish hatchery, net gains are expressed in figure 28 as percentages of total inflow to the river between the fish hatchery 355.8 and station 334.4. Net gains to the irrigation reach ranged from 30 percent in 1964 to about 80 percent in 1967 when rainfall during part of the growing season far exceeded consumptive use (table 2). From 1968 to 1971, when irrigation water was used more efficiently, the percentage remained relatively constant between 62 and 69 percent.
Figure 28--Net gains in the irrigation reach, expressed as percentages of the total inflow to the Smoky Hill River between Cedar Bluff Fish Hatchery (355.8) and station 334.4.
Progressive increases in net gains in the spring of each year, when excess water from the preceding season had ample time to drain from the aquifer, attest to the increase in storage shown by higher water levels adjacent to the irrigated area during successive years. The lowest net gain in the irrigation reach was 1.4 cfs (0.04 m3/s) in October 1964. After 1967, net gains fluctuated between 8 and 10 cfs (0.23 and 0.28 m3/s) in the spring and fall, respectively, as the water table fell and rose in response to drainage from and infiltration of excess water to ground-water storage.
Net gains consist of measured tributary inflow plus net seepage. Tributary inflow, measured near the mouths of the streams, consisted mainly of effluent ground water from the aquifer underlying the high terrace. Net seepage gains represent unmeasured inflow mainly from small tributaries and from marshy areas along the river. Net seepage losses represent unmeasured withdrawals, including evapotranspiration, from the main stem or from tributaries downstream from data-collection sites. Measured withdrawals during the surveys in the spring and fall were negligible.
In April 1964, seepage was the major component of net gains of less than 2 cfs (0.06 m3/s). As more water was applied to more acres, the ground-water reservoir under the high terrace filled and tributary inflow comprised a generally increasing percentage of net gains in the irrigation reach (fig. 28). Flow of natural tributaries increased and was augmented by drains constructed in marshy areas that formerly contributed to seepage.
Net seepage gain or loss between adjacent stations probably is significant only when the magnitude exceeds about 6 percent of the measured discharge at the terminus of the reach. The effect of small errors in measurement and analysis at each station is minimized when seepage gains and losses are accumulated in a downstream direction. Cumulative net seepage losses of nitrate in the irrigation reach were recorded during all surveys, but gains were recorded for water and all the other major ions. Net seepage losses of nitrate that ranged from about 45 to 53 kilograms of nitrate (10 to 12 kg. of nitrogen) per day during the last four surveys would represent improbably high unmeasured withdrawals of about 21 acre-feet (10.6 cfs-days) per day of river water at the observed concentration of about 2 mg/l. The losses probably represent interception and consumption of much smaller quantities of highly concentrated tributary inflow by abundant phreatophytes on the flood plain as well as withdrawal of nitrate by aquatic plants.
Figure 29--Specific conductance, sodium-adsorption ratio, and magnitude and composition of net gains in the irrigation reach during seepage-salinity surveys, 1964-71.
Although the maximum net gains in water discharge to the irrigation reach after each growing season was measured during the survey in October 1969, the net gain in chemical discharge increased progressively from 1964 to 1971 (fig. 29). However, the rate of annual increase in chemical discharge diminished from year to year.
As shown in figure 29, the percent composition of the ionic load in net gains remained relatively constant after 1965. Calcium and sulfate were the predominant ions, but the percent sulfate was much lower than in the irrigation water. After 1965, the percent sulfate in net gains and in the irrigation water generally increased with a corresponding decrease in percent. However, the percentages of sodium and chloride in net gains remained higher than the percentages in the irrigation water. Significant relations between the ions are readily described in terms of concentrations and concentration ratios.
The specific conductance of water discharge in the irrigation reach increased from 880 to 900 micromhos per centimeter at 25°C in the fall of 1964. In the fall of 1971, the specific conductance in the same reach increased from 1,070 to 1,230 micromhos per centimeter at 25°C. The concentration ratio for specific conductance increased from 1.01 in 1964 to 1.15 in 1971.
The concentration in milliequivalents per liter of the ions in net gains was calculated by dividing the net gains in chemical discharge shown in figure 29 y the product of the net gains in water discharge and the unit constant 2.446. The concentrations of the ions in net gains, net seepage, and tributary inflow during each of the surveys is shown in table 8; progressive changes are illustrated graphically in figure 30. Because concentrations of nitrate were very low and the range of concentration ratios was very wide, it was not practical to include nitrate in figure 30.
Table 8--Quantity and chemical quality of net gains, tributary inflow, and net seepage in the irrigation reach 350.0-338.0 during seepage-salinity surveys, April 1964-November 1971.
| Date of survey |
Discharge in cfs (0.028 m3/s) |
Seepage as percent net gain |
Specific conductance net gains |
Dissolved solids net gains (residue at 180°C) |
Boron (B) | Phosphate (PO4) | pH net gain |
SO4 mg/l net gain |
SAR2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Net gain |
Tributary | Net seepage |
micromhos/cm at 25°C |
CR1 | mg/l | CR1 | mg/l | CR1 | mg/l | CR1 | Units | CR1 | ||||
| 4/30/64 | 1.66 | 0.15 | 1.51 | 91.0 | 1,054 | 1.31 | 771 | 1.41 | 0.16 | 0.94 | 0.20 | 1.43 | 1.11 | 1.58 | ||
| 10/21/643 | 1.40 | .93 | .47 | 33.6 | 880 | 1.13 | 628 | 1.17 | .19 | neg. | 7.6 | 13 | 1.73 | 2.47 | ||
| 9/ 1/654 | 14.72 | 9.96 | 4.76 | 32.3 | 855 | 1.05 | 571 | 1.06 | .19 | .76 | .83 | 1.19 | ||||
| 11/16/65 | 4.85 | 2.04 | 2.81 | 57.9 | 869 | 1.07 | 597 | 1.10 | .14 | .56 | .10 | .94 | 1.34 | |||
| 3/22/66 | 10.42 | 3.49 | 6.93 | 66.5 | 888 | 1.09 | 626 | 1.16 | .18 | .72 | .86 | 1.23 | ||||
| 11/16/66 | 7.19 | 3.29 | 3.90 | 54.2 | 981 | 1.22 | 632 | 1.16 | .24 | 1.41 | .91 | 1.30 | ||||
| 3/30/67 | 5.96 | 3.04 | 2.92 | 49.0 | 1,005 | 1.25 | 684 | 1.26 | .16 | .94 | 1.08 | 1.54 | ||||
| 11/21/67 | 9.00 | 6.16 | 2.84 | 31.6 | 1,057 | 1.28 | 719 | 1.22 | .26 | .79 | .10 | 5.00 | 1.27 | 1.59 | ||
| 10/30/68 | 9.58 | 5.81 | 3.77 | 39.4 | 1,086 | 1.26 | 746 | 1.24 | .23 | .08 | .80 | 1.39 | 1.99 | |||
| 4/10/69 | 7.80 | 5.09 | 2.71 | 34.7 | 1,139 | 1.32 | 746 | 1.24 | .26 | 1.45 | 2.07 | |||||
| 10/28/69 | 10.64 | 6.84 | 3.80 | 35.7 | 1,154 | 1.30 | 808 | 1.28 | .21 | 1.24 | 1.42 | 1.78 | ||||
| 3/25/70 | 7.95 | 4.47 | 3.48 | 43.8 | 1,141 | 1.29 | 767 | 1.22 | .18 | 1.06 | .05 | 1.25 | 7.8 | 12 | 1.28 | 1.60 |
| 11/ 5/70 | 10.22 | 8.46 | 1.76 | 17.2 | 1,259 | 1.39 | 876 | 1.35 | .18 | 1.29 | .06 | .60 | 7.7 | 15 | 1.56 | 1.95 |
| 4/ 6/71 | 9.34 | 5.74 | 3.60 | 38.5 | 1,255 | 1.38 | 853 | 1.32 | 1.47 | 1.83 | ||||||
| 8/31/714 | 10.68 | 9.11 | 1.57 | 14.7 | 1,142 | 1.16 | 812 | 1.17 | .21 | 1.17 | .09 | .90 | 7.9 | 12 | 1.48 | 1.85 |
| 11/10/71 | 9.93 | 7.06 | 2.87 | 28.9 | 1,302 | 1.32 | 872 | 1.26 | .20 | 1.11 | .06 | .60 | 7.8 | 20 | 1.66 | 2.08 |
| Date of survey |
Net Gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
|||||||||
| Calcium (Ca) | Magnesium (Mg) | Sodium (Na) | Potassium (K) | |||||||||||||||||
| 4/30/64 | 8.66 | 1.69 | 67.4 | 4.44 | 9.08 | 1.39 | 0.78 | 10.8 | 1.06 | 1.4 | 2.49 | 1.92 | 19.4 | 0.76 | 2.66 | 0.31 | 0.84 | 2.41 | 0.16 | 0.33 |
| 10/21/64 | 5.18 | 1.02 | 65.9 | 3.80 | 7.96 | .50 | .36 | 6.3 | 6.47 | 1.86 | 1.55 | 23.6 | 6.08 | .33 | .80 | 4.18 | .25 | .47 | ||
| 9/ 1/653 | 5.27 | 1.02 | 56.7 | 5.28 | 5.25 | 2.02 | 1.28 | 21.7 | 1.33 | 3.47 | 1.59 | 1.21 | 17.1 | 1.37 | 2.04 | .41 | 1.00 | 4.41 | .44 | .36 |
| 11/16/65 | 6.50 | 1.25 | 61.6 | 5.60 | 7.16 | 1.88 | 1.19 | 17.8 | .94 | 2.56 | 1.92 | 1.46 | 18.2 | 1.81 | 2.00 | .26 | .63 | 2.46 | .20 | .30 |
| 3/22/66 | 5.92 | 1.14 | 59.7 | 5.54 | 6.11 | 2.02 | 1.28 | 20.4 | 1.33 | 2.36 | 1.71 | 1.30 | 17.3 | 1.94 | 1.59 | .26 | .63 | 2.62 | .18 | .30 |
| 11/16/66 | 6.25 | 1.17 | 60.6 | 5.74 | 6.68 | 1.97 | 1.12 | 19.1 | 1.54 | 2.33 | 1.85 | 1.39 | 17.9 | 2.54 | 1.26 | .24 | .60 | 2.32 | .21 | .27 |
| 3/30/67 | 6.74 | 1.27 | 57.9 | 5.53 | 7.99 | 2.01 | 1.14 | 17.9 | 1.16 | 2.90 | 2.26 | 1.70 | 20.1 | 2.18 | 2.34 | .24 | .60 | 2.13 | .17 | .31 |
| 11/21/67 | 7.25 | 1.32 | 66.2 | 6.76 | 8.32 | .90 | .50 | 8.2 | 1.22 | .21 | 2.57 | 1.69 | 23.4 | 2.73 | 2.20 | .24 | .58 | 2.19 | .19 | .34 |
| 10/30/68 | 6.62 | 1.26 | 55.4 | 7.20 | 5.73 | 2.14 | .90 | 17.9 | 1.50 | 3.72 | 2.91 | 2.02 | 24.4 | 3.20 | 2.46 | .27 | .58 | 2.26 | .22 | .3,1 |
| 4/10/69 | 6.67 | 1.27 | 54.9 | 6.61 | 7.05 | 2.21 | .93 | 18.1 | 1.33 | 3.87 | 3.06 | 2.12 | 25.2 | 3.23 | 2.74 | .21 | .46 | 1.73 | .18 | .2 |
| 10/28/69 | 7.57 | 1.36 | 61.3 | 6.56 | 9.03 | 1.48 | .67 | 12.0 | 1.31 | 1.71 | 3.03 | 1.98 | 24.5 | 3.34 | 2.34 | .27 | .59 | 2.19 | .22 | .35 |
| 3/25/70 | 7.25 | 1.30 | 57.9 | 7.11 | 7.42 | 2.27 | 1.02 | 18.1 | 1.45 | 3.22 | 2.78 | 1.82 | 22.2 | 3.23 | 2.20 | .22 | .48 | 1.76 | .36 | .26 |
| 11/ 5/70 | 8.53 | 1.42 | 60.4 | 7.78 | 12.14 | 1.81 | .91 | 12.8 | 2.90 | 3.53 | 2.13 | 25.0 | 3.54 | 3.49 | .25 | .53 | 1.77 | .21 | .41 | |
| 4/ 6/71 | 8.31 | 1.38 | 60.4 | 7.41 | 9.71 | 1.89 | .95 | 13.7 | 1.53 | 2.47 | 3.32 | 2.00 | 24.1 | 3.41 | 3.19 | .24 | .51 | 1.74 | .20 | .28 |
| 8/31/713 | 7.55 | 1.18 | 58.4 | 7.44 | 8.17 | 1.82 | .92 | 14.1 | 1.70 | 2.53 | 3.20 | 1.88 | 24.8 | 3.01 | 4.33 | .36 | .73 | 2.78 | .36 | .42 |
| 11/10/71 | 8.52 | 1.33 | 60.9 | 8.06 | 9.64 | 1.50 | .76 | 10.7 | 1.57 | 1.34 | 3.72 | 2.19 | 26.6 | 3.59 | 4.04 | .49 | .49 | 1.72 | .22 | .28 |
| Date of survey |
Net Gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
Net gains | Tributaries me/l |
Net seepage me/l |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
me/l | CR1 | percent cations |
|||||||||
| Bicarbonate (HCO3) | Sulfate (SO4) | Chloride (Cl) | Nitrate (NO3) | |||||||||||||||||
| 4/30/64 | 4.96 | 1.86 | 34.2 | 4.31 | 5.03 | 6.88 | 1.28 | 47.4 | 1.23 | 7.33 | 2.63 | 5.37 | 18.1 | .84 | 2.81 | loss | 0.09 | loss | ||
| 10/21/64 | 1.66 | .69 | 19.7 | 5.13 | 5.29 | 1.10 | 62.9 | 3.25 | 9.32 | 1.43 | 2.80 | 17.0 | 2.43 | loss | ||||||
| 9/ 1/653 | 2.91 | 1.20 | 32.5 | 2.52 | 3.73 | 5.04 | .91 | 56.4 | 5.21 | 4.68 | .93 | 1.72 | 10.4 | .61 | 1.60 | .02 | .22 | 04 | loss | |
| 11/16/65 | 4.74 | 1.95 | 43.2 | 4.06 | 5.24 | 4.44 | .80 | 40.5 | 2.82 | 5.62 | 1.72 | 3.18 | 15.0 | 1.53 | 1.86 | .04 | 2.60 | .36 | .15 | loss |
| 3/22/66 | 3.62 | 1.49 | 37.2 | 3.99 | 3.44 | 4.63 | .84 | 47.6 | 3.11 | 5.40 | 1.43 | 2.65 | 14.7 | 1.74 | 1.28 | .02 | .70 | .21 | .09 | loss |
| 11/16/66 | 3.30 | 1.36 | 32.8 | 3.52 | 3.11 | 4.75 | .83 | 47.2 | 4.42 | 5.02 | 1.96 | 3.56 | 19.5 | 2.03 | 1.90 | .04 | 1.33 | .40 | .09 | loss |
| 3/30/67 | 3.55 | 1.47 | 31.8 | 3.18 | 3.93 | 5.29 | .92 | 47.4 | 3.58 | 7.06 | 2.29 | 4.16 | 20.5 | 2.02 | 2.56 | .02 | .67 | .18 | .11 | loss |
| 11/21/67 | 4.03 | 1.74 | 35.7 | 4.30 | 3.43 | 4.83 | .77 | 42.7 | 4.26 | 6.05 | 2.36 | 4.21 | 20.9 | 2.13 | 2.86 | .06 | 6.00 | .53 | .14 | loss |
| 10/30/68 | 3.37 | 1.56 | 28.3 | 4.03 | 2.35 | 6.14 | .93 | 51.5 | 5.57 | 7.01 | 2.35 | 4.20 | 19.7 | 2.29 | 2.43 | .06 | 2.00 | .50 | .12 | loss |
| 4/10/69 | 3.29 | 1.52 | 27.4 | 3.51 | 2.88 | 5.85 | .88 | 48.7 | 5.24 | 7.00 | 2.81 | 5.02 | 23.4 | 2.41 | 3.57 | .03 | 1.00 | .25 | .08 | loss |
| 10/28/69 | 3.96 | 1.84 | 31.8 | 3.26 | 5.04 | 5.81 | .85 | 46.7 | 5.41 | 6.26 | 2.57 | 4.14 | 20.7 | 2.30 | 2.94 | .08 | 8.00 | .64 | .13 | loss |
| 3/25/70 | 3.62 | 1.68 | 29.6 | 3.82 | 3.37 | 5.82 | .85 | 47.6 | 5.44 | 6.32 | 2.69 | 4.34 | 22.0 | 2.47 | 2.98 | .07 | 7.00 | .57 | .02 | loss |
| 11/ 5/70 | 4.35 | 1.96 | 31.2 | 4.23 | 4.96 | 6.70 | .96 | 48.1 | 5.99 | 10.13 | 2.77 | 4.40 | 19.9 | 2.51 | 4.03 | .08 | 8.00 | .57 | .14 | loss |
| 4/ 6/71 | 3.85 | 1.73 | 28.4 | 3.65 | 4.18 | 6.72 | .96 | 49.6 | 5.83 | 8.13 | 2.85 | 4.52 | 21.0 | 2.83 | 2.95 | .10 | 10.00 | .74 | .15 | loss |
| 8/31/713 | 3.29 | 1.62 | 25.6 | 3.60 | 1.48 | 7.11 | .91 | 55.4 | 6.57 | 10.23 | 2.35 | 3.22 | 18.3 | 2.09 | 3.85 | .03 | 1.50 | .23 | .07 | loss |
| 11/10/71 | 4.10 | 2.02 | 29.1 | 4.25 | 3.73 | 7.03 | .90 | 50.0 | 6.35 | 8.69 | 2.85 | 3.90 | 20.3 | 2.62 | 3.42 | .02 | 1.00 | .14 | .08 | |
| 1 CR = Concentration ratio--Ratio of concentration or property in net gain to that in irrigation water. 2 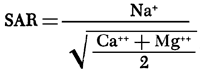 3 Reach 348.9-340.3. 4 Surveys made during irrigation season. |
||||||||||||||||||||
The specific conductance and the concentrations of most of the major ions in net gains generally increased progressively after 1964, but were not directly related to the rate of water discharge. Anomalously high concentrations were recorded for the survey in April 1964 when small net gains consisted chiefly of seepage. In October 1964, sharply lower concentrations in net gains that consisted mainly of tributary inflow (fig. 28) probably reflect the first arrival of excess water from irrigation of land on the high terrace. The concentrations of most of the ions in seepage (excluding sodium) exceeded the concentrations in tributary inflow, possibly as a result of transpiration of water by phreatophytes and evaporation of water from marshy areas near the river. Despite the paucity of sodium, the possibility can not be discounted entirely that seepage is augmented by saline water from abandoned wells that tap artesian aquifers at depth.
The relatively constant concentration ratios for calcium and sulfate suggest that the concentrations of those ions in net gains and in the irrigation water are related. The ratios for calcium were similar to those for the specific conductance which increased from 1.07 in November 1965 to 1.39 in November 1970, but the ratios for sulfate were less than one. Low ratios for sulfate, magnesium, and potassium indicate dilution, organic removal, or precipitation of the salts of those ions. Some of the magnesium and potassium may have replaced sodium in the soil by ion exchange.
Disproportionately high concentration ratios for sodium and chloride indicate a source for those ions that is supplementary to the irrigation water. Although the concentrations continued to increase through 1971, the trends in the ionic loads shown in figure 29 and in the ratios shown in figure 30 suggest depletion of that source. The ratios of the concentration of sodium to the concentration of chloride (in me/l) generally exceeded one; the sodium to chloride ratio in oil-field brine normally is less than one (Leonard, 1972). Recently produced brine does not seem to be the principal source of the sodium and chloride unless it has become enriched with sodium displaced from the soil by calcium and magnesium from the irrigation water. The ratios seem to substantiate the evidence given in preceding sections that naturally accumulated salts in the soil are the common source.
Figure 30--Concentrations and concentration ratios of ions in net gains in the irrigation reach during seepage-salinity surveys, 1964-71.
Concentrations of the other ions appear to have achieved some sort of temporary equilibrium with the environment at concentrations about equal to or less than those predicted from consumptive use (table 2). If meteorologic conditions, quality of the irrigation water, and management of the district do not change drastically, the specific conductance and concentrations of sodium and chloride in low flow should not significantly exceed the concentrations of ions in transient storage in the ground water or the maximums shown in figure 30 for extended periods of time as a result of irrigation.
Seven sets of samples were collected during periods of low flow at stations 355.8, 352.8, 347.4, 340.3, and 334.4 (pl. 1) between August 1964 and November 1967 for determination of pesticides and carbon extractables. Qualitative and quantitative analyses for halogenated and sulfur containing phosphatic organic and phenoxy-type pesticides plus carbon chloroform (CCE) and carbon alcohol extractables (CAE) were performed on 1-gallon (3.8-l) samples of river water and on packed granular carbon columns representing 100-gallon (380-l) volumes of river water. Analysis was by electron gas capture chromatography and micro coulometric titration gas chromatography at the Southeast Water Laboratory of the Environmental Protection Agency at Athens, Georgia. No identifiable pesticides were detected at low flow (Leonard and Stoltenberg, 1972, table 10); however, some pesticides may be carried from the irrigated area to the river by surface runoff resulting from heavy rainfall.
Only trace levels (less than 0.1 part per billion) of chlorinated hydrocarbons were reported by Knutson and others (1971, p. 25) in samples of water from the irrigation canal, Cedar Bluff Reservoir, the Smoky Hill River, and in runoff from Plot 1 collected between 1966 and 1969. Higher concentrations (up to 13 parts per billion) of Dieldren were found in the suspended sediment in surface runoff from plot 1. During 1969, no residues were detected in silt or water from either the reservoir or the river, perhaps as a result of the decreased use of chlorinated hydrocarbons.
Table 9--Non-silicate trace element content of composited samples of bed material, suspended sediment, and water at sites 338.0, 338.5, and 339.1 on the Smoky Hill River, April 4, 1971. [Analyses by E. A. Jenne, U.S. Geological Survey.]
| Type of sample | Particle size (microns) |
Percent of total sample |
Beryllium (Be) |
Cadmium (Cd) |
Cobalt (Co) |
Copper (Cu) |
Iron (Fe) |
Lead (Pb) |
Manganese (Mn) |
Mercury (Hg) |
Molybdenum (Mo) |
Nickel (Ni) |
Silver (Ag) |
Zinc (Zn) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration, in micrograms per gram1 | ||||||||||||||
| Bed material core 0-.5 inch |
>20 | 100 | 0.2 | 0.6 | 4.2 | <0.18 | 9,000 | 880 | 0.07 | 1.2 | 11 | <0.27 | 40 | |
| Bed material core 0-4 inches |
.45-20 | 2 | .09 | .4 | 2.4 | 10 | 5,100 | 1.7 | 1,200 | .13 | 1.2 | 7.6 | <.03 | 40 |
| 20-63 | 4 | .2 | .6 | 2.7 | 74 | 7,900 | 4.6 | 1,000 | 2.7 | 11 | 1.0 | 90 | ||
| 63-125 | 2 | .2 | 2.5 | 3.9 | 74 | 9,400 | 15 | 1,100 | 2.2 | 14 | .8 | 70 | ||
| 125-150 | 31 | .1 | .4 | 2.4 | 1.4 | 5,500 | <1.7 | 580 | 0.9 | 7.2 | .3 | 30 | ||
| 250-2,000 | 61 | .02 | .05 | .8 | <.03 | 1,600 | <0.5 | 130 | .2 | 1.7 | .1 | 6.5 | ||
| Suspended sediment | >.10 | 100 | .04 | 1.7 | 1.1 | 300 | 8,400 | 44 | 970 | .68 | 8.9 | 32 | .59 | 670 |
| Concentration, in micrograms per liter | ||||||||||||||
| Water2 | <0.5 | 1.4 | 0.7 | 7.9 | 20 | 7.2 | 50 | 0.3 | 2.4 | 5.7 | 0.8 | 17 | ||
| Suspended sediment | >.10 | .001 | .04 | .03 | 7.6 | 210 | 1.1 | 24 | .02 | .22 | .80 | .01 | 17 | |
| 1 Oven dried at 110°C. 2 Filtered through Gelman 0.1 micron membrane. Soluble organic material combusted by ultraviolet radiation prior to analysis. 3 Suspended sediment concentration 25 mg/l. |
||||||||||||||
Table 10--Average specific conductance and concentrations of selected ions in discrete samples from stations on the Smoky Hill River during water years 1966-71. [Samples collected when discharge <15 cfs at station 352.8, <20 cfs at station 345.5, and <30 cfs at station 334.4]
| Constituents | Number of analyses at stations | Mean at stations, in milligrams per liter |
Standard deviation at stations, in milligrams per liter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 355.9 | 352.8 | 345.5 | 334.4 | 355.9 | 352.8 | 345.5 | 334.4 | 355.9 | 352.8 | 345.5 | 334.4 | |
| Specific conductance (micromhos/cm at 25°C) | ||||||||||||
| 1966 | 13 | 29 | 34 | 888 | 879 | 897 | 880 | 29 | 39 | 38 | ||
| 1967 | 12 | 42 | 36 | 901 | 899 | 940 | 951 | 64 | 42 | 41 | ||
| 1968 | 16 | 26 | 17 | 898 | 938 | 980 | 982 | 28 | 37 | 39 | ||
| 1969 | 11 | 9 | 21 | 29 | 920 | 986 | 1,022 | 1,029 | 37 | 24 | 42 | 49 |
| 1970 | 22 | 24 | 23 | 24 | 971 | 1,035 | 1,092 | 1,082 | 52 | 81 | 47 | 66 |
| 1971 | 11 | 10 | 10 | 16 | 996 | 1,070 | 1,116 | 1,139 | 20 | 35 | 31 | 34 |
| Dissolved solids | ||||||||||||
| 1966 | 9 | 28 | 31 | 12 | 640 | 625 | 634 | 614 | 79.8 | 23.9 | 41.4 | 28.4 |
| 1967 | 10 | 45 | 36 | 12 | 637 | 640 | 664 | 649 | 36.7 | 18.8 | 33.3 | 29.3 |
| 1968 | 16 | 28 | 17 | 16 | 645 | 670 | 694 | 680 | 15.8 | 31.6 | 20.4 | 25.3 |
| 1969 | 21 | 29 | 30 | 17 | 663 | 702 | 708 | 713 | 24.3 | 24.5 | 57.2 | 39.2 |
| 1970 | 24 | 23 | 34 | 22 | 703 | 747 | 777 | 754 | 16.1 | 20.3 | 37.6 | 54.1 |
| 1971 | 8 | 9 | 25 | 10 | 720 | 776 | 797 | 791 | 16.0 | 14.0 | 16.0 | 26.0 |
| Calcium (Ca) | ||||||||||||
| 1966 | 12 | 9 | 28 | 31 | 117 | 123 | 120 | 119 | 12.9 | 7.5 | 11.9 | 10.0 |
| 1967 | 12 | 10 | 45 | 36 | 118 | 124 | 128 | 130 | 8.9 | 6.9 | 9.3 | 9.5 |
| 1968 | 16 | 16 | 29 | 17 | 119 | 129 | 133 | 129 | 4.3 | 9.7 | 7.3 | 11.1 |
| 1969 | 17 | 21 | 29 | 30 | 120 | 134 | 134 | 135 | 4.4 | 9.7 | 15.8 | 11.7 |
| 1970 | 21 | 24 | 23 | 34 | 126 | 145 | 149 | 141 | 8.6 | 14.4 | 9.7 | 10.3 |
| 1971 | 11 | 10 | 11 | 16 | 133 | 154 | 156 | 151 | 5.5 | 3.1 | 7.0 | 9.4 |
| Magnesium (Mg) | ||||||||||||
| 1966 | 12 | 9 | 28 | 30 | 26 | 21 | 22 | 20 | 4.9 | 3.2 | 3.5 | 2.9 |
| 1967 | 12 | 10 | 45 | 36 | 25 | 20 | 23 | 20 | 6.0 | 4.1 | 3.3 | 3.1 |
| 1968 | 16 | 16 | 29 | 17 | 25 | 23 | 25 | 22 | 2.9 | 2.8 | 2.2 | 4.1 |
| 1969 | 17 | 21 | 29 | 30 | 27 | 25 | 23 | 23 | 2.8 | 2.5 | 3.4 | 2.3 |
| 1970 | 22 | 24 | 23 | 34 | 28 | 25 | 26 | 24 | 3.5 | 3.6 | 2.9 | 2.8 |
| 1971 | 11 | 10 | 11 | 16 | 28 | 26 | 26 | 23 | 2.6 | 1.9 | 1.7 | 3.0 |
| Sodium (Na) | ||||||||||||
| 1966 | 12 | 9 | 19 | 22 | 36 | 37 | 39 | 39 | 2.4 | 3.5 | 4.4 | 1.8 |
| 1967 | 11 | 8 | 13 | 36 | 36 | 41 | 41 | 47 | 3.3 | 2.2 | 2.2 | 6.2 |
| 1968 | 16 | 15 | 10 | 17 | 36 | 41 | 46 | 51 | 8.1 | 3.1 | 5.0 | 4.0 |
| 1969 | 17 | 14 | 17 | 15 | 37 | 44 | 50 | 55 | 2.9 | 2.4 | 2.8 | 3.0 |
| 1970 | 16 | 17 | 17 | 19 | 41 | 47 | 56 | 60 | 5.5 | 4.8 | 4.6 | 3.6 |
| 1971 | 11 | 10 | 11 | 16 | 40 | 46 | 54 | 65 | 2.1 | 2.6 | 3.7 | 3.1 |
| Potassium (K) | ||||||||||||
| 1966 | 12 | 9 | 19 | 21 | 15 | 12 | 12 | 11 | 1.3 | 3.4 | 1.4 | 1.4 |
| 1967 | 11 | 8 | 13 | 11 | 14 | 12 | 11 | 9.8 | 1.1 | 2.2 | 1.2 | 1.2 |
| 1968 | 16 | 15 | 10 | 17 | 16 | 13 | 11 | 11 | 3.8 | 2.5 | 1.7 | 1.9 |
| 1969 | 17 | 14 | 14 | 15 | 17 | 12 | 12 | 11 | 1.6 | 1.0 | 1.2 | 1.6 |
| 1970 | 15 | 16 | 15 | 15 | 17 | 13 | 12 | 11 | 0.5 | 1.0 | 1.3 | 1.6 |
| 1971 | 11 | 10 | 11 | 16 | 17 | 13 | 12 | 11 | 1.1 | 1.2 | 1.3 | 1.6 |
| Bicarbonate (HCO3) | ||||||||||||
| 1966 | 12 | 9 | 28 | 31 | 167 | 196 | 190 | 192 | 20.8 | 24.5 | 28.3 | 28.7 |
| 1967 | 12 | 9 | 35 | 36 | 157 | 193 | 211 | 213 | 15.2 | 22.1 | 55.7 | 32.3 |
| 1968 | 16 | 16 | 22 | 27 | 151 | 191 | 206 | 210 | 9.2 | 32.3 | 33.6 | 30.0 |
| 1969 | 17 | 21 | 29 | 30 | 142 | 193 | 195 | 205 | 8.7 | 25.2 | 38.5 | 32.9 |
| 1970 | 21 | 23 | 22 | 33 | 148 | 211 | 227 | 217 | 12.9 | 19.5 | 21.1 | 30.4 |
| 1971 | 11 | 10 | 11 | 16 | 144 | 233 | 237 | 216 | 11.0 | 13.0 | 13.0 | 32.0 |
| Sulfate (SO4) | ||||||||||||
| 1966 | 13 | 9 | 31 | 31 | 320 | 280 | 280 | 250 | 47.3 | 7.0 | 20.2 | 15.3 |
| 1967 | 12 | 10 | 47 | 36 | 320 | 290 | 280 | 250 | 29.1 | 11.6 | 13.6 | 14.4 |
| 1968 | 16 | 16 | 29 | 17 | 330 | 310 | 300 | 270 | 13.2 | 21.7 | 24.2 | 25.0 |
| 1969 | 17 | 21 | 29 | 30 | 340 | 330 | 320 | 290 | 12.0 | 13.7 | 25.4 | 16.6 |
| 1970 | 22 | 24 | 23 | 34 | 370 | 350 | 330 | 300 | 8.7 | 35.8 | 12.1 | 29.2 |
| 1971 | 11 | 10 | 11 | 16 | 380 | 360 | 350 | 330 | 11.0 | 7.1 | 8.5 | 13.4 |
| Chloride (Cl) | ||||||||||||
| 1966 | 13 | 9 | 31 | 31 | 21 | 23 | 30 | 38 | 3.2 | 2.7 | 3.5 | 7.3 |
| 1967 | 12 | 10 | 47 | 35 | 21 | 24 | 39 | 56 | 1.2 | 1.2 | 4.7 | 11.2 |
| 1968 | 16 | 16 | 29 | 17 | 22 | 25 | 41 | 56 | 1.3 | 2.6 | 6.8 | 8.1 |
| 1969 | 17 | 21 | 28 | 30 | 23 | 26 | 44 | 62 | 1.2 | 1.4 | 5.8 | 6.1 |
| 1970 | 22 | 24 | 23 | 34 | 24 | 29 | 49 | 67 | 1.4 | 3.2 | 6.0 | 7.2 |
| 1971 | 11 | 10 | 11 | 16 | 25 | 30 | 49 | 73 | 1.3 | 1.5 | 3.2 | 4.7 |
| Nitrate (NO3) | ||||||||||||
| 1966 | 12 | 9 | 19 | 22 | 1.2 | 1.6 | 1.8 | 1.9 | 0.8 | 1.6 | 2.0 | 1.3 |
| 1967 | 11 | 9 | 24 | 11 | 0.9 | 0.7 | 0.7 | 1.7 | .4 | 0.4 | 0.4 | 0.8 |
| 1968 | 16 | 15 | 20 | 17 | .8 | 1.2 | 1.1 | 2.2 | .4 | 2.2 | .6 | 1.3 |
| 1969 | 17 | 14 | 16 | 15 | .6 | 1.8 | 1.1 | 2.5 | .5 | 1.2 | .3 | 1.6 |
| 1970 | 16 | 18 | 16 | 17 | .6 | 1.0 | 1.1 | 2.6 | .6 | .7 | .6 | 1.4 |
| 1971 | 11 | 10 | 11 | 16 | 1.1 | 1.6 | 1.6 | 3.5 | .5 | .4 | .7 | 1.3 |
| Phosphate (PO4) | ||||||||||||
| 1966 | 8 | 20 | 15 | 0.11 | 0.24 | 0.16 | 0.04 | 0.22 | 0.05 | |||
| 1967 | 8 | 7 | .18 | .13 | .14 | .01 | ||||||
| 1968 | 16 | 10 | 17 | .19 | .11 | .80 | .16 | .03 | .24 | |||
| 1969 | 8 | 7 | 8 | .12 | .10 | .12 | .07 | .04 | .03 | |||
| 1970 | 16 | 11 | 12 | 6 | .14 | 0.05 | .10 | .07 | .16 | 0.001 | .10 | .02 |
| 1971 | 11 | 10 | 11 | 16 | .10 | .10 | .10 | .10 | 0 | 0 | 0 | 0 |
| Boron (B)1 | ||||||||||||
| 1966 | 12 | 18 | 25 | 208 | 180 | 187 | 54 | 70 | 50 | |||
| 1967 | 11 | 9 | 11 | 215 | 211 | 183 | 52 | 60 | 43 | |||
| 1968 | 16 | 10 | 17 | 214 | 207 | 217 | 35 | 17 | 44 | |||
| 1969 | 11 | 8 | 15 | 14 | 187 | 199 | 215 | 214 | 43 | 30 | 14 | 63 |
| 1970 | 16 | 16 | 16 | 19 | 202 | 204 | 203 | 185 | 49 | 40 | 42 | 47 |
| 1971 | 11 | 10 | 11 | 16 | ||||||||
| Sodium-adsorption ratio (SAR) | ||||||||||||
| 1966 | 0.8 | 0.8 | 0.9 | 1.2 | ||||||||
| 1967 | .8 | .9 | .9 | 1.0 | ||||||||
| 1968 | .8 | .9 | 1.1 | 1.1 | ||||||||
| 1969 | .8 | 1.0 | 1.0 | 1.2 | ||||||||
| 1970 | .8 | 1.0 | 1.1 | 1.2 | ||||||||
| 1Concentrations of boron given in micrograms per liter. | ||||||||||||
On March 26, 1971, when the water discharge of the Smoky Hill River adjacent to the district was representative of the low flow that is exceeded about 60 percent of the time, samples of water, suspended sediment, and bed material were collected at three sites (338.0, 338.5, and 339.1). The non-silicate trace element content of composited samples (table 9) were analyzed by E. A. Jenne, U.S. Geological Survey (1971). The analyses indicate that appreciable quantities of trace elements are associated with sediment that would be incorporated in streamflow during periods of storm runoff. Anomalously high concentrations of copper in the suspended sediment probably were derived from copper-bearing algicides used in the canal. The chemistry of direct runoff from the district warrants additional study.
Data collected for the seepage-salinity surveys under idealized conditions during only 6 days are presumed to represent changes in the quantity and chemical quality of low flow during a period of more than 7 years. Efforts were made to standardize the data at the time of collection and analysis, but the effects of short-term fluctuations during the surveys and unmeasured variations could obscure significant relations. To describe the quality of flow in the river over a wider range of conditions, samples were collected for chemical analysis at monthly or more frequent intervals at five of the stations during water years 1966-71. Discharge measurements accompanied most of the samples.
In addition to the river mile designation used for stations in this report, some stations have been assigned a number (shown in parentheses) in downstream order according to the identification system used by the U.S. Geological Survey. Records for the Smoky Hill River near Schoenchen 344.4 (0686270) and for the Smoky Hill River at Cedar Bluff Dam 355.9 (0686200) are published in the series of annual reports "Water Resources Data for Kansas." Both stations are equipped with continuous stage recorders. A graphic conductivity recorder was maintained at the downstream station during water years 1966-70. Records for the two intermediate project stations 352.8 (0686210) and 345.5 (0686220) and for the Fish Hatchery outfall 355.8 are included in the basic data report (Leonard and Stoltenberg, 1972).
During infrequent periods of high runoff from the 220 square miles (570 km2) of drainage area to the reach of the river between the dam and station 334.4, contributions from the approximately 6,000 acres (2,400 ha) of irrigated land normally comprised an insignificant part of streamflow in the reach. The effects of storm runoff, accumulated salts flushed from the land surface outside of the irrigated area, and releases from the dam to the river far outweighed the effects of irrigation on the chemical quality of high flow.
Statistical analysis of the data for discrete samples from stations along the main stem showed that omission of a small number of samples collected at high rates of discharge drastically reduced the standard deviations from the means and the standard errors of estimate for linear and polynomial regressions relating discharge, specific conductance, and concentrations of the ions. The statistics shown in table 10 and related illustrations represent samples collected at rates of discharge less than 15, 20, and 30 cfs (0.42, 0.57, and 0.85 m3/s) at stations 352.8, 345.5, and 344.4, respectively. Statistics for station 355.9, at Cedar Bluff Dam, are included in table 10, but are generally omitted from the illustrations because the quality of water is similar to the irrigation water. The mean values shown for specific conductance and discharge probably are higher and lower, respectively, than the actual values, but they represent conditions that prevailed during more than 80 percent of the time.
In general, the mean specific conductance increased from year to year at each of the stations and in a downstream direction. The specific conductance of natural streamflow normally varies inversely with the rate of water discharge as a result of dilutant rainfall. However, the relations of specific conductance to water discharge shown in figure 31 indicate that the specific conductance (and concentration of dissolved solids) corresponding to a given rate of discharge increased from year to year. To minimize the effect of the progressive increase in the specific conductance of the irrigation water, the values shown in the figure are expressed as concentration ratios to the specific conductance of the irrigation water during the preceding irrigation season. At the downstream station 344.4, the ratios increased from 1.08 in 1966 to 1.25 in 1971, slightly less than the ratios for net gains in the irrigation reach during the seep age-s alinity surveys.
Figure 31--Relations of the average specific conductance (expressed as concentration ratios) to the corresponding water discharge for discrete samples at three stations on the Smoky Hill River for water years 1966-71.
The concentration ratios for calcium remained relatively constant and similar to the ratios for specific conductance at all three stations (fig. 32). The ratios for sulfate remained relatively constant from year to year at each station and decreased in a downstream direction to values less than one. The concentration ratios for sodium and chloride increased in a downstream direction to disproportionately high values with respect to specific conductance. The concentration ratios representing the mean concentration of chloride increased progressively through 1971, but the concentration ratios for sodium were exceeded during several preceding years. Changes in the other ions are shown in table 10.
Figure 32--Average concentration ratios for selected ions in discrete samples at three stations on the Smoky Hill River during water years 1966-71.
Despite minor discrepancies attributable to sampling over a wider range of discharge, the recorded changes substantiate the results of the seepage-salinity surveys. Inflow between the three stations varied widely in quantity and quality during each year. During periods of low to moderate flow, drainage from the irrigation district apparently was the major factor that determined the rate and quality of flow at station 344.4 near Schoenchen.Results of analyses of samples collected at semimonthly or more frequent intervals were combined with continuous records of stage and specific conductance to relate variations in chemical quality to water discharge and to time at station 334.4. Continuous specific conductivity records were available for water years 1966-70. A specific conductivity record for 1971 was synthesized by combining the specific conductance of samples collected at weekly or more frequent intervals with the continuous discharge records. Simulated records calculated from similar data collected during preceding years compared favorably with the actual records except during periods of extremely high discharge.
Relations of daily mean specific conductance to daily mean discharge and time (fig. 33) show that successively higher values of specific conductance generally prevailed for similar periods of time during water years 1966-71. Except during the dry 1968 water year, the specific conductance of streamflow exceeded the specific conductance of the irrigation water for progressively longer periods ranging from 82 percent of the 1966 water year to 97 percent of the 1971 water year. During the same periods, the water discharge was less than about 30 cfs (0.85 m3/s), the rate at which streamflow was evidently diluted by overland runoff or releases from the reservoir. Only during the 1966 and 1967 water years, when substantial releases were made to the river from the reservoir, did the daily mean discharge exceed 30 cfs (0.85 m3/s) more than 10 percent of the time. Largely because drainage from the irrigated land sustained base flow, the daily mean discharge equalled or exceeded 90 and 50 percent of the time remained relatively constant.
Figure 33--Daily mean specific conductance and daily mean discharge of the Smoky Hill River at station 334.4 near Schoenchen during 90, 50, and 10 percent of water years 1966-71.
Relations between specific conductance, concentrations of the ions, and discharge were poorly defined for the relatively few samples collected when the discharge exceeded 30 cfs (0.85 m3/s), because the high rates of discharge were normally associated with storm runoff. During each rise, the specific conductance corresponding to a given rate of discharge generally was higher than during the subsequent recession. Linear relations of the specific conductance of discrete samples to the corresponding rates of discharge equal to or less than 30 cfs (0.85 m3/s) (fig. 34) illustrate that the specific conductance (and concentration of dissolved solids) corresponding to a given rate of discharge increased progressively for water years 1966-71 despite random variations in precipitation. Although the coefficients of correlation are low, the maximum standard error of estimate is only about 6 percent of the corresponding mean specific conductance for each water year.
Figure 34--Relations of the specific conductance of discrete samples to the corresponding discharge of the Smoky Hill River at station 334.4 near Schoenchen for water years 1966-71.
During periods of stable flow, the specific conductance of discrete samples was similar to the daily mean specific conductance. The concentrations of the ions in discrete samples taken during those periods of each water year were related to the specific conductance of the samples by the least squares method using polynomial regressions of the third degree. The concentrations of the ions corresponding to the daily mean specific conductance E10, E50, and E90, shown in figure 33, were then determined from the regression equations.
Near equivalence of the sums of the cations to the sums of the anions (in me/l) for each of the synthetic analyses (table 11) determined from independent equations for each of the ions attests to the validity of the method and results. The values for the concentrations of the ions at E50 are nearly equal to the mean concentrations of the corresponding ions calculated directly from the analyses of the samples. The specific conductance, the sodium-adsorption ratio, and the concentration ratios for the ions corresponding to E50 are shown graphically in figure 35.
Table 11--Concentrations of the ions and percent composition of the ionic load corresponding to the daily mean specific conductance equalled or exceeded 10, 50, and 90 percent of the time in the Smoky Hill River at station 334.4 near Schoenchen during water years 1966-71. [Load computed using third-degree polynomial equations that define the relation of specific conductance (independent variable) to the concentration of the ions for discharges less than 30 cfs (0.85 m3/s).]
| Water year |
Specific conductance | Calcium (Ca) | Magnesium (Mg) | Sodium (Na) | Potassium (K) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 90 | 10 | 50 | 90 | 10 | 50 | 90 | 10 | 50 | 90 | ||||
| Micromhos per centimeter at 25°C |
Concentrations, in milligrams per liter | ||||||||||||||
| 1966 | 920 | 880 | 765 | 128 | 118 | 102 | 21 | 21 | 17 | 39 | 38 | 32 | 11 | 12 | 13 |
| 1967 | 940 | 920 | 730 | 128 | 125 | 109 | 20 | 20 | 17 | 46 | 45 | 36 | 10 | 10 | 10 |
| 1968 | 1,030 | 990 | 920 | 129 | 134 | 111 | 27 | 20 | 26 | 52 | 52 | 44 | 11 | 10 | 13 |
| 1969 | 1,040 | 980 | 880 | 139 | 124 | 104 | 23 | 23 | 21 | 56 | 52 | 46 | 11 | 12 | 9 |
| 1970 | 1,120 | 1,090 | 1,030 | 150 | 143 | 130 | 24 | 24 | 24 | 64 | 61 | 55 | 10 | 11 | 12 |
| 1971 | 1,195 | 1,135 | 1,070 | 161 | 149 | 139 | 23 | 23 | 24 | 68 | 66 | 61 | 10 | 12 | 11 |
| Sodium-adsorption ratio | Percent composition | ||||||||||||||
| 1966 | 0.9 | 0.9 | 0.8 | 63.5 | 61.7 | 62.4 | 16.9 | 17.8 | 10.6 | 17.0 | 17.5 | 19.4 | 2.7 | 3.0 | 7.7 |
| 1967 | 1.0 | 1.0 | .8 | 62.2 | 61.8 | 63.6 | 16.0 | 16.3 | 9.7 | 19.4 | 19.3 | 21.5 | 2.5 | 2.6 | 5.2 |
| 1968 | 1.1 | 1.1 | 1.0 | 57.5 | 61.6 | 57.5 | 19.8 | 15.2 | 13.3 | 20.2 | 20.8 | 22.8 | 2.5 | 2.4 | 6.4 |
| 1969 | 1.2 | 1.1 | 1.0 | 60.1 | 57.9 | 58.1 | 16.3 | 18.0 | 11.4 | 21.3 | 21.3 | 25.6 | 2.3 | 2.8 | 4.9 |
| 1970 | 1.3 | 1.2 | 1.2 | 60.1 | 59.4 | 58.2 | 15.7 | 16.3 | 17.5 | 22.2 | 22.0 | 21.5 | 2.0 | 2.3 | 2.8 |
| 1971 | 1.3 | 1.3 | 1.3 | 61.2 | 59.6 | 58.6 | 14.4 | 15.1 | 16.6 | 22.4 | 22.9 | 22.4 | 2.0 | 2.4 | 2.4 |
| Water year |
Dissolved solids (DS) | Bicarbonate (HCO3) | Sulfate (SO4) | Chloride (Cl) | Nitrate (NO3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 50 | 90 | 10 | 50 | 90 | 10 | 50 | 90 | 10 | 50 | 90 | ||||
| Concentrations, in milligrams per liter | |||||||||||||||
| 1966 | 635 | 613 | 538 | 215 | 186 | 164 | 256 | 253 | 219 | 39 | 39 | 32 | 2.0 | 1.8 | 1.1 |
| 1967 | 640 | 632 | 501 | 210 | 211 | 197 | 245 | 243 | 215 | 54 | 51 | 28 | 1.3 | 1.8 | 3.9 |
| 1968 | 696 | 687 | 648 | 211 | 223 | 147 | 292 | 264 | 292 | 55 | 59 | 44 | 2.3 | 2.4 | 1.4 |
| 1969 | 721 | 674 | 614 | 215 | 183 | 127 | 292 | 285 | 247 | 63 | 56 | 49 | 2.5 | 2.3 | 3.3 |
| 1970 | 782 | 759 | 714 | 237 | 220 | 189 | 306 | 304 | 292 | 72 | 69 | 61 | 3.9 | 3.0 | 1.8 |
| 1971 | 825 | 791 | 249 | 203 | 190 | 327 | 331 | 318 | 76 | 72 | 70 | 4.3 | 3.5 | 2.4 | |
| Percent composition | |||||||||||||||
| 1966 | 35.3 | 32.3 | 39.5 | 53.5 | 55.8 | 52.4 | 10.1 | 11.6 | 7.5 | 0.3 | 0.3 | 0.6 | |||
| 1967 | 34.1 | 34.7 | 44.4 | 50.5 | 50.7 | 48.5 | 15.2 | 14.3 | 6.2 | .2 | .3 | .9 | |||
| 1968 | 31.1 | 33.6 | 30.4 | 54.7 | 50.7 | 60.4 | 13.8 | 15.3 | 9.1 | .4 | 4 | .1 | |||
| 1969 | 30.8 | 28.4 | 23.5 | 53.3 | 56.2 | 59.8 | 15.5 | 15.0 | 15.9 | .4 | .4 | .8 | |||
| 1970 | 31.4 | 30.1 | 28.3 | 51.7 | 53.1 | 55.7 | 16.5 | 16.4 | 15.7 | .5 | .4 | .3 | |||
| 1971 | 31.1 | 27.0 | 26.5 | 52.0 | 56.0 | 56.4 | 16.4 | 16.5 | 16.8 | .5 | .5 | .3 | |||
Figure 35--Specific conductance, sodium-adsorption ratios, and concentration ratios of the ions that were equalled or exceeded 50 percent of the time in the Smoky Hill River at station 334.4 near Schoenchen during water years 1966-71.
Part of the general increase in the specific conductance and concentrations of the ions downstream from the district reflects similar changes in the quality of water in Cedar Bluff Reservoir. Although the concentrations of sodium and chloride ions increased appreciably as a result of irrigation, they remained well below recommended maximums for drinking water (Kansas State Board of Health, 1973). Concentrations of dissolved solids and sulfate generally exceed the recommended maximum limits in the irrigation water and in the river water downstream from the district.
Annual discharge at the gage near Schoenchen is a variable mixture of drainage from the irrigation district (including irrigation return flow) and a larger quantity of water of differing composition from other sources. The similarity of the curves shown in figure 35 to those shown in figure 30 for net gains indicate that drainage from the district measured during the seepage-salinity surveys continued during the rest of the year and was a major factor governing the chemical quality of water downstream. By use of simplifying assumptions, independent estimates of the quantity and quality of drainage attributable to irrigation during each year were calculated from records of streamflow in and releases to the river (net drainage), and from irrigation requirements and distribution of the irrigation water (consumptive use). Much of the data were collected as part of the normal operation and management of the reservoir and irrigation district. The results are compiled in table 12 for comparison with results from the seepage-salinity surveys.
Table 12--Calculated quantity and chemical quality of net drainage to the Smoky Hill River from Cedar Bluff Irrigation District during calendar years 1964-71. [Reach between fish-hatchery outfall (355.8) and the gage near Schoenchen (334.4).]
| Year | Method of computation |
Predicted annual concentration ratio CR=N/Xn |
Net drainage | Specific conductance micromhos/ cm at 25°C |
Concentrations, in milligrams per liter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cfs-days | Cfs | Silica (SiO2) |
Dissolved solids (calculated) |
Calcium (Ca) |
Magnesium (Mg) |
Sodium (Na) |
Potassium (K) |
Bicarbonate (HCO3) |
Sulfate (SO4) |
Chloride (Cl) |
Nitrate (NO3) |
Boron (B) |
Fluoride (F) |
||||
| 1964 | A1 | ||||||||||||||||
| B2 | 4.44 | 1,004 | 709 | 143 | 21 | 50 | 8 | 278 | 254 | 51 | 0.3 | ||||||
| C3 | 1.33 | 3,284 | 1,037 | 716 | 135 | 23 | 37 | 19 | 194 | 306 | 24 | .8 | 0.8 | ||||
| 1965 | A1 | 4,268 | 11.7 | ||||||||||||||
| B2 | 11.0 | 792 | 13 | 556 | 114 | 19 | 40 | 9 | 218 | 212 | 44 | .7 | |||||
| C3 | 1.11 | 4,394 | 966 | 5.9 | 654 | 124 | 22 | 35 | 18 | 178 | 300 | 22 | .8 | 0.28 | .7 | ||
| 1966 | A1 | 3,715 | 10.2 | 914 | 635 | 125 | 18 | 45 | 7 | 235 | 216 | 55 | 1.9 | ||||
| B2 | 10.7 | 116 | 20 | 42 | 8 | 172 | 235 | 57 | 2.0 | ||||||||
| C3 | 1.43 | 2,940 | 1,024 | 8.8 | 692 | 133 | 25 | 38 | 20 | 188 | 329 | 24 | 1.4 | .26 | .8 | ||
| 1967 | A1 | 3,986 | 10.9 | 1,085 | 640 | 125 | 20 | 48 | 8 | 221 | 234 | 56 | 2.1 | ||||
| B2 | 9.4 | 995 | 683 | 138 | 18 | 52 | 9 | 240 | 232 | 66 | 2.4 | ||||||
| C3 | 1.19 | 3,744 | 1,013 | 7.5 | 694 | 133 | 25 | 39 | 20 | 183 | 335 | 24 | 1.3 | .31 | .8 | ||
| 1968 | A1 | 3,990 | 11.0 | 1,086 | 640 | 122 | 17 | 53 | 5 | 223 | 218 | 67 | 2.1 | ||||
| B2 | 15.1 | 1,060 | 740 | 144 | 22 | 58 | 10 | 229 | 290 | 66 | 2.3 | ||||||
| C3 | 1.49 | 3,935 | 1,076 | 7.4 | 742 | 138 | 29 | 41 | 21 | 187 | 368 | 26 | 1.7 | .30 | .8 | ||
| 1969 | A1 | 5,519 | 15.1 | 1,019 | 740 | 127 | 21 | 56 | 8 | 206 | 264 | 69 | 3.0 | ||||
| B2 | 14.4 | 1,086 | 741 | 139 | 22 | 63 | 9 | 217 | 289 | 73 | 2.4 | ||||||
| C3 | 1.61 | 4,618 | 1,118 | 7.4 | 774 | 143 | 31 | 43 | 22 | 189 | 387 | 27 | 1.5 | .28 | .9 | ||
| 1970 | A1 | 4,838 | 13.3 | 1,054 | 790 | 138 | 22 | 62 | 9 | 215 | 280 | 74 | 3.3 | ||||
| B2 | 13.9 | 1,134 | 14 | 784 | 146 | 25 | 65 | 10 | 230 | 296 | 76 | 3.1 | .4 | ||||
| C3 | 1.50 | 4,750 | 1,154 | 7.5 | 804 | 149 | 32 | 45 | 23 | 191 | 404 | 28 | 1.4 | .28 | .9 | ||
| 1971 | A1 | 4,693 | 12.9 | 1,156 | 799 | 154 | 23 | 70 | 9 | 242 | 303 | 84 | 4.0 | ||||
| B2 | 14.1 | 1,218 | 18 | 790 | 161 | 22 | 72 | 9 | 240 | 325 | 84 | 3.8 | .5 | ||||
| C3 | 1.88 | 4,129 | 1,222 | 8.1 | 853 | 158 | 33 | 48 | 24 | 195 | 434 | 30 | 1.5 | .28 | 1.0 | ||
|
1 Net drainage. Streamflow at gage near Schoenchen (334.4) minus storm runoff, releases from dam to river, and waste from fish hatchery, irrigation canal, and laterals. No record before 1966. 2 See page 44 salinity survey. Net inflow to reach. Average of spring and fall surveys. 3 Consumptive use. Excess water (Ax) = inflow = excess rainfall (Pa - Pe) plus excess irrigation water (I-R) = Pa + I - C. (See table 2 and figure 9). Specific conductance of net drainage Ex = ΣEiN/ΣXn where Ei is the specific conductance of the irrigation water (see table 2). |
|||||||||||||||||
Streamflow at the gage near Schoenchen (334.4) consists of a variable high-flow component, and a relatively stable low-flow component that prevailed most of the time. The variable component consists mainly of storm runoff and direct releases to the river from Cedar Bluff Reservoir. Flow is mostly regulated by the dam, but the maximum discharge recorded near Schoenchen since the recording stage gage was installed in July 1964 was 20,400 cfs (580 m3/s) on June 14, 1970 when discharge recorded at the dam (355.9) was less than 5 cfs (0.14 m3/s) . As the scope of the project did not include adequate data collection for measuring the quantity and quality of storm runoff from the irrigated area, which represents less than 5 percent of the drainage area, efforts were confined to evaluation of the stable component of streamflow.
The stable component consists mainly of subsurface drainage of excess water from the irrigated cropland that is augmented by seepage from the dam, fish hatchery ponds, canal and laterals, bank storage, and a small amount of natural ground-water runoff not directly associated with irrigation. During the growing season, natural streamflow was depleted by evaporation, transpiration by phreatophytes and other vegetation that proliferated along draws, tributaries, and the river channel in response to the increased availability of water, and by withdrawals for irrigation. Depending on magnitude and timing, measured releases from the fish hatchery and waste from the canal and laterals augmented both stable and variable components.
Releases from the dam and fish hatchery were subtracted from the discharge measured at the gage near Schoenchen (334.4) for corresponding 5-day intervals during the period of record, 1965-71. The cumulation of differences against time (ΣQ) shown on figure 36 is a series of straight-line segments representing periods of relatively stable low flow separated by intervals during which the discharge fluctuated rapidly as a result of storm runoff. The slope of each of the linear segments presumably represents the rate of inflow to the reach between the fish hatchery and the gage. If the information collected during the seepage-salinity surveys applies to conditions that prevail during the remainder of each year, the inflow consisted chiefly of effluent ground water and waste from the irrigated area (the combination that conforms to the generally accepted definition of irrigation return flow). The hypothesis would be substantiated if the chemical quality of the inflow minus waste (hereafter termed net drainage, Xd) determined indirectly from the records at the gage near Schoenchen were similar to the net gains in the same reach during the seepage-salinity surveys.
Figure 36--Components of cumulative discharge of the Smoky Hill River at station 334.4 near Schoenchen, 1965-71.
By assuming that the average rate of ground-water inflow during each period of major fluctuation in discharge was equal to the mean of the rates prevailing before and after the period, cumulative net drainage (ΣXd) was calculated for the period of record. To illustrate the calculation graphically, successive linear segments were extended and translated vertically to form the relatively smooth curve that represents cumulative net drainage to the river with storm runoff, releases from the dam and fish hatchery, and canal and lateral waste (overflow) removed (fig. 36). The average daily net drainage ranged from 10.2 cfs (0.29 m3/s) per day in 1966 to 15.1 cfs (0.43 m3/s) per day in 1969. High values in 1965 and 1969 may reflect above average rainfall during those years. For all years, the values were lower than Q50 (fig. 34), but some were nearly equal to net gains measured for the reach between the fish hatchery outfall (355.8) and the gage near Schoenchen (334.4) during the seepage-salinity surveys (table 12).
The chemical discharge (or load) of the major ions that passed the gage during the periods of relatively stable flow, shown in figure 36, was calculated for each calendar year from the daily mean water discharge, the daily mean specific conductance, and regression equations relating the specific conductance to the concentrations of the ions in discrete samples. Digital-computer programs developed specifically for this phase of the project (written commun., D. Maddy, 1971) facilitated the lengthy calculations. The chemical discharge in measured releases from the dam, fish hatchery, and wasteways during the same periods was evaluated readily because the chemical quality was nearly similar to the irrigation water for each year (table 3). The differences between the chemical and water discharge at the station and in the releases and waste during the same periods are assumed to represent the chemical and water discharge of net drainage for those periods. The specific conductance and concentrations of the major ions in net drainage (table 12) were calculated from the differences.
Although the calculated values for specific conductance and concentrations of the ions in net drainage were derived by indirect methods using relatively imprecise data collected during the entire year, they are remarkably similar to the values for net gains between the fish hatchery (355.8) and the gage near Schoenchen (334.4) that were based on direct measurements during seepage- salinity surveys representing periods of one or two days under idealized conditions.
Evidently the results of the seepage-salinity surveys approximately represent conditions of low How throughout the year. On the other hand, unless the more detailed information provided by the surveys is required, changes in the quantity and chemical quality of drainage from the irrigated areas can be estimated from continuous records at the downstream site supplemented with data collected as part of the normal operation of the reservoir and irrigation district.
Theoretically, the quantity and quality of net drainage could be estimated from the consumptive use and distribution of precipitation and irrigation water (table 2) and the quality of the irrigation water (table 3) if there were no other sources of the ions or chemical reactions between the ions in the soils and aquifers. Results of the early seepage-salinity surveys justify the assumption that without irrigation there would be virtually no net drainage.
As shown by the cumulative curves in figure 36, net drainage for most years was nearly equal to the sum of the excess rainfall and irrigation water (Ax, table 2) on the irrigated acreage. Annual values for Ax are used for "inflow" in table 13. The empirical relation may be useful for prediction, but the implication that net drainage, including dissolved salts, originated only in the irrigated acreage is an oversimplification. Cumulative deliveries of irrigation water to the farms (ΣI) (table 2) were nearly equal to cumulative net drainage (ΣXd) (fig. 36) for the period 1965-71, but the similarity is probably coincidental because the annual values differed widely.
Excess input (Xn, table 2) to the system probably represents a more realistic estimate than Ax of actual recharge to the aquifers from which effluent ground water sustains net drainage. As the ground-water reservoir filled from 1965 to 1968, excess input (Xn) far exceeded net drainage. By the end of 1971, the cumulative excess input (ΣEn) exceeded cumulative net drainage (ΣXd) by less than 3 percent (fig. 36). If drainage from about 660 acres (270 ha) of land outside of the district that were irrigated by estimated diversions of about 870 acre-feet (1.1 hm3) per year (records of Kansas State Department of Agriculture) were added to cumulative net drainage, the difference would be even smaller. Therefore, ΣXn appears to be a reasonable estimate of ΣXd.
The specific conductance (Ex) and the concentrations of the ions in the excess input (Xn) were calculated using the expression
Ex = (ΣEiN) / (ΣXn)
as shown in table 2, and analogous expressions using the concentrations of the ions in the irrigation water. The specific conductance and the concentrations of dissolved solids and calcium were remarkably similar to corresponding values determined by the other two methods (table 12). However the relatively low predicted concentrations of sodium, chloride, bicarbonate, and nitrate substantiate the hypothesis that the higher concentrations of those ions in measured inflow to the river were derived from supplementary sources.
Lack of adequate data describing overland runoff precluded evaluation of a comprehensive salt balance for the district; however, the similarities described above indicate that the estimates of the quantity of water and salts in net drainage (Xd) (fig. 36) can be related with some degree of confidence to corresponding quantities in net input of irrigation water (N). Because most of the infiltrated water probably remained in the aquifers longer than one year, relations of cumulative input to cumulative drainage (fig. 37) probably are more significant than relations between annual values.
Figure 37--Cumulative loads of selected ions in cumulative net drainage (ΣXd) expressed as percentage of the corresponding ions in cumulative net input (ΣN) of irrigation water, 1966-71.
Cumulative net drainage of water was equal to about 60 percent of cumulative net input of irrigation water, but the percentages representing the various ions differed widely. As shown by the parallel configuration of the curves, the fluctuation in chemical discharge generally depended mainly on the water discharge. If all of the ions in net drainage were derived from the irrigation water, and there were no significant losses of ions to (or contributions from) the soil, aquifers, or storm runoff, the curves for all of the ions would coincide and approach 100 percent.
During the entire 1966-71 period, the chloride in net drainage exceeded the chloride in deliveries of the irrigation water, whereas the net input of magnesium, potassium, and sulfate in the irrigation water exceeded net drainage. More sodium, bicarbonate, and nitrate was carried out of the system in net drainage than was delivered in the irrigation water. The configuration of the curves shown in figure 37 substantiates the evidence from changes in the chemistry of the soil, ground water, and net gains during seepage-salinity surveys that net drainage consists mainly of effluent ground water containing accumulated sodium, chloride, and nitrate leached from the soil and underlying aquifers by excess irrigation water. Part of the calcium, magnesium, and sulfate carried by the irrigation water was transported to the river in overland runoff and part remains in the soil or ground-water reservoir. A relatively small part of the ionic load, including potassium, probably was consumed by vegetation.
With continued irrigation under existing conditions, exchangeable cations in the soil and irrigation water will reach unstable equilibrium, the soluble salts that accumulated in the soil and aquifers before irrigation will become depleted, and most of the altered ground water containing the leachate will have been displaced to the river. The quality of drainage from the irrigated land would then depend almost entirely on the quality and rate of application and consumptive use of the applied irrigation water. Unless conditions change drastically, the composition of ground water under the irrigated area and drainage from the irrigated land eventually should become nearly uniform and similar in composition to the applied water; however, the concentrations of the major ions should be from 1.5 to 2.0 times higher. The analyses of the soils and well waters show that significant quantities of soluble salts remain in transient storage, but that, with the exception of nitrate from fertilizer, they pose no significant threat to the downstream user.
Conversion from range land dryland farming to intensive cultivation under irrigation with water from Cedar Bluff Reservoir upset the natural equilibrium of the semiarid hydrologic environment of the area in and adjacent to the Cedar Bluff Irrigation District No. 6, west-central Kansas. Progressive changes in the chemical quality of ground water beneath the irrigated land and in water in the Smoky Hill River adjacent to the district depend on the composition and configuration of the geohydrologic system as well as on the composition, distribution, and consumption of the irrigation water.
The irrigated area is underlain by unconsolidated fluvial deposits of Pleistocene age that overlap and fill channels eroded in relatively impermeable limestone and shale of Cretaceous age. The Cretaceous rocks function chiefly as a lower boundary for ground-water circulation; however, included soluble minerals are a source of ions in the ground water.
The texture and related physical properties of the fluvial deposits and overlying soils vary widely, both vertically and laterally, but permeability is generally adequate to permit infiltration, deep percolation, and circulation of excess rainfall and irrigation water. Deposits of sand and gravel near the bedrock surface are the chief aquifers.
The water table in the valley-fill deposits under most of the irrigated land slopes gently to the south and east toward the Smoky Hill River. Erosional remnants of Cretaceous rock in some places retard the flow of water from the aquifer under the high terraces to the aquifer under the flood plain. Relief on the erosional surface affected the thickness and textural characteristics of the overlying sediments at the time of deposition, and is a significant factor in the thickness of the saturated section, the direction and rate of movement of ground water, and the distribution of waters of differing chemical composition.
During the period 1964-71, annual rainfall ranged from about 17 to 29 inches (43 to 74 cm). The mean was about 22 inches (56 cm), about 75 percent of which occurred during the growing season when consumptive use by crops exceeded rainfall. As the moisture deficiency was overcome by irrigation, an average of from 5 to 14 inches (13 to 36 cm) of excess water was available for leaching and recharge in the irrigated area. Part of the precipitation during the remainder of the year, as well as seepage from the irrigation canal and laterals, augmented the excess water available to percolate below the root zone and into the ground-water reservoir.
The specific conductance of the irrigation water generally increased progressively from about 800 to 980 micromhos per centimeter at 25°C between 1964 and 1971. Excepting a progressive increase in sulfate at the expense of bicarbonate, the relative proportions of the ions in the calcium-sulfate type water remained nearly constant. Sodium comprised less than 17 percent of the cations and the sodium-adsorption ratio (SAR) was less than 0.8. With moderate leaching, the water is well suited for irrigation of field crops in medium-textured soil.
Seasonal fluctuations in a generally rising water table reflected infiltration of excess water into and drainage from the aquifers beneath and adjacent to the irrigated land. Springs and marshes developed in some topographic depressions, but the water table under most of the irrigated land remained well below the root zone.
Based on rainfall, consumptive use, and the quality and quantity of the applied irrigation water, the average specific conductance of infiltrated water on the irrigated land should range from 1.2 to 3.2 times the specific conductance of the irrigation water. If seepage losses from the canal and laterals were included, the ratios would range from 1.1 to 1.9. To facilitate evaluation of the effect of the irrigation water on the quality of sampled water, the specific conductance and concentrations of the ions in well waters, soil extracts, and surface water are expressed as concentration ratios to corresponding quantities in the irrigation water.
The locally variable chemical composition of natural waters in the area was determined chiefly by soluble minerals in the soil and bedrock. With the addition of excess water from irrigation, the specific conductance of most of the well waters increased and calcium and sulfate became the predominant ions. Increases in the concentrations of sodium and chloride in many of the wells were disproportionately higher with respect to the other ions than would be predicted from simple mixing with irrigation water or from estimates based on consumptive use. Widespread distribution of significant amounts of chloride in transient storage in the aquifer suggested a more extensive and abundant source than the irrigation water or the known localized oil-field and livestock operations in the area.
Detailed information from three experimental plots that typify the varied geologic conditions beneath the irrigated land shows that the rate and magnitude of continuing changes in the quality of ground water under the irrigated area, and eventually in drainage to the river, are dependent on locally variable conditions that are not readily generalized. Annual applications of irrigation water exceeded the irrigation requirement for all three plots.
Plot 1, located near the northern boundary of the high terrace, is underlain by predominantly fine-grained colluvial and fluvial deposits with lenses of sand and gravel. The thickness of unconsolidated sediments increased from about 10 to 70 feet (3 to 21 m) in a southeastward direction. As a result of wide variation in the depth of the well screens and inhomogeneity of the aquifer, changes in water levels in the 14 observation wells were neither simultaneous nor of equal magnitude. Infiltrated calcium-sulfate type irrigation water evidently mixed with and displaced other types of water to the south and east. Tracer dye injected at the surface appeared in well waters down gradient, but residues of chlorinated hydrocarbon pesticides applied generously to crops remained in the upper foot of the soil profile.
Sodium and chloride in some well waters increased to concentrations far higher than in the irrigation water during the first two years of irrigation. Subsequent decreases, and convergence with time of the concentration ratios for the specific conductance and concentrations of most of the ions toward a common value less than two, indicated depletion of soluble salts in the soils and aquifers. The quantity and average concentration of chloride in the ground water decreased from 1965 to 1971, but the persistence of high concentrations of sodium in some well waters may represent continuing displacement of sodium from the fine-grained sediment by ion exchange for abundant calcium in the percolating waters. Significant increases in the concentrations of nitrate in 1969 probably reflect transport of fertilizer nitrogen below the root zone by excessive applications of irrigation water during the preceding growing season, but concentration ratios for potassium, another plant nutrient, were consistently less than one.
Plot 3 is located on the high terrace about 1.5 miles (2.4 km) south of and down the ground-water gradient from plot 1. The saturated zone lines entirely within relatively homogeneous sand and gravel that underlies 10 to 15 feet (3.0 to 4.6 m) of silt. From 1965 to 1971, the local water table rose about 5 feet (1.5 m) to a minimum recorded depth to water of 29 feet (8.8 m). In 1971, response of the water level to deep percolation in the center of the field lagged the start and finish of the irrigation by about 19 days.
The predominant ions in 11 closely spaced wells varied widely in 1965, but calcium and sulfate were the predominant ions in all the well waters by 1969. Progressive southeastward migration of water types, increases in specific conductance, and reduction of sodium-adsorption ratios in the well waters indicated infiltration to the aquifer of excess water carrying salts from the irrigation water and soil and the movement of ground water toward the river.
After 1968, the concentration ratios for specific conductance remained relatively constant between 1.5 and 2, but the ratios for chloride reached maximums of more than 10 (more than 260 mg/l) in 1969. Lack of a conspicuous tendency for the concentration ratios of the ions to converge toward a common value suggests that changes caused by irrigation in the plot are superimposed on and masked by changes caused by lateral migration of ground water from adjacent irrigated areas. Concentration ratios for sulfate and potassium consistently less than one indicate loss of those ions to plants and soil or dilution by waters containing lower concentrations of those ions than in the irrigation water. As in plot 1, leaching of fertilizer probably caused a significant increase in the concentration of nitrate in most of the well waters after 3 years of irrigation.
Plot 2 is located on a low terrace overlying a buried alluvial channel about 600 feet (180 m) north of the Smoky Hill River. Measured water levels fluctuated within a range of about 2 feet (0.6 m) at a depth of about 16 feet (4.9 m). Salts from the irrigation water and calcium and bicarbonate from the sandy alluvium seem to be the main sources of ions in the well waters. Other soluble salts probably were washed away before irrigation began. The concentration ratios for specific conductance ranged between 1.2 and 1.6, slightly lower than was predicted from estimates of rainfall and consumptive use. Concentration ratios for nitrate were variable, possibly as a result of the nitrogen-fixing activity of alfalfa. As was observed in the other plots, concentration ratios for potassium were less than one.
There was no evidence of significant leaching of sodium and chloride in the soil as was observed for the other plots. After 1968, the concentration ratios for chloride were less than one. The chemistry of the ground water beneath the plot may closely resemble the chemistry of ground water beneath the irrigated area (and drainage to the river) after prolonged application of irrigation water.
Particle-size analyses show that the soil profiles to a depth of 128 inches (325 cm) in plots 1 and 3 consist mainly of silt and clay and are similar to the soil profiles in adjacent nonirrigated areas. Chemical analyses of soil saturation extracts revealed leaching and translocation of soluble salts by percolating water. Calcareous zones in the profiles are continuing sources of calcium and bicarbonate in percolating water; therefore, the concentrations of those ions depend chiefly on the solubility of calcium carbonate. Concentration ratios less than one for specific conductance above the calcareous zone probably represent leaching by rainfall.
In plot 1, the concentration of soluble sodium in the extracts was only slightly lower, but the concentration of chloride was much lower in the irrigated than in the nonirrigated soil profile. In plot 3, high concentrations of sodium and chloride appear to have been displaced downward by excess water. High concentration ratios for nitrate near the surface of irrigated plots 1 and 3 reflect application of anhydrous ammonia; high ratios near the base of the sections probably represent fertilizer that was transported below the root zone by excess water.
Calcium, magnesium, and sulfate from the irrigation water apparently augmented the natural accumulation of salts in the upper part of the irrigated profile. At depths greater than 4 feet (1.2 m), the salt content of the irrigated profiles generally was less than for the corresponding nonirrigated profiles. If differences in the soluble salt content of the profiles actually represent leaching of salts by excess irrigation water, the concentrations of the drainage from plot 1 would have increased by about 240 mg/l for chloride and 40 mg/l for sodium during the period 1965-71. Comparable increases for plot 3 would be about 110 mg/l for both sodium and chloride.
The soluble cation content generally represented less than 10 percent of the extractable cation content of the profiles; the calculated increases for sodium based on differences in the extractable ion contents would be greater. Naturally accumulated salts leached from the soil profile apparently supplied enough sodium and chloride to account for the observed changes in the ground water beneath the plots.
The content of nitrate in the irrigated soil profiles was greater than for the nonirrigated profiles in all plots. In plot 1, the difference was equivalent to about 178 kilograms of nitrate (40 kg of nitrogen) per acre, mainly below the root zone. The increase, which probably is related to increased concentrations of nitrate in the well waters, may indicate excessive or poorly timed application of fertilizer, water, or both.
Because the approximately 6000 acres (2,400 ha) of irrigated land normally contributed an insignificant part of storm runoff from the 220 square miles (570 km2) of drainage area to the reach of the Smoky Hill River adjacent to the irrigation district, the effects of irrigation on the river were most conspicuous during periods of low flow. However, additional study of the quality of direct runoff from the district over a wide range of discharge is warranted.
Sixteen seepage-salinity surveys of the reach revealed progressive changes in the quantity and quality of low flow in the main stem, of tributary inflow, and of seepage directly into the main stem between 1964 and 1971. Accelerated drainage of ground water from the irrigated acreage progressively raised the concentration of chloride in a downstream direction to values far higher than chloride in the irrigation water. However, the concentration of sulfate generally decreased in a downstream direction from values higher to values lower than the irrigation water.
After 1965, net gains between successive stations during the surveys consisted mainly of ground-water discharge from the irrigated area north of the river. However, the rate of inflow per mile varied from reach to reach in response to local variations in the distribution of irrigation water and in the geohydrology.
Net gains in the irrigation reach that comprised from 30 percent of the inflow to the river between the fish hatchery and the downstream station (334.4) in 1964 to about 80 percent in 1967. From 1968 to 1971, the percentages ranged between 62 and 69 percent despite wide and relatively random variation in antecedent rainfall. The lowest net gain of 1.40 cfs (0.04 m3/s) was measured in October 1964. After 1967, net gains fluctuated between 8 and 10 cfs (0.23 and 0.28 m3/s) in the spring and fall, respectively, in response to fluctuations in the drainage of excess water from the irrigated acreage.
Net gains consist of measured tributary inflow plus net seepage. In April 1964, seepage was the major component of net gains. As water levels under the irrigated acreage on the high terrace rose in response to irrigation, tributary inflow comprised a larger percentage of net gains in the irrigation reach. In general, the concentrations of the ions in net seepage gains (excluding sodium) exceeded corresponding concentrations in tributary inflow. Net seepage losses represent unmeasured withdrawals of water or ions from the river or from tributaries downstream from data-collection stations. Cumulative net seepage losses for nitrate in the irrigation reach accompanied gains for water and the other major ions during all surveys. During the last four surveys, net seepage losses of from 45 to 53 kilograms of nitrate per day were recorded. Interception and consumption of more highly concentrated tributary inflow by phreatophytes on the flood plain and selective consumption of nitrate by terrestrial and aquatic plants probably caused the losses.
The chemical discharge in net gains to the irrigation reach (consisting mainly of irrigation return flow) increased from year to year between 1964 and 1971, but the rate of increase diminished as the system seemed to approach equilibrium. Calcium and sulfate were the predominant ions in the net gains, but the percent sulfate was lower and the percent sodium and chloride were higher than in the irrigation water. The specific conductance and concentrations of the ions generally increased progressively after 1964, but were not directly related to the rate of water discharge.
The specific conductance of water discharge in the irrigation reach increased from 880 to 900 micromhos per centimeter at 25°C in the fall of 1964. In the fall of 1971, the specific conductance in the same reach increased from 1,070 to 1,230 micromhos per centimeter at 25°C. The concentration ratio for specific conductance increased from 1.01 in 1964 to 1.15 in 1971.
Concentration ratios for calcium in net gains were similar to those for specific conductance, but the ratios were less than one for sulfate and potassium during 1965-71 and for magnesium during the last three years. Relatively small changes or declines in the disproportionately high concentration ratios for sodium and chloride after 1967 may indicate depletion of a source of those ions supplementary to the irrigation water. Readily apparent similarities of the changes in the chemistry of net gains to changes in the ground water show that the quality of low flow in the river depends to a large extent on the ions in transient storage in the aquifers receiving excess water from irrigation. Maximum concentrations of the ions in the ground water probably exceed maximums that will occur in the Smoky Hill River as a result of irrigation.
Chemical analyses of discrete samples representing a wide range of discharge at four stations on the river adjacent to the district generally substantiated information from the seepage-salinity surveys. The mean specific conductance at the three downstream stations and the specific conductance corresponding to a given rate of water discharge increased from year to year. Disproportionately high ratios for sodium and chloride and low ratios for sulfate characterized the waters containing drainage from the irrigated land.
At the station downstream from the district near Schoenchen (334.4), concentration ratios for the mean specific conductance increased from 1.08 in 1966 to 1.25 in 1971, slightly less than corresponding ratios for net gains in the irrigation reach during the seepage-salinity surveys. Successively higher values of specific conductance generally prevailed for progressively longer periods during water years 1966-71, but the discharge equalled or exceeded 50 and 90 percent of the time (Q50, Q90) remained relatively constant from year to year because drainage from the irrigated land sustained base flow during dry periods.
The concentrations of the ions corresponding to measured values of daily mean specific conductance that were equalled or exceeded 10, 50, and 90 percent of the time (E10, E50, E90) during each water year were calculated using polynomial regression equations relating the concentrations of the ions to the specific conductance of discrete samples. Although the concentrations of some ions, notably sodium and chloride, increased appreciably as a result of irrigation, they remained well below recommended maximums for drinking water. The concentrations of dissolved solids and sulfate exceeded recommended maximums even before irrigation began.
Estimates of the quantity and quality of annual net drainage (drainage excluding waste and storm runoff) from the irrigated land based on releases to the river and on the data collected at the gage near Schoenchen (334.4) are remarkably similar to net gains between the fish hatchery and the gage during the seepage-salinity surveys. Reasonable estimates evidently can be made using either of the two methods. During periods of stable streamflow, the estimated average net drainage from the district ranged from 10.2 cfs (0.29 m3/s) per day in 1966 to 15.1 cfs (0.43 m3/s) per day in 1970.
The quantity and quality of net drainage from the district also was estimated from the distribution and consumptive use of precipitation and irrigation water. Annual values for excess water on the irrigated acreage were generally similar to the magnitude of net drainage estimated from the continuous records at the gage near Schoenchen. However, the chemistry of net drainage, and comparison of cumulative values indicates that excess input of irrigation water to the system (including seepage from the canal and laterals) is a more realistic estimate of net drainage than is the excess water on the irrigated acreage. The predicted values for specific conductance and the concentrations of dissolved solids and calcium were similar to corresponding values determined by the other two methods, but the concentrations of sodium, chloride, bicarbonate, and nitrate were much lower.
During the period 1966-71, net input of chloride to the system was less than net drainage, whereas net input of magnesium, potassium, and sulfate delivered in the irrigation water exceeded net drainage. From 1969 to 1971, more sodium, bicarbonate, and nitrate were carried out of the system in net drainage than was delivered in the irrigation water.
In 1971, significant quantities of soluble salts remained in transient storage in the aquifers beneath and adjacent to the irrigated land. With continued irrigation the supply of natural salts in the soil and aquifers will be depleted and the quality of drainage from the irrigated land will depend chiefly on the quality, distribution, and consumption of the irrigation water. If conditions do not change drastically, the composition of ground water under the irrigated land and drainage to the river will eventually become more uniform and similar to the irrigation water. However, the concentrations of the major ions probably will be from 1.5 to 2 times higher than in the irrigation water. Phreatophytes, which have proliferated in response to the increased availability of water may significantly reduce recharge to the ground-water reservoir from excess water. Use of water for irrigation and augmentation of base flow by drainage from the irrigation district have not caused serious degradation of the chemical quality of streamflow in the Smoky Hill River.
American Public Health Association and others, 1971, Standard methods for the examination of water, sewage, and industrial wastes: 13th ed., New York, Am. Public Health Assoc. Inc., 874 p.
Bidwell, O. W., 1956, Major soils in Kansas: Kansas Agr. Expt. Sta., Circ. 336, 16 p.
Blaney, H. F., and Criddle, W. D., 1950, Determining water requirements in irrigated areas from climatological and irrigation data: U.S. Dept. Agriculture, Soil Conserv. Serv., Research Div. of Irrig. and Water Conserv., 48 p.
Byrne, F. E., Coombs, V. B., and Bearman, C. H., 1947, Construction materials in the Cedar Bluff area, Trego County, Kansas: U.S. Geol. Survey Circ. 15, 21 p.
Eldridge, E. F., 1960, Return irrigation water-characteristics and effects: U.S. Public Health Serv., Portland, Ore., 120 p.
Hanson, R. E., and Meyer, W. R., 1953, Irrigation requirements, estimates for Kansas: Kansas State Univ. Bull. 69, 24 p.
Harbeck, G. E., Jr., and Meyers, J. S., 1970, Present day evaporation measurement techniques: Hydraulics Div. jour., July, p. 1381-1389.
Herpich, Russell, and McKinney, R. D., 1959, Irrigation farming for profit: Kansas Agr. Expt. Sta. Circ. 372, 34 p.
Hodson, W. G., 1965, Geology and ground-water resources of Trego County, Kansas: Kansas Geol. Survey Bull. 174, 80 p. [available online]
Jackson, M. L., 1958, Soil chemical analysis: Englewood Cliffs, N.J., Prentice-Hall Inc., 498 p.
Jacobs, H. S., 1963, Irrigation water quality charts for Kansas: Kansas Agr. Expt. Sta. Tech. Bull. 133, 16 p.
Jacobs, H. S., Dixon, R. M., Lynn, W. C., and Naddih, B. I., 1964, Irrigation effects on Kansas soils: Kansas Agr. Expt. Sta. Tech. Bull. 134, 94 p.
Jacobs, H. S., Naddih, B. I., and Dixon, R. M., 1961, Correlation between constituents in irrigation waters and irrigated soils in Kansas: Soil Sci. Soc. America Proc., v. 25, no. 5, p. 404-407.
Jacobs, H. S., and Whitney, D. A., 1968, Determining water quality for irrigation: Kansas State Univ., Coop. Ext. Serv., Aug., 22 p.
Kansas State Board of Agriculture, 1968, Procedure for determining quantities of water for irrigation use: Water, Oct., 26 p.
Kansas State Board of Health, 1973, Water quality criteria for interstate and intrastate waters of Kansas: Kansas State Board of Health Regulations 28-16-28, 5 p.
Kansas State University, Department of Economics, 1970, Economics of irrigation: Kansas State Univ., Coop. Ext. Serv., Sept., 22 p.
Kansas Water Resources Board, 1967a, Irrigation in Kansas--Planning for development, 701 Project, Kansas, No. P-43: Kansas Water Resources Board Rept. 16(e), 233 p.
Kansas Water Resources Board, 1967b, A Kansas water atlas: Kansas Water Resources Board, 701 Project, Kansas, Dec., 57 p.
Knutson, Herbert, Kadoum, A. M., Hopkins, T. L., Swoyer, G. F., and Harvey, T. L., 1971, Insecticide usage and residues in a newly developed Great Plains Irrigation District: U.S. Public Health Serv., Pesticides in Soil, v. 5, no. 1, p. 17-27.
Leonard, A. R., and Berry, D. W., 1961, Geology and groundwater resources of southern Ellis County and parts of Trego and Rush Counties, Kansas: Kansas. Geol. Survey Bull. 149, 156 p.
Leonard, R. B., 1969a, Effect of irrigation on the chemical quality of low streamflow adjacent to Cedar Bluff Irrigation District, Kansas--a progress report: Kansas State Dept. Health Bull. 1-10, 17 p.
Leonard, R. B., 1969b, Variations in the chemical quality of ground water beneath an irrigation field, Cedar Bluff Irrigation District, Kansas--an interim report: Kansas State Dept. Health Bull. 1-11, 20 p.
Leonard, R. B., 1970, Effect of irrigation on the chemical quality of ground and surface water, Cedar Bluff Irrigation District, Kansas; in, Relations of agriculture to soil and water pollution: Cornell Univ. Agr. Waste Management Conf., Rochester, N.Y., Jan., p. 147-163.
Leonard, R. B., 1972, Chemical quality of water in the Walnut River Basin, south-central Kansas: U.S. Geol. Survey Water-Supply Paper 1982, 109 p.
Leonard, R. B., and Morgan, C. O., 1970, Application of computer techniques to seepage-salinity surveys in Kansas: Kansas Geol. Survey, Spec. Distrib. Pub. 47, 44 p.
Leonard, R. B., and Stoltenberg, G. A., 1972, Compilation of data for water-quality investigation, Cedar Bluff Irrigation District, Kansas: Kansas State Dept. Health Bull. 1-12, 14 p., 10 tables.
Lowell, B. H., Morgan, C. O., and McNellis, J. M., 1971, Brief descriptions of and examples of output from computer programs developed for use with water data in Kansas: Kansas Geol. Survey, Spec. Distrib. Pub. 28, 54 p.
Musick, J. T., and Grimes, D. W., 1961, Water management and consumptive use by irrigated grain sorghum in western Kansas: Kansas Agr. Expt. Sta. Tech. Bull. 113, 20 p.
Reeve, R. C., and Fireman, Milton, 1967, Salt problems in relation to irrigation; in, Irrigation of Agricultural Lands: Am. Soc. Agronomy no. 11, p. 988-1008.
Riggs, H. C., 1966, Frequency curves: U.S. Geol. Survey, Techniques of Water Res. Inv., Bk. 2, Ch. 3, 17 p.
Searcy, J. K., and Hardison, C. H., 1960, Double-mass curves, in Manual of Hydrology: Pt. 1, General Surface Water Techniques: U.S. Geol. Surve, Water-Supply Paper 1541-B, 66 p.
Sylvester, R. O., and Seabloom, R. W., 1963, Quality and significance of irrigation return flow: Irrig. and Drainage Div., Am. Soc. Civil Engineers jour., v. 89, no. IR3, paper 3624, 27 p.
U.S. Bureau of Reclamation, Region VII, 1958 (Rev. 1960), Definite plan report, Cedar Bluff Unit, Kansas: General plan of development, Kansas River Projects, v. 1, open-file report, 112 p.
U.S. Bureau of Reclamation, Region VII, 1963, Reservoir management plan, Cedar Bluff Reservoir, Kansas: Kansas River Projects, McCook, Nebr., open-file report, 175 p.
U.S. Bureau of Reclamation, Region VII, 1963-71, Monthly water distribution report for the Cedar Bluff Irrigation District, 1963-71: Form 7-322: U.S. Dept. Interior, v. 3, p. 422.
U.S. Department of Agriculture, 1954, Quality of irrigation water, Diagnosis and improvement of saline and alkali soils: U.S. Dept. Agriculture Handbook no. 60, p. 6982.U.S. Department of Agriculture, Soil Conservation Service, 1962, Identification and nomenclature of soil horizons: Soil Survey Manual, Supplement to U.S. Dept. Agriculture Handbook no. 18, p. 173-188.
U.S. Department of Commerce, 1964-72, Climatological data, Kansas, Annual summaries, 1963-71: U.S. Dept. Commerce, National Oceanic and Atmospheric Admin., Environmental Data Serv.
U.S. Geological Survey, 1970, Methods for collection and analysis of water samples for dissolved minerals and gases: U.S. Geol. Survey, Techniques of Water Res. Inv., Bk. 5, Ch. 1, 160 p.
U.S. Geological Survey, 1972, Streamflow and ground-water conditions: Water Resources Review, Apr., 10 p.
U.S. Office of Water Resources Research, 1967, Water Resources Research catalog, Project no. 5.0465: U.S. Dept. Interior, v. 3, p. 422.
Utah State University Foundation, 1969, Characteristics and pollution problems of irrigation return flow: U.S. Dept. Interior, Fed. Water Pollution Control Admin., Ada, Okla., 237 p.
Wilcox, L. V., 1963, Salt balance and leaching requirement in irrigated lands: U.S. Dept. Agriculture Tech. Bull. 1290, 23 p.
Wilcox, L. V., and Durum, W. H., 1967, Quality of irrigation water; in, Irrigation of Agricultural lands: Am. Soc. Agronomy, no. 11, p. 104-120.