
Kansas Geological Survey, Bulletin 96, pt. 7, originally published in 1952

Originally published in 1952 as Kansas Geological Survey Bulletin 96, pt. 7. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (4 MB) is also available.
The development of the lime-sinter process of extracting alumina from clay and the potential use of this method for beneficiation of Kansas refractory clays have prompted the Geological Survey to make further investigations of alumina extraction from Kansas clays. The Geological Survey's original studies of the soda-lime-sinter process were conducted under conditions of stress during World War II and were directed exclusively toward the ultimate production of aluminum metal. The present study tests the applicability of the lime-sinter process to Kansas clays and is directed primarily toward the development of high-alumina refractory materials with the possible utility of Kansas clay as a source of ore for aluminum metal as a secondary consideration. Laboratory tests indicate that Kansas clays are amenable to treatment by the lime-sinter process for both of these ultimate products, and that 80 to 84 percent of the alumina contained in the samples tested can be extracted as a high-grade product by this relatively simple process.
In 1943 the State Geological Survey of Kansas published a report on the results of studies directed toward the development of a method of extracting alumina from Kansas clay (Kinney, 1943). This early investigation was prompted by conditions existing during the war years, and although the experimental work was successful it did not lead to commercial development. During the subsequent years additional work has been carried on intermittently on this same problem.
The present investigation was undertaken to determine what extraction of alumina of metallurgical grade could be made from Kansas clay using the lime-sinter process (Archibald and Jackson, 1944) followed by purification of the resulting extract. An important new factor prompting further study of this problem was the desire to determine the utility of the process as an inexpensive method for the manufacture of high-alumina refractories. Such refractories may be produced by using alumina of lower grade obtained by this process, in raising the alumina content of ordinary fire clay to a suitable composition. There are extensive clay deposits in Kansas suitable for this method of treatment.
The production of aluminum in the United States has increased from 175,000 tons in 1939 to an output of about 1,200,000 tons in 1951. Bauxite, the common ore, contains 40 to 60 percent alumina (Al2O3). To extract aluminum from bauxite or from clay (which has only half the alumina content of bauxite) it is first necessary to separate alumina, which is then subjected to electrolysis in an electric furnace for reduction to metal. Conventional smelting cannot be employed. After calcination the ore is treated by wet methods to obtain alumina. Bauxite is leached directly, but clay commonly must be sintered with fluxes to form a soluble aluminate before leaching.
Because of its higher alumina content and cheaper processing, bauxite is the ore for practically all aluminum production at the present time. However, on account of the rapid depletion of bauxite deposits in this country, attention in recent years has been directed to the possible utilization of clay to augment production. Bauxite deposits in the United States are estimated at only 60 million tons, of which 4 million tons are high grade (Reynolds, 1951). Yearly consumption is 7 million tons, more than half of which is imported from the Guianas, South America. The condition of bauxite reserves has been changed recently with the discovery in Haiti of what is claimed to be the world's largest deposits of high-grade ore, reported as 350,000,000 tons (Reynolds, 1951). While cheap water transportation is available from Dutch Guiana to Mobile, Alabama, a distance of 2,500 miles, cargoes must be transshipped from shallow river boats to deep-water vessels at Trinidad. From Haiti the distance is only 1,000 miles and no transshipment is required. Increased supplies of bauxite may be expected from Haiti for many years.
Although the easing of the bauxite ore situation may temporarily delay any program for the use of clay as an ore of alumina, there is little doubt as to its eventual use. Furthermore, if during an emergency foreign sources of bauxite are closed to us, it will certainly become necessary to utilize clay as a substitute source. It is judged that the most eminent value of the present study will be in its application to the production of refractories.
Thanks are expressed to Norman Plummer and William Hladik, who supplied clay samples and information on the beneficiation of clay, and to Russell Runnels and staff of the geochemistry laboratory who made all chemical analyses.
Clays typical of known Kansas high-alumina deposits were selected for study (Plummer and Romary, 1947). Samples of clay from four localities in Ellsworth, Ottawa, and Wilson Counties, Kansas, were used. The location and laboratory number of these clay samples are given in Table 1. For comparison purposes samples of commercial-grade Georgia kaolin were included in all tests.
Table 1—Kansas clays used in the investigation
| Clay no. | Location | County | Type of clay |
|---|---|---|---|
| O-38-4 | NW NW 8-9-2W | Ottawa | Kaolinitic |
| EL-69-2 | W2 NW 30-15-6W | Ellsworth | Kaolinitic |
| EL-60-6 | SW 19-15-9W | Ellsworth | Kaolinitic |
| WL-1 | Excelsior Brick Co., Fredonia, Kansas |
Wilson | Illite |
Clay from locality O-38-4 was tested in both the natural state and after beneficiation by sedimentation. Clay samples from Ellsworth County (EL-69-2 and EL-60-6) were beneficiated. Wilson County clay (WL-1) was tested both in the natural state and after leaching with hydrochloric acid to remove some iron. The Georgia kaolin was used in the natural condition. All samples were calcined at 800° C. as a preliminary operation.
Chemical analyses of clay samples are shown in Table 2. Certain impurities present in the clay or flux used in the lime-sinter process may seriously affect extraction of alumina (Grim, Machin, and Bradley, 1945). Quantities of magnesium oxide (MgO) in the sinter in amounts greater than 2 percent and of phosphorous pentoxide (P2O5) greater than 0.3 percent are particularly damaging. Ferric oxide (Fe2O3) can be tolerated to about 3 percent. Except for the illite type, Kansas clays are relatively free from excessive amounts of impurities.
Table 2—Chemical composition of samples used in the study. (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Clay | Treatment | SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | SO3 | MgO | P2O5 | K2O* + Na2O |
Loss on ignition, 1000° C. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O-38-4 | Dried 140° C. | 64.78 | 22.24 | 0.27 | 1.58 | 1.32 | 0.05 | 0.66 | Nil | 1.66 | 7.49 |
| O-38-4 | Calcined 880° C. | 70.02 | 24.04 | 0.29 | 1.71 | 1.43 | 0.05 | 0.71 | Nil | 1.79 | |
| O-38-4 | Concentrated | 50.20 | 32.26 | 0.43 | 2.34 | 1.23 | 0.79 | 2.11 | 10.64 | ||
| O-38-4 | Conc. & calcined | 56.17 | 36.11 | 0.48 | 2.62 | 1.37 | 0.88 | ||||
| EL-69-2 | Dried 140° C.' | 66.08 | 23.70 | 2.03 | 0.46 | *** | 6.62 | ||||
| EL-69-2 | Concentrated | 49.29 | 33.03 | 0.37 | 2.14 | 1.13 | 0.97 | 2.13 | 10.91 | ||
| EL-69-2 | Concentrated** | 47.75 | 32.23 | 0.30 | 2.12 | 1.20 | 0.05 | 0.71 | 0.05 | 2.82 | 12.82 |
| EL-69-2 | Conc. & calcined | 55.33 | 37.07 | 0.41 | 2.41 | 1.27 | 1.08 | ||||
| EL-69-2 | Conc. & calcined** | 54.73 | 36.94 | 0.34 | 2.43 | 1.38 | 0.06 | 0.81 | 0.06 | 3.23 | 0.07 |
| EL-60-6 | Dried 140° C. | 71.80 | 19.12 | 0_18 | 0.88 | 1.30 | 0.16 | 0.82 | 6.05 | ||
| EL-60-6 | Concentrated | 46.92 | 36.53 | 0.19 | 1.42 | 1.57 | 0.12 | 13.58 | |||
| EL-60-6 | Concentrated** | 46.22 | 35.58 | 0.12 | 1.30 | 1.79 | 0.05 | 0.07 | 0.03 | 0.31 | 14.59 |
| EL-60-6 | Conc. & calcined | 54.30 | 42.27 | 0.22 | 1.64 | 1.81 | 0.14 | ||||
| EL-60-6 | Conc. & calcined** | 53.56 | 41.23 | 0_14 | 1.51 | 2.07 | 0.06 | 0.07 | 0.03 | 0.36 | 0.88 |
| WL-1 | Dried 140° C. | 55.94 | 22.42 | 0.49 | 7.47 | 1.76 | 1.93 | 0.20 | 2.44 | 7.40 | |
| WL-1 | Calcined | 60.41 | 24.21 | 0.53 | 8.07 | 1.90 | 2.08 | 0.20 | |||
| WL-1 | Leached & calcined | 63.97 | 25.67 | 0.56 | 2.64 | 2.01 | 2.21 | ||||
| Georgia kaolin | Dried 140° C. | 44.67 | 38.40 | 0.19 | 1.97 | 14.75 | |||||
| Georgia kaolin | Calcined | 52.40 | 45.04 | 0.22 | 2.31 | ||||||
| * Undetermined difference ** 1951 samples *** Reported with Al2O3. |
|||||||||||
The lime necessary for the chemical reactions in the sinter is added in the form of calcium carbonate. For this study commercial grades of ceramic whiting were used. However, deposits of high-purity limestone suitable for this purpose are available in Kansas (Runnels, 1951).
The amount of silica present as an impurity in the calcium carbonate must be known so that suitable corrections for it can be made in the process. Chemical analyses of the two batches of whiting used are given in Table 3. Whiting no. 1 was used in sinters 1 to 21 (Table 6), whereas whiting no. 2 was used in all other tests.
Table 3—Analyses of whitings used in the tests
| Constituent | No. 1 | No. 2 |
|---|---|---|
| Percent CaCO3 | 98.40 | 98.00 |
| Percent SiO2 | 0.63 | 1.83 |
The beneficiation of raw clay raises the amount of alumina in the product to be treated by eliminating some of the silica and concentrating the alumina in a mass weighing less than the original clay. It reduces the amount of material to be processed for a given amount of alumina, saves on freight and fluxes, and the beneficiated clay yields a higher percentage of its alumina on extraction than does untreated clay. This is shown by a comparison of extraction results for clay O-38-4 in both the beneficiated and unbeneficiated condition (Table 6). The extraction of 80 to 84 percent alumina mentioned in this bulletin is from beneficiated clay.
The main constituents of the clay. studied are quartz (SiO2) and kaolinite (Al2O3 · 2SiO2 · 2H2O) with specific gravities of approximately 2.65 and 2.61 respectively. Quartz occurs in granular form, kaolinite more or less in thin flakes. Beneficiation is possible by reason of the slower settling rate of kaolinite compared to quartz when the clay is ground, agitated in water, and allowed to settle. Quartz tends to concentrate at the bottom of the settler, kaolinite in the supernatant emulsion. The latter is drawn off and the clay, now enriched in alumina, is recovered by prolonged settling. Clay can also be concentrated in classifiers or centrifuges. Results are influenced by the ratio of quartz to kaolinite and by the grain size of the clay treated. The greater the amount of quartz and the finer its size, the poorer the concentration. Usually the alumina in the concentrate is increased 30 to 40 percent.
Beneficiation of clay in its present state of development is by no means a perfect process. The ratio of concentration is in the order of 2 to 2.5 of clay to 1 of concentrate; not in itself a great handicap, as clay at the mine is cheap, but costs rise with increase in amount of raw material processed for a given amount of product. A redeeming feature is that the residues, or tailings, still contain enough clay to be plastic and can be used in the manufacture of face brick or siliceous fire clay brick.
Concentrated clays mentioned in this report were prepared in the Geological Survey laboratory using agitation in water followed by settling in glass cylinders. A very small amount of ammonium hydroxide was added to help deflocculate the clay. Some results are shown in Table 4.
Table 4—Results of beneficiation tests on three Kansas clays
| No. | Original composition, percent |
Weights of agitation mixture, percent |
Concentrate | Tailings | Settling time, hours |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Percent of original clay |
Al2O3 percent |
Percent of original clay |
Al2O3 percent |
|||||||
| Al2O3 | SiO2 | Clay | Water | NH4OH | ||||||
| O-38-4 | 22.24 | 68.74 | 13.00 | 86.9 | 0.01 | 52 | 36 | 48 | 19 | |
| EL-69-2 | 23.70 | 66.08 | 13.00 | 86.7 | 0.03 | 40 | 60 | 22 | ||
| EL-60-6 | 19.12 | 71.80 | 16.70 | 83.2 | 0.01 | 35 | 65 | 23 | ||
The lime-sinter process consists of heating a mixture of lime and calcined clay to a sintering temperature, removing the soluble calcium aluminate by leaching with sodium carbonate solution, and precipitating the alumina with carbon dioxide gas.
The ground siliceous alumina-bearing ore and ground calcium carbonate are mixed in calculated amount (ignoring volatile constituents) to give a mixture corresponding in percentage composition to dicalcium silicate (2 CaO . SiO2) and pentacalcium trialuminate (5 CaO · 3 Al2O3), or other calcium aluminates approaching it in composition. The dicalcium silicate gram mole ratio (CaO : SiO2 :: 2 : 1) is essential for the success of the process. The calcium aluminate, depending on the ore, may be pentacalcium trialuminate, with a mole ratio (CaO : Al2O3 :: 1.66 : 1), or in any event the ratio in the case of clays will be somewhere between 1.5 and 2.0. The mixture is then heated to suitable sintering temperature and for such a time as may be necessary to develop the compounds. The ground sinter is then leached with dilute sodium carbonate to dissolve alumina as sodium aluminate, which is removed by filtering, leaving the silica and lime in the sinter residue as calcium silicate and calcium carbonate. The dicalcium silicate has two functions: (1) it holds nearly all the silica in insoluble form, and (2) it undergoes crystallographic transformation, on cooling below about 675° C., with an increase in volume which results in a disintegration of the sinter to a fine powder easily attacked by the leaching agent without further grinding. This phenomenon is called "dusting." Carbon dioxide gas is added to the aluminate solution to precipitate aluminum as aluminum hydroxide. Reactions are as follows:
Leaching: 5CaO · 3Al2O3 + 5Na2CO3 + 2H2O = 5CaCO3 + 3Na2Al2O4 + 4NaOH
Precipitation: Na2Al2O4 + CO2 + 3H2O = 2Al(OH)3 + Na2O3 and 4NaOH + 2CO2 = 2Na2CO3 + 2H2O
The sodium carbonate is regenerated and the solution used for further leaching.
The following sequence of operations represents the procedure followed in the original tests:
Preliminary work on the process was done in the laboratories of the Geological Survey in 1950 to determine the amount of lime to use, the sintering temperature, and the time of leaching to give optimum results. At that time sodium carbonate solutions used for leaching gave satisfactory extraction results, but the silica content of the aluminate solutions was as much as 3.5 percent. Most of the silica is precipitated with alumina when carbon dioxide is added and 3.5 percent is far too much to enable alumina of high grade to be made, although sufficiently pure to produce a product satisfactory for the ceramic industry. The results of leaching the sinters with simple sodium carbonate solutions are shown in Table 6 and in Figures 1 and 2. A summary of Table 6 showing factors involved in giving best extraction results for each clay tested is shown in Table 5.
In 1951, it was found that seeding with sodalite, in the presence of sodium chloride added to the sodium carbonate leaching solution, was effective in removing silica from the leached solutions, and from then on this method was adopted in our work.
Figure 1—Percent alumina extracted versus mole ratio CaO : Al2O3.
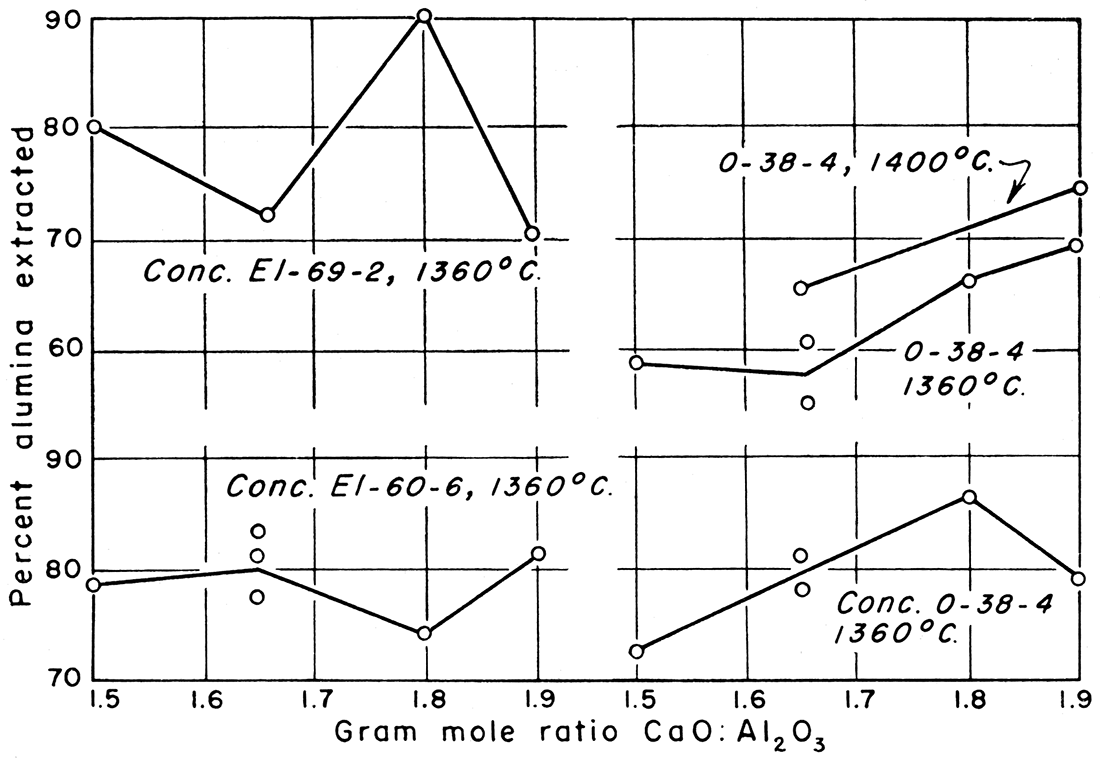
Figure 2—Percent alumina extracted versus sintering temperature.
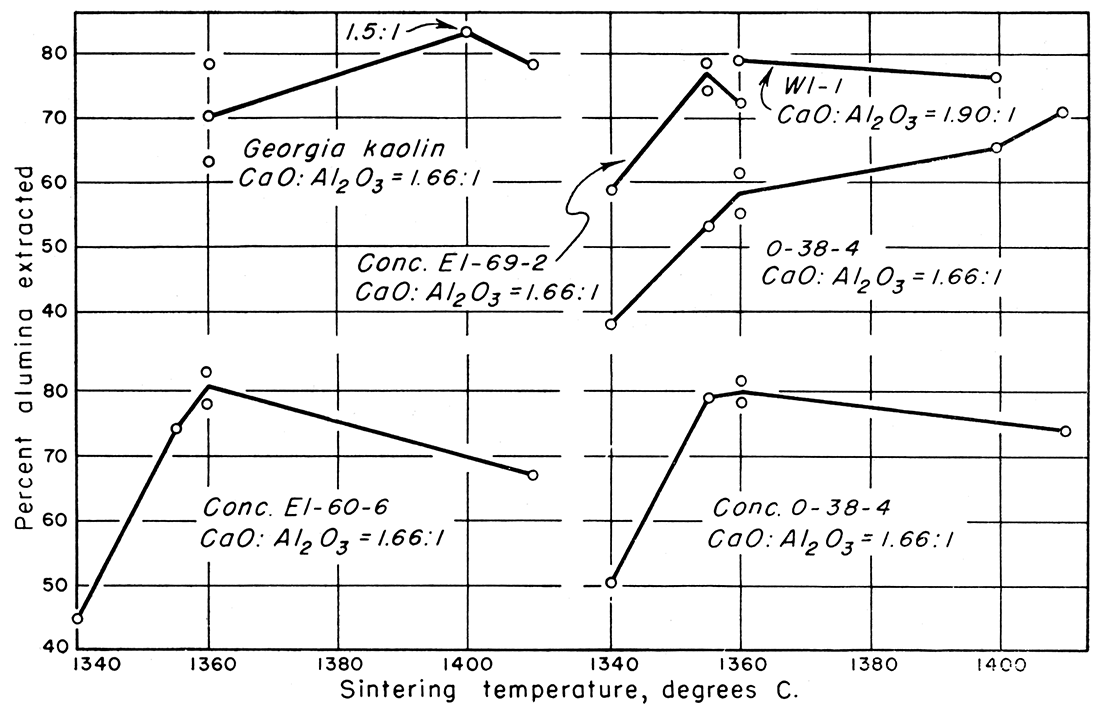
Table 5—Summary of best results after leaching sinter with 3 percent sodium carbonate solutions
| Clay | Alumina extraction, percent |
Ratio CaO : Al2O3 in sinter |
Sintering temperature, degrees C. |
|---|---|---|---|
| EL-69-2 beneficiated | 90.4 | 1.80 | 1360 |
| EL-60-6 beneficiated | 83.3 | 1.66 | 1360 |
| O-38-4 beneficiated | 86.1 | 1.80 | 1360 |
| O-38-4 | 74.2 | 1.90 | 1400 |
| Georgia kaolin | 84.5 | 1.50 | 1400 |
| WL-1-illite | 79.1 | 1.90 | 1360 |
Table 6—Sinter composition and extraction data after leaching with 3 percent sodium carbonate solutions (Final solutions not purified) (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Sample designation |
Percent | Weight Al2O3 from 10 gms. |
Alumina extraction percent |
Weight SiO2 from 10 gms. |
(100 × SiO2) / (SiO2 + Al2O3) in extract |
More ratio CaO : Al2O3 in sinter |
Sinter temp degrees C. |
Remarks | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | MgO | ||||||||
| EL-69-2 | |||||||||||||
| Enriched and calcined | 55.33 | 37.07 | 0.41 | 2.41 | 1.27 | 1.08 | |||||||
| Sinter 10 | 23.07 | 15.10 | 57.66 | 1.71 | 0.99 | 1.17 | 79.38 | 0.031 | 2.60 | 1.66 | 1,355 | ||
| Sinter 14 | 23.07 | 15.10 | 57.66 | 1.71 | 1.16 | 72.20 | 0.036 | 3.02 | 1.66 | 1,360 | 2 : 1 Na2CO3: Al2O3 in leach | ||
| Sinter 18 | 22.89 | 14.77 | 58.30 | 1.85 | 0.87 | 58.80 | 0.031 | 3.45 | 1.66 | 1,340 | |||
| Sinter 10 B | 23.07 | 15.10 | 57.66 | 1.71 | 0.99 | 1.12 | 75.95 | 0.070 | 5.60 | 1.66 | 1,355 | ||
| Sinter 23 | 23.96 | 15.37 | 57.27 | 1.07 | 0.82 | 1.23 | 80.20 | 0.035 | 2.70 | 1.50 | 1,360 | ||
| Sinter 28 | 23.89 | 13.93 | 59.10 | 2.72 | 1.26 | 90.40 | 0.031 | 2.42 | 1.80 | 1,360 | |||
| Sinter 34 | 22.94 | 14.64 | 58.53 | 1.57 | 1.03 | 70.56 | 0.018 | 1.70 | 1.90 | 1,360 | |||
| EL-60-6 | |||||||||||||
| Enriched and calcined | 54.30 | 42.27 | 0.22 | 1.64 | 1.81 | 0.14 | |||||||
| Sinter 9 | 22.53 | 16.74 | 57.96 | 1.98 | 0.74 | 1.23 | 73.60 | 0.043 | 3.42 | 1.66 | 1,355 | ||
| Sinter 15 | 22.53 | 16.74 | 57.96 | 1.98 | 1.30 | 77.50 | 0.041 | 3.08 | 1.6C | 1,360 | 25 min. leach | ||
| Sinter 15A | 22.53 | 16.74 | 57.96 | 1.98 | 1.40 | 83.30 | 0.061 | 4.20 | 1.66 | 1,360 | |||
| Sinter 5 | 22.53 | 16.74 | 57.96 | 1.98 | 1.13 | 67.44 | 0.028 | 2.48 | 1.66 | 1,410 | |||
| Sinter 15B | 22.53 | 16.74 | 57.96 | 1.98 | 1.35 | 80.64 | 0.058 | 4.10 | 1.66 | 1,360 | 2 : 1 :: Na2CO3 : Al2O3 in leach | ||
| Sinter 19 | 22.53 | 16.74 | 57.96 | 1.98 | 0.74 | 43.96 | 0.042 | 5.40 | 1.66 | 1.340 | |||
| Sinter 24 | 23.20 | 17.21 | 57.37 | 0.66 | 0.79 | 1.36 | 78.90 | 0.042 | 3.00 | 1.50 | 1,360 | ||
| Sinter 29 | 22.88 | 16.81 | 58.41 | 1.44 | 1.25 | 74.65 | 0.060 | 4.56 | 1.80 | 1,360 | |||
| Sinter 33 | 21.87 | 16.24 | 58.63 | 1.38 | 1.32 | 81.03 | 0.052 | 3.80 | 1.90 | 1,360 | |||
| O-38-4 (test 1) | |||||||||||||
| Calcined | 70.02 | 24.04 | 0.29 | 1.71 | 1.43 | 0.71 | K2O Na2O 1.79 percent | ||||||
| Sinter 16 | 27.30 | 9.64 | 59.54 | 1.60 | 0.37 | 38.30 | 0.045 | 10.80 | 1.66 | 1,340 | |||
| Sinter 11 | 27.57 | 9.68 | 59.35 | 1.60 | 1.04 | 0.53 | 54.50 | 0.025 | 4.50 | 1.66 | 1,355 | ||
| Sinter 21 | 27.57 | 9.76 | 59.46 | 0.76 | 0.67 | 0.54 | 55.07 | 0.045 | 7.70 | 1.66 | 1,360 | ||
| Sinter 12 | 27.57 | 9.68 | 59.84 | 1.60 | 0.60 | 61.80 | 0.040 | 6.20 | 1.66 | 1,360 | |||
| Sinter 2 | 27.30 | 9.64 | 59.84 | 1.60 | 0.67 | 70.01 | 0.016 | 2.35 | 1.66 | 1,410 | |||
| Sinter 35 | 27.31 | 9.99 | 60.09 | 1.49 | 0.65 | 65.31 | 0.043 | 6.30 | 1.66 | 1,400 | |||
| Sinter 25 | 28.22 | 9.81 | 59.07 | 1.39 | 0.58 | 58.99 | 0.020 | 3.30 | 1.50 | 1,360 | |||
| Sinter 26 | 28.44 | 9.79 | 59.86 | 1.56 | 0.65 | 66.26 | 0.022 | 3.35 | 1.80 | 1,360 | |||
| Sinter 31 | 27.75 | 9.74 | 59.73 | 1.38 | 0.68 | 69.71 | 0.061 | 8.20 | 1.90 | 1,360 | |||
| Sinter 36 | 27.20 | 9.95 | 60.56 | 1.33 | 0.74 | 74.25 | 0.051 | 6.50 | 1.90 | 1,400 | |||
| O-38-4 (test 2) | |||||||||||||
| Enriched and calcined | 56.17 | 36.11 | 0.48 | 2.62 | 1.37 | 0.88 | |||||||
| Sinter 8 | 23.21 | 14.85 | 57.21 | 1.13 | 0.64 | 1.17 | 78.80 | 0.032 | 2.70 | 1.66 | 1,355 | ||
| Sinter 13 | 23.26 | 14.85 | 57.21 | 1.13 | 0.64 | 1.18 | 79.40 | 0.041 | 3.30 | 1.66 | 1,360 | 25 min. leach | |
| Sinter 13A | 23.26 | 14.85 | 57.21 | 1.13 | 0.64 | 1.19 | 80.00 | 0.065 | 5.20 | 1.66 | 1,360 | ||
| Sinter 4 | 23.26 | 14.85 | 57.21 | 1.13 | 0.64 | 1.11 | 74.95 | 0.017 | 1.55 | 1.66 | 1,410 | 2 : 1 :: Na2CO3 : Al2O3 in leach | |
| Sinter 13B | 23.26 | 14.85 | 57.21 | 1.13 | 0.64 | 1.20 | 80.80 | 0.058 | 4.60 | 1.66 | 1,360 | ||
| Sinter 17 | 23.26 | 14.85 | 57.21 | 1.13 | 0.64 | 0.75 | 50.50 | 0.046 | 5.80 | 1.66 | 1,340 | ||
| Sinter 22 | 24.38 | 15.02 | 57.01 | 1.70 | 1.09 | 72.30 | 0.035 | 3.10 | 1.50 | 1,360 | |||
| Sinter 27 | 24.24 | 14.75 | 59.09 | 1.77 | 1.27 | 86.10 | 0.038 | 2.95 | 1.80 | 1,360 | |||
| Sinter 32 | 23.43 | 14.53 | 58.41 | 1.57 | 1.16 | 79.56 | 0.046 | 3.80 | 1.90 | 1,360 | |||
| Georgia kaolin | |||||||||||||
| Calcined | 52.40 | 45.04 | 0.22 | 2.31 | |||||||||
| Sinter 7 | 21.90 | 17.73 | 58.43 | 1.48 | 1.13 | 63.73 | 0.023 | 2.10 | 1.66 | 1,360 | |||
| Sinter 20 | 21.90 | 17.73 | 58.43 | 1.48 | 1.40 | 78.30 | 0.043 | 3.04 | 1.66 | 1,360 | |||
| Sinter 37 | 22.70 | 18.56 | 56.64 | 1.27 | 1.56 | 84.46 | 0.070 | 4.20 | 1.50 | 1,400 | |||
| Sinter 3 | 21.90 | 17.73 | 58.43 | 1.48 | 1.39 | 78.40 | 0.038 | 2.71 | 1.66 | 1,410 | |||
| WL-1 (Illite) | |||||||||||||
| Calcined | 60.41 | 24.21 | 0.53 | 8.07 | 1.90 | 2.08 | |||||||
| Sample leached with HCl | |||||||||||||
| Sinter 30 | 26.07 | 9.82 | 57.95 | 4.03 | 0.78 | 79.13 | 0.041 | 5.00 | 1.90 | 1,360 | |||
| Sinter 38 | 26.94 | 9.60 | 59.63 | 1.67 | 0.74 | 77.34 | 0.026 | 3.40 | 1.90 | 1,400 | |||
The sodium aluminate solutions obtained by leaching the sinter of the lime-sinter process with sodium carbonate solutions will contain appreciable silica as sodium silicate. Unless this is removed first it will seriously contaminate the aluminum hydroxide, when the latter is precipitated, and the alumina and aluminum made will carry prohibitive amounts of silica. This is especially true of dilute aluminate solutions such as considered in this report. The upper limit for silica in high-grade alumina is 0.06 percent. This degree of purity cannot be obtained without a preliminary purification of the aluminate solutions.
Many processes have been suggested for the removal of silica from aluminate solutions. With the exception of the sodalite seeding process, developed by the National Bureau of Standards (Flint and others, 1946), none have been entirely successful.
The composition of the mineral sodalite is 3Na2O · 3Al2O3 · 6SiO2 · 2NaCl. When sodalite powder is added to an aluminate solution containing sodium chloride, under proper conditions it will start to grow without change of composition due to assimilation of alumina, silica, and sodium chloride from the surrounding liquid. Removal of the sodalite seed by filtration reduces the silica content of the solution, but at the expense of a small amount of alumina and sodium chloride. This loss is not particularly important as seed can be used repeatedly or recycled with the ore.
It should be recalled that in precipitating aluminum hydroxide with carbon dioxide, sodium carbonate is regenerated and the solutions are recycled for further leaching. If seeding is practiced there will also be sodium chloride in the aluminate solutions so that sodalite will develop spontaneously in small amount in the solutions during leaching. To limit the development of this insoluble compound which would otherwise be discarded with the leached ore, causing a loss of alumina, the time of leaching is made as short as possible.
Using sodium chloride solutions the extraction of alumina will be slightly lower than where straight sodium carbonate is employed, but the advantage of having a sound process for the removal of silica far outweighs this disadvantage.
Desiliconization of sodium aluminate solutions is favored by the following factors: (1) the presence of a high concentration of sodium chloride in the solution; (2) the presence or formation of a seed charge of sodalite; (3) a boiling temperature; and (4) sufficient time for precipitation of sodalite to approach completion.
In our work of evaluating the lime-sinter process, using sodium carbonate-sodium chloride leaching solutions and seeding, the effect of the added salt on extraction of alumina was first determined followed by tests to determine the efficiency of sodalite seed in removing silica from the extract.
Sintering—In making the sinter mixture of calcined clay and limestone the following gram mole ratios were employed: for sample EL-60-6, CaO : Al2O3 :: 1.66 : 1, CaO : SiO2 = 2 : 1; and for sample EL-69-2, CaO : Al2O3 :: 1.75 : 1, CaO : SiO2 = 2 : 1. No allowance was made in the fluxing calculations for compounds in the calcined clay other than silica, alumina, and lime.
The sinter mixture briquettes were heated slowly and kept at 1360° C. for 1 hour. They were then cooled to 1100° C. in 30 minutes. On further cooling sample EL-60-6 dusted completely in 20 minutes. Sample EL-69-2 dusted completely in 30 minutes. Partial analyses of the sinters are given in Table 7.
Table 7—Partial chemical analyses of sinters
| Sinter | SiO2, percent |
Al2O3, percent |
|---|---|---|
| EL-60-6 | 22.47 | 17.11 |
| EL-69-2 | 23.34 | 15.22 |
Table 8—Partial analyses of annealed sinters*
| Sample | SiO2, percent |
Al2O3, percent |
|---|---|---|
| EL-60-6a | 23.14 | 17.48 |
| EL-60-6a' | 16.48 | |
| EL-69-2a | 23.20 | 16.20 |
| EL-69-2a' | 16.20 | |
| * Extraction data of annealed sinters are given in Table 10. | ||
Leaching the sinter with sodium carbonate solutions containing sodium chloride—In all leaching tests, except those noted, 20 gram samples of sinter were leached for 15 minutes with 6 percent sodium carbonate solutions containing salt at 65° C., using high-speed agitation. The residue was washed four times by decantation using sodium chloride solutions of the same concentration, and approximately the same volume, as used in leaching. For purposes of comparison a few sinters were leached with sodium carbonate solution without salt and washed with water. Also some were leached with 15 percent sodium carbonate solutions and salt (Table 9).
Table 9—Sinter extraction data after leaching with sodium carbonate solutions containing sodium chloride only (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Sample | Sinter | Solvent | Salt in solvent g/l |
Mole ratio Na2CO3 in solvent to Al2O3 in sinter |
Unpurified filtrate | Recovery of Al2O3 percent |
|||
|---|---|---|---|---|---|---|---|---|---|
| Volume, cc | Al2O3, g/l | SiO2, g/l | (100 × SiO2) / (SiO2 + Al2O3) |
||||||
| 1 | EL-60-6 | 6% Na2CO3 | 1.8:1 | 184 | 15.06 | 0.2 | 1.31 | 81.3 | |
| 2 | EL-60-6 | 6% Na2CO3 | 75 | 1.66:1 | 140 | 18.49 | 0.156 | 0.82 | 75.6 |
| 3 | EL-60-6 | 6% Na2CO3 | 75 | 2:1 | 185 | 15.5 | 0.16 | 1.02 | 83.8 |
| 4 | EL-60-6 | 6% Na2CO3 | 150 | 1.66:1 | 164 | 15.89 | 0.096 | 0.60 | 76.2 |
| 5 | EL-60-6 | 6% Na2CO3 | 150 | 2:1 | 201 | 14.33 | 0.104 | 0.72 | 84.3 |
| 37 | EL-69-2 | 6% Na2CO3 | 2.1 | 201 | 12.67 | 0.172 | 1.34 | 83.6 | |
| 28 | EL-69-2 | 6% Na2CO3 | 75 | 2:1 | 217 | 11.30 | 0.132 | 1.15 | 80.4 |
| 23 | EL-69-2 | 6% Na2CO3 | 75 | 2:1 | 192 | 12.35 | 0.144 | 1.15 | 77.8 |
| 29 | EL-69-2 | 6% Na2CO3 | 150 | 2:1 | 171 | 12.50 | 0.124 | 0.99 | 70.1 |
| 24 | EL-69-2 | 6% Na2CO3 | 150 | 2:1 | 191 | 11.39 | 0.132 | 1.15 | 71.2 |
| 25 | EL-69-2 | 6% Na2CO3 | 2.4:1 | 206 | 12.60 | 0.048 | 0.38 | 85.3 | |
| 26 | EL-69-2 | 6% Na2CO3 | 75 | 2.4:1 | 229.5 | 11.10 | 0.112 | 0.99 | 83.6 |
| 33 | EL-69-2 | 6% Na2CO3 | 75 | 2.4:1 | 230 | 10.58 | 0.132 | 1.22 | 80.9 |
| 34 | EL-69-2 | 6% Na2CO3 | 150 | 2.4:1 | 212.5 | 10.61 | 0.108 | 1.01 | 74.2 |
| 6 | EL-60-6 | 15% Na2CO3 | 1.66:1 | 184 | 13.06 | 0.144 | 1.09 | 70.3 | |
| 7 | EL-60-6 | 15% Na2CO3 | 2:1 | 196 | 13.9 | 0.20 | 1.42 | 79.8 | |
| 9 | EL-60-6 | 15% Na2CO3 | 75 | 2:1 | 175 | 15.06 | 0.184 | 1.21 | 76.8 |
| 8 | EL-60-6 | 15% Na2CO3 | 150 | 2:1 | 170 | 12.33 | 0.04 | 0.32 | 68.0 |
| 11 | EL-60-6 | 15% Na2CO3 | 150 | 1.66:1 | 178 | 13.24 | 0.108 | 0.73 | 68.7 |
The highest single extraction of alumina, 85.3 percent, was made on a sinter of clay EL-69-2 using a solution without salt. Next highest, 84.3 percent, was on a sinter of clay EL-60-6, with salt. The insoluble compound sodalite (3Na2O · 3Al2O3 · 6SiO2 · 2NaCl) is believed to form more or less during the leach, when salt is used in the leaching solution, thus reducing alumina extraction. For the same reason the percentage of silica to alumina
(SiO2 × 100) /
(SiO2 + Al2O3)
in the aluminate extract is lower where salt is employed. Compare results shown in Table 6, where no salt was used, with those of Table 9. Results also indicate that for best extraction the mole ratio of Na2O in the leaching solution to Al2O3 in the sinter should be from 2 : 1 to 2 : 4. Sinters leached with 15 percent sodium carbonate and salt solutions showed rather poor extraction due to the low volume of leaching- agent compared to the mass of sinter. A thick pulp formed making filtering difficult. It is believed trouble from this source would be less in a plant of commercial size.
The effect of various amounts of sodium chloride on alumina extraction and the silica content of the extract, when added to sodium carbonate leaching solutions, is shown in Tables 9, 10, and 11. Sodium chloride in excess of 150 grams per liter is believed to reduce greatly alumina extraction by forming insoluble sodalite which remains in the solid residue, and such excess was not used.
Table 10—Annealed sinter extraction data after leaching annealed sinters with sodium carbonate solutions containing sodium chloride only (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Sample | Sinter | Solvent | Salt in solvent g/l |
Mole ratio Na2CO3 in solvent to Al2O3 in sinter |
Unpurified filtrate | Recovery of Al2O3 percent |
|||
|---|---|---|---|---|---|---|---|---|---|
| Volume, cc | Al2O3, g/l | SiO2, g/l | (SiO2 × 100) / (SiO2 + Al2O3) |
||||||
| 12 | EL-60-6a* | 6% Na2CO3 | 2:1 | 216 | 13.16 | 0.032 | 0.24 | 81.3 | |
| 13 | EL-60-6a* | 6% Na2CO3 | 75 | 1.66:1 | 223 | 12.02 | 0.064 | 0.53 | 76.9 |
| 14 | EL-60-6a* | 6% Na2CO3 | 75 | 2:1 | 203 | 13.64 | 0.12 | 0.87 | 79.3 |
| 15 | EL-60-6a* | 6% Na2CO3 | 150 | 1.66:1 | 194 | 13.52 | 0.096 | 0.70 | 75.1 |
| 16 | EL-60-6a* | 6% Na2CO3 | 150 | 2:1 | 210 | 13.53 | 0.064 | 0.40 | 81.3 |
| 35 | EL-60-6a* | 6% Na2CO3 | 75 | 2.4:1 | 217 | 12.57 | 0.112 | 0.87 | 78.0 |
| 36 | EL-60-6a* | 6% Na2CO3 | 150 | 2.4:1 | 207 | 13.47 | 0.10 | 0.74 | 79.7 |
| 53 | EL-60-6a'** 14.2 gms. |
6% Na2CO3 | 150 | 2:1 | 209 | 8.3 | 74.1 | ||
| 54 | EL-60-6a'** 14.2 gms. |
6% Na2CO3 | 150 | 2.4:1 | 218 | 8.02 | 74.8 | ||
| 17 | EL-60-6a' | 15% Na2CO3 | 2:1 | 220 | 12.2 | 0.08 | 0.65 | 76.9 | |
| 18 | EL-60-6a' | 15% Na2CO3 | 75 | 1.66:1 | 202 | 9.73 | 0.024 | 0.24 | 56.3 |
| 19 | EL-60-6a' | 15% Na2CO3 | 75 | 2:1 | 234 | 12.27 | 0.08 | 0.65 | 82.2 |
| 20 | EL-60-6a' | 15% Na2CO3 | 150 | 1.66:1 | 188 | 11.90 | 0.012 | 0.10 | 63.9 |
| 21 | EL-60-6a' | 15% Na2CO3 | 150 | 2:1 | 200 | 11.46 | 0.04 | 0.35 | 65.6 |
| 30 | EL-69-2a | 6% Na2CO3 | 2:1 | 231 | 10.77 | 0.172 | 1.58 | 76.6 | |
| 31 | EL-69-2a | 6% Na2CO3 | 75 | 2:1 | 185 | 12.36 | 0.108 | 0.86 | 70.6 |
| 32 | EL-69-2a | 6% Na2CO3 | 150 | 2:1 | 228 | 9.84 | 0.088 | 0.88 | 70.0 |
| 55 | EL-69-2a' | do | 2:1 | 232 | 9.99 | 71.5 | |||
| 56 | EL-69-2a | 6% Na2CO3 | 75 | 2:1 | 217 | 10.52 | 0.116 | 1.05 | 70.4 |
| 57 | EL-69-2a | 6% Na2CO3 | 75 | 2.4:1 | 208 | 10.70 | 68.9 | ||
| * a Indicates annealed, cooled to 1200° C. in one-half hour. ** a' Indicates annealed, cooled to 1200° C. in 1 1/2 hours. |
|||||||||
Table 11—Effect of sodium chloride in sodium carbonate leaching solutions (average of all tests) (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Sintered clay |
Sodium carbonate solution, percent |
No NaCl | NaCl, 75 g/l | NaCl, 150 g/l | |||
|---|---|---|---|---|---|---|---|
| Al2O3 percent |
(SiO2 × 100*) / (SiO2 + Al2O3) in extract |
Al2O3 percent |
(SiO2 × 100*) / (SiO2 + Al2O3) in extract |
Al2O3 percent |
(SiO2 × 100*) / (SiO2 + Al2O3) in extract |
||
| EL-60-6 | 6 | 81.3 | 1.33 | 79.7 | 0.93 | 80.2 | 0.66 |
| EL-60-6 | 15 | 75.0 | 1.27 | 76.8 | 1.22 | 68.4 | 0.53 |
| EL-60-6a | 6 | 81.3 | 0.24 | 78.1 | 0.79 | 79.0 | 0.65 |
| EL-60-6a | 15 | 76.9 | 0.65 | 69.2 | 0.44 | 64.8 | 0.23 |
| EL-69-2 | 6 | 84.5 | 0.68 | 80.7 | 1.14 | 71.8 | 1.01 |
| EL-69-2a | 6 | 76.6 | 1.58 | 70.6 | 0.87 | 70.0 | 0.89 |
| Averages | 79.3 | 0.96 | 75.4 | 0.90 | 72.4 | 0.66 | |
| * Extract not purified. | |||||||
Table 12—Summary of leaching sinters with sodium carbonate and sodium chloride solutions, showing highest and lowest individual recoveries of Al2O3 from sinter and conditions involved (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Sample | Sinter | Na2CO3, percent |
High extraction | Low extraction | Al2O3 recovery, percent |
||
|---|---|---|---|---|---|---|---|
| Salt, g/l |
Mole ratio Na2CO3 in solvent to Al2O3 in sinter |
Salt, g/l |
Mole ratio Na2CO3 in solvent to Al2O3 in sinter |
||||
| 5 | EL-60-6 | 6 | 150 | 2:1 | 84.3, highest | ||
| 2 | EL-60-6 | 6 | 75 | 1.66:1 | 75.6, lowest | ||
| 16 | EL-60-6a* | 6 | 150 | 2:1 | 81.3, highest | ||
| 15 | EL-60-6a | 6 | 150 | 1.66:1 | 75.1, lowest | ||
| 53 | EL-60-6a'** | 6 | 150 | 2.4:1 | 74.8, highest | ||
| 54 | EL-60-6a' | 6 | 150 | 2:1 | 74.1, lowest | ||
| 19 | EL-60-6 | 15 | 75 | 2:1 | 82.2, highest | ||
| 18 | EL-60-6 | 15 | 75 | 1.66:1 | 56.3, lowest | ||
| 26 | EL-69-2 | 6 | 75 | 2.4:1 | 83.6, highest | ||
| 29 | EL-69-2 | 6 | 150 | 2:1 | 70.1, lowest | ||
| 31 | EL-69-2a | 6 | 75 | 2:1 | 70.6, highest | ||
| 32 | EL-69-2a | 6 | 150 | 2:1 | 70.0, lowest | ||
| 56 | EL-69-2a' | 6 | 75 | 2:1 | 70.4, highest | ||
| 57 | EL-69-2a' | 6 | 75 | 2.4:1 | 68.9, lowest | ||
| * a Indicates annealed, cooled at 1200° C. in one-half hour. ** a' Indicates annealed, cooled to 1200° C. in 1 1/2 hours. |
|||||||
On heating a mixture to sintering temperature a certain amount of viscous or molten material is formed which is probably a solution of calcium aluminate in beta dicalcium silicate. On slow cooling through the solidus line and below, the beta silicate inverts to the gamma allotropic form at 675° C. with an expansion of volume. This causes dusting and the liberation of calcium aluminate. If the cooling is rapid, inversion is retarded and aluminate may be held in glassy beta silicate. In annealing, the sinter is reheated to 1300° C. for 1 hour and then the temperature is slowly dropped to 1200° C. over a period of 1 1/2 hours. The treatment is supposed to promote the change of beta silicate from glassy to granular form, give more complete dusting, and provide more efficient leaching because of better separation of the aluminate.
Two sets of sinters were annealed but gave lower extraction results, especially sample EL-69-2a (Table 8), than the unannealed samples. The time in cooling from 1300° C. to 1200° C. for the first group, designated "a", was 30 minutes and for the second group, designated "a'," was 11/2 hours. The dusting characteristics differed. The first group dusted completely; sample EL-60-6a in 3 hours, sample EL-69-2a in 15 hours. The second group dusted poorly—sample EL-69-2a' required 2 days. Sample EL-60-6a' dusted rapidly to the extent of 84 percent and the balance, which failed to dust, was removed before leaching. The second group briquettes, where in contact with the carbon discs on which they rested, were of a much lighter color than the first group, indicating reduction. The material which failed to dust, or dusted poorly, came from this section. These sinters contain small amounts of iron, titanium, and magnesium as oxides. At the prolonged annealing temperature they probably reacted to form slag which tended to inhibit dusting.
The average silica content of the extract from annealed sinters is lower than that from the unannealed sinters, owing probably to the formation of more insoluble calcium silicate in the sinter with the prolonged time of sintering. The poorest extraction was obtained when the ratio of Na2O in the solvent to Al2O3 in the sinter was lowest.
The desiliconization experiments indicate the high efficiency of sodalite in removing silica. Of 32 tests made, 18, or 56 percent, yielded solutions with silica to alumina ratios of 0.06 percent or lower, the amount allowable in high-grade alumina. Considering the factors involved-the use of different amounts of seed and salt and the time of boiling, all of which were varied-the results appear satisfactory and indicate desiliconization can be accomplished by observing proper precautions. A factor not conducive to good results was the use of pyrex beakers from which silica could have dissolved on boiling the alkaline solutions. Some special corrosion resistant beakers were also used and should have been used entirely. Glass would not be used as acontainer if this operation were conducted on a commercial scale. Complete desiliconization tests are shown in Table 13.
Table 13—Desiliconization of sodium aluminate solutions (Analyses in State Geological Survey geochemistry laboratory under supervision of Russell Runnels)
| Original solution | Desiliconized solution | Boiling time, hours |
Seed g/l |
SiO2 removed, percent |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample no. |
Al2O3, g/l | SiO2, g/l | (SiO2 × 100*) / (SiO2 + Al2O3) |
Al2O3, g/l | SiO2, g/l | (SiO2 × 100*) / (SiO2 + Al2O3) |
NaCl, g/l | |||
| 2 | 18.47 | 0.156 | 0.82 | 18.00 | 0.012 | 0.06 | 75 | 1 | 10 | 92 |
| 3 | 15.50 | 0.16 | 1.02 | 15.19 | 0.009 | 0.06 | 75 | 1 | 7 | 94 |
| 9 | 15.01 | 0.184 | 1.21 | 15.02 | 0.008 | 0.053 | 75 | 1 | 7 | 95 |
| 14 | 13.64 | 0.12 | 0.87 | 12.78 | 0.017 | 0.13 | 75 | 1 | 6 | 86 |
| 15 | 13.52 | 0.096 | 0.70 | 13.20 | 0.022 | 0.16 | 150 | 1 | 7 | 77 |
| 19 | 12.27 | 0.08 | 0.65 | 12.70 | 0.001 | 0.008 | 75 | 1 | 5 | 98 |
| 21 | 11.46 | 0.04 | 0.35 | 11.19 | 0.001 | 0.008 | 150 | 1 | 6 | 97 |
| 4 | 15.S9 | 0.096 | 0.60 | 14.41 | 0.007 | 0.048 | 150 | 1 | 4 | 92 |
| 11 | 13.24 | 0.108 | 0.73 | 12.94 | 0.003 | 0.023 | 150 | 1 | 4 | 97 |
| 16 | 13.52 | 0.064 | 0.40 | 13.13 | 0.018 | 0.13 | 150 | 1 | 3 | 72 |
| 31 | 12.36 | 0.108 | 0.86 | 10.19 | 0.028 | 0.27 | 75 | 1 | 3 | 74 |
| 32 | 9.84 | 0.088 | 0.88 | 9.01 | 0.025 | 0.27 | 150 | 1 | 3 | 72 |
| 50 | 13.46 | 0.134 | 0.99 | 13.46 | 0.003 | 0.022 | 75 | 1 | 26 | 98* |
| 58 | 12.38 | 0.163 | 1.30 | 10.03 | 0.001 | 0.010 | 150 | 1 | 10 | 99* |
| 59 | 12.38 | 0.163 | 1.30 | 10.03 | 0.004 | 0.039 | 150 | 1 | 16 | 97* |
| 23 | 12.35 | 0.144 | 1.15 | 10.92 | 0.002 | 0.019 | 75 | 1.5 | 7 | 98 |
| 24 | 13.85 | 0.132 | 1.15 | 10.91 | 0.001 | 0.009 | 150 | 1.5 | 7 | 99 |
| 35 | 12.57 | 0.112 | 0.87 | 12.00 | 0.032 | 0.260 | 75 | 1.5 | 6 | 71 |
| 36 | 13.47 | 0.10 | 0.74 | 12.41 | 0.014 | 0.11 | 150 | 1.5 | 6 | 86 |
| 33 | 10.58 | 0.132 | 1.22 | 8.81 | 0.045 | 0.51 | 75 | 1.5 | 6 | 66** |
| 34 | 10.61 | 0.108 | 1.01 | 10.34 | 0.023 | 0.22 | 150 | 1.5 | 6 | 79** |
| 26 | 11.10 | 0.112 | 0.99 | 9.98 | 0.026 | 0.26 | 75 | 1.5 | 3 | 77 |
| 28 | 11.30 | 0.132 | 1.15 | 11.37 | 0.033 | 0.29 | 75 | 1.5 | 3 | 75** |
| 29 | 12.50 | 0.124 | 0.99 | 12.71 | 0.009 | 0.07 | 150 | 1.5 | 4 | 93 |
| 51 | 13.49 | 0.134 | 0.99 | 13.47 | 0.008 | 0.059 | 75 | 1.5 | 26 | 94* |
| 60 | 12.38 | 0.163 | 1.31 | 10.03 | 0.001 | 0.01 | 150 | 1.5 | 15 | 99* |
| 43 | 13.76 | 0.144 | 1.04 | 13.76 | 0.008 | 0.058 | 150 | 2.0 | 10 | 94** |
| 44 | 13.76 | 0.144 | 1.04 | 13.76 | 0.006 | 0.043 | 150 | 2.0 | 10 | 96 |
| 45 | 13.76 | 0.144 | 1.04 | 13.76 | 0.006 | 0.043 | 150 | 2.0 | 10 | 96 |
| 52 | 13.46 | 0.134 | 0.99 | 13.47 | 0.022 | 0.163 | 75 | 2.0 | 26 | 83* |
| 61 | 12.38 | 0.163 | 1.31 | 10.03 | 0.003 | 0.029 | 150 | 2.0 | 15 | 98* |
| 49 | 13.46 | 0.134 | 0.99 | 13.47 | 0.012 | 0.089 | 75 | 0.5 | 26 | 91 |
| * Indicates chemically resistant beaker used. ** Indicates seed had been used before. |
||||||||||
Definite conclusions were not reached as to the length of time the solution with seed should be boiled. It depends somewhat on the quantity of silica to be removed and the amount of seed used. Best results were obtained by boiling from 1 to 1 1/2 hours (Table 14).
Loss of alumina in seeding—The approximate loss of alumina in seeding calculated from the 32 tests completed is 5 percent of the alumina content of the solution at the start of seeding. This alumina contributes to the formation of more seed. It is really not a total loss as it can be used for further seeding operations or, because it contains about 35 percent alumina, after calcination to remove water it can be recycled as ore.
Synthetic sodalite—The sodalite used in these experiments was made as follows. Kaolin was first activated by heating at 700° C. for 1 hour. A batch of 200 grams of this dehydrated kaolin, 150 grams of NaCl, 100 grams of NaOH, and 400 cc of water, was kept at 150° C. in an autoclave for 4 days. The product was then removed, washed with hot water, and dried at 110° C.
Table 14—Seed boiling time on .successful desiliconizing experiments
| Number of tests |
Weight of seed added g/l |
Hours of boiling |
Average (SiO2 × 100) / (SiO2 + Al2O3) |
|---|---|---|---|
| 10 | 9.5 | 1 | .033 |
| 4 | 12.0 | 1.5 | .024 |
| 4 | 11.0 | 2.0 | .043 |
Other processes—Many unsuccessful attempts were made to purify aluminate solutions from the lime sinter process by other methods. These include the use of lime, both solid and as milk of lime, and carbon dioxide gas. A considerable portion of the silica could be removed but never enough to give the desired purity. Carbon dioxide will effectively remove silica from solutions of the soda-lime process by fractional precipitation as silicic acid (Kinney, 1943), but not from those of the lime-sinter process, because although both contain sodium aluminate, the limesinter filtrate also has considerable sodium hydroxide. Since using sodalite seeding other methods of purification have been abandoned.
About 7 tons of residue are produced in extracting 1 ton of alumina; therefore the possibilities for profitable disposal of the residue are of interest. Its suitability for the manufacture of Portland cement is well known. Other uses are for fertilizer, plastics, industrial fillers, and as a clarifying filter for certain solutions. Analyses of residual sinters after leaching are shown in Table 15.
Table 15—Analyses of sinter residues
| Constituent | EL-60-6 | EL-69-2 |
|---|---|---|
| SiO2 | 18.45 | 20.76 |
| Al2O3 | 3.02 | 2.32 |
| Fe2O3 | 0.54 | 0.93 |
| TiO2 | 0.55 | 0.42 |
| CaO | 48.46 | 52.97 |
| MgO | 0.50 | 0.82 |
| K2O (total) | 0.18 | 0.28 |
| Na2O (total) | 2.75 | 1.43 |
| Cl (chloride) | 3.14 | 1.64 |
| Remainder by difference (mostly CO2 and H2O) |
22.41 | 18.43 |
Based on the amounts of alumina in sinter and residue, the losses of alumina in the lime-sinter process are 17.6 percent and 15.2 percent in sinters from clays EL-60-6 and EL-69-2 respectively. The amounts of soda ash and salt lost are' largely dependent on the efficiency of washing. Such losses are low but could not be accurately determined as the sodium oxide shown in the analyses comes from both the sodium chloride and carbonate used as reagents as well as from the small amount present in the original ore.
The concentration of alumina in solutions should preferably be from 40 to 80 grams per liter. Obviously the greater the concentration the less volume required for the same output. Another important reason is that alumina precipitated from concentrated solutions contains less silica. In practice, only threefourths of the alumina contained is precipitated in anyone cycle. The solution is then recycled for further leaching. Under these conditions high-grade alumina can be precipitated even though the solution contains as much as 0.13 gram per liter silica (Archibald, 1948).
In the experimental work covered by this report the alumina averaged 15 grams per liter. In the laboratory there are difficulties in obtaining concentrated solutions which are less prevalent in plant operation. Extraction results, however, even though working with dilute solutions, are an accurate measure of what can be accomplished on a larger scale, and with richer solutions.
Considering results obtained in testing Kansas clays by both the lime-sinter and the soda-lime-sinter processes (Kinney, 1943) the former appears definitely the better for the following reasons. (1) The extraction of alumina is about 15 percent greater. (2) Consumption of soda ash is less. Also, in the lime-sinter process soda ash is regenerated in solution and can be recycled at once, while in the soda-lime-sinter method evaporation of solutions is necessary because the ash is used in solid form. (3) Desiliconization is probably cheaper. Both processes have effective means of removing silica, but sodalite seeding requires less control and would probably be less expensive than fractional precipitation by carbon dioxide as required in the soda-lime process.
Melting point cones were prepared by adding ground alumina to clay using polyvinyl alcohol as a binder. Melting points were determined by comparing with Seger cones. The alumina was obtained as described by precipitation from impure solutions with carbon dioxide and probably contained as much as 3.5 percent silica. Results of these tests are shown in Table 16.
Table 16—Melting points of day EL-69-2 with and without added alumina
| Clay | Alumina added, percent |
Total Al2O3, percent |
Conditions under which sample failed to melt |
||
|---|---|---|---|---|---|
| Seger cone | Temp. ° F. | ||||
| EL-69-2 | none | 23.70 | 27 | 2921 | |
| EL-69-2 | beneficiated | none | 32.23 | 31 | 3056 |
| EL-69-2 | beneficiated | 5 | 35.45 | 32 1/2 | 3137 |
| EL-69-2 | beneficiated | 10 | 38.39 | 33 | 3173 |
| EL-69-2 | beneficiated | 15 | 41.07 | 34 | Insufficient heat to test at cone 34. |
| EL-69-2 | beneficiated | 20 | 43.50 | 34 | Insufficient heat to test at cone 34. |
The melting point of the raw clay EL-69-2, containing 23.70 percent alumina, used in the preceding test is not particularly high, being a little more than 2921° F. Other Kansas clays which might have been used have melting points of 3000° F. or more before benefication. Table 17 shows a classification of fire-clay refractories. Comparing it with Table 16 it is evident that any type of fire-clay brick, including super-duty fire-clay brick, can be made from ordinary Kansas fire clay by first beneficiating the clay and then adding as much as 10 percent crude alumina as produced by the lime-sinter process without desiliconization. These tests also suggest that sillimanite or other high-alumina refractories could also be made from beneficiated Kansas clay enriched with alumina produced by the lime-sinter process. Pure sillimanite contains 62.85 percent alumina and 37.15 percent silica and melts at 3280° F.
Table 17—A.S.T.M. standard classification of fire-day refractories (A.S.T.M., 1949, Designation: C 27-41)
| Classification | Description |
|---|---|
| Super-duty fireclay brick: | A fireclay brick having a pyrometric cone equivalent not lower than cone 33. |
| High-duty fireclay brick: | Pyrometric cone equivalent (P.C.E.) not lower than cone 31-32. |
| Intermediate-duty firecaly brick: | Pyrometric cone equivalent not lower than cone 29. |
| Low-duty fireclay brick: | Pyrometric cone equivalent not lower than cone 19. |
A tentative flow sheet of the lime-sinter process for the extraction of alumina from clay is suggested in Figure 3. It should be tried for defects in a pilot plant before considering larger scale operation.
One apparent problem is that of limiting the volume of sodium carbonate leaching solution, which is regenerated in the process and must be recycled, despite additions of wash water in every cycle. Some wash water is of course discarded with the sinter residue. The use of sensible heat in the discharged kiln gases to eliminate the excess by evaporation may be the answer. Some pure water condensed in this operation could be used to wash the precipitated aluminum hydroxide.
The flow sheet shows conventional filtering being used to separate the leached sinter from aluminate solution. It is possible the use of counter current decantation washing of the residue, after it leaves the settling tank, would be more satisfactory. Aside from those mentioned, there seem to be no problems of consequence associated with the flow sheet.
Figure 3—Flow sheet of lime-sinter process for extracting alumina from Kansas clay.
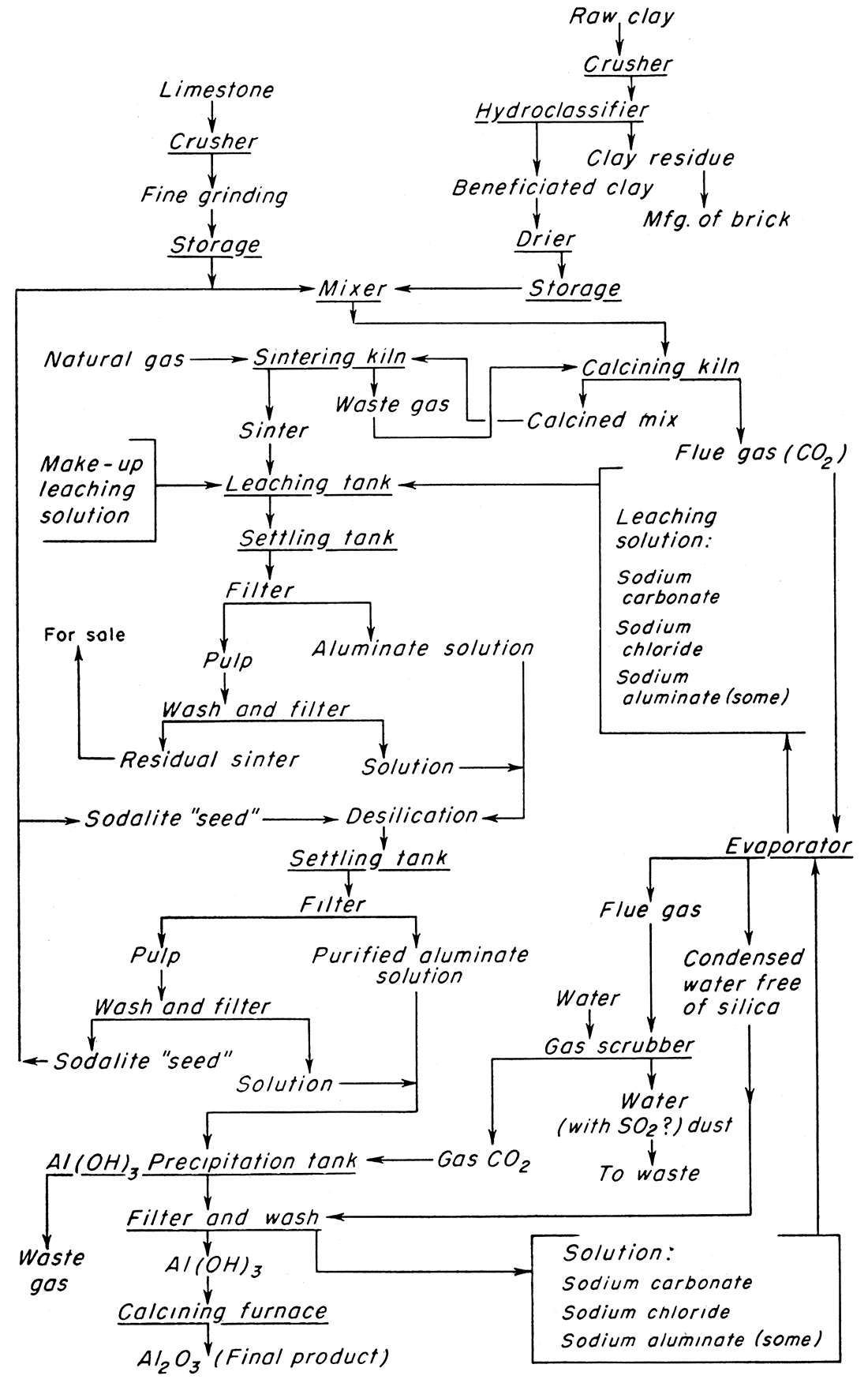
In appraising the lime-sinter process, tests have been conducted under varying conditions to determine the best method of operation. Probably no one set of conditions is suitable for every Kansas clay, but in general, by following procedures giving the best results, as shown in this report, net recoveries of 80 to 84 percent of the alumina, in the purest commercial form, can be obtained from beneficiated Kansas clays. Further tests with annealed sinters might show greater recoveries on certain clays.
For the lime-sinter process, clay, limestone, water, fuel, and soda ash are required. Kansas has the first four in abundance and the manufacture of soda ash may soon start in the State. Compared with other states Kansas is in a favorable position to produce alumina when and if clay can compete with bauxite as an ore.
The advantages of the lime-sinter process, besides giving good extraction, are (1) small loss of alkali, (2) all steps can be carried out at atmospheric pressure, and (3) the process is simple and no unusual equipment is required. A disadvantage is the limestone needed-about 2.5 tons per ton of clay.
The competitive production of metallic aluminum under existing economic conditions has never been seriously considered as a possibility in Kansas, partly because of a lack of abundant cheap power in the State. Even this view may need revision in the face of recent developments where, in two new aluminum plants in Texas, Diesel engines to supply power for the aluminum reduction cells are operated with natural gas-a mineral resource with which Kansas is abundantly endowed. The production of alumina of metallurgical grade to be shipped elsewhere for conversion to metallic aluminum or production of alumina of a lower grade to be used locally as an addition to existing clay in the manufacture of high-alumina refractories are potentialities that may be worthy of serious consideration by Kansas industry in the not too distant future.
American Society for Testing Materials (1949) A.S.T.M. Standards, part 3, cement. concrete, ceramics, thermal insulation, road materials, waterproofing, soils: Am. Soc. Testing Materials, Baltimore, Md., pp. 1-1344.
Archibald, F. R. and Jackson, C. F. (1944) Alumina from clay by the limesinter method: Am. Inst. Mining and Metal. Engineers Trans., vol. 159, pp. 227-238, figs. 1-4.
Archibald, F. R. and Nicholson, C. M. (1948) Alumina from clay by the lime-sinter method II: Am. Inst. Mining and Metal. Engineers, Metals Technology, Tech. Paper 2390, pp. 1-25, figs. 1-13.
Edwards, J. D., Frary, F. C., and Jeffries, Zay (1930) The aluminum in dustry, aluminum and its production: McGraw-Hill Book Co., New York, vol. 1, pp. 1-358, figs. 1-63.
Flint, E. P., and others (1946) Extraction of alumina from clays and highsilica bauxites: Natl. Bur. Standards, Jour. Research, vol. 36, pp. 63-106. figs. 1-18.
Grim, R. E., Machin, J. S., and Bradley, W. F. (1945) Amenability of various types of clay minerals to alumina extraction by the lime-sinter and lime-soda sinter process: Illinois Geol. Survey, Bull. 69, pp. 1-77, figs. 1-24.
Kinney, E. D. (1943) A process for extracting alumina from Kansas clay: Kansas Geol. Survey, Bull. 47, pt. 4, pp. 113-136. [available online]
Plummer, Norman, and Romary, J. F. (1947) Kansas clay, Dakota formation: Kansas Geol. Survey, Bull. 67, pp. 1-241, figs. 1-17, pls. 1-7.
Reynolds, R. J. Jr. (1951) Reynolds Metal Company, Annual Report for 1950, pp. 1-22.
Runnels, R. T. (1951) Some high-calcium limestones in Kansas: Kansas Geol. Survey, Bull. 90, pt. 5, pp. 77-104, figs. 1-3, pls. 1-2. [available online]
Kansas Geological Survey
Placed on web Jan. 4, 2019; originally published Oct. 15, 1952.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/96_7/index.html