
Kansas Geological Survey, Bulletin 47, pt. 2, originally published in 1943

Originally published in 1943 as Kansas Geological Survey Bulletin 47, pt. 2. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (6.3 MB) is also available.
This report is a brief study of the Kansas oil field brines, especially as related to their magnesium content: It has been known in a general way that some of the Kansas oil field brines have magnesium content greater than that of sea water from which, in recent years, metallic magnesium is being extracted successfully on a commercial scale. Similarly, metallic magnesium is also being extracted from oil field brines in Michigan. The chief purpose of the investigation was to ascertain the chemical nature of the waste brines, which are now very expensive to dispose of, and to determine whether metallic magnesium, an essential strategic material for war purposes, might not be extracted from these brines. During the summer of 1942 brine samples and other pertinent data were collected from 79 of the major and more important oil fields in the state. The brines were analyzed in the chemical laboratories at the University of Kansas.
On the basis of the analyses, at least six oil-producing formations (Layton, Stalnaker, Kansas City-Lansing, Hoover, Topeka, and Peru pay zones) have an average magnesium content greater than that of ordinary sea water. Certain other formations, such as "chat," Bartlesville, "conglomerate," and Simpson ("Wilcox") formations, yield brines essentially the same in their magnesium content as sea water. The "Mississippi lime," Gorham, Viola, "Hunton," and Arbuckle formations are deficient in this ingredient. The report includes tables giving the complete analyses of the brines studied.
The report includes a brief description of metallic magnesium and its compounds including their occurrences, uses, and derivation from oil field brines. Sources of required processing materials, costs, and prices of magnesium and its compounds are briefly outlined.
After discussing the factors controlling the commercial practicability of recovering magnesium from oil field brines, the report concludes with an analysis of Kansas oil fields worthy of consideration for magnesium recovery and recommended for further detailed study. The Kansas oil fields most worthy of consideration on the basis of data on hand are: Burrton field, Reno county; Bornholdt field, McPherson county; Zenith field, Stafford and Reno counties; Welch field, Rice county; Hall-Gurney field, Russell county; and Oxford field, Sumner county.
The present studies indicate that metallic magnesium extraction from oil field brines as a new and specialized industry in Kansas is not feasible. The information at hand does, however, suggest strongly that more detailed study may show the practicability of extracting magnesium from the brines as an auxiliary phase of the oil industry, to the extent of offsetting the high cost of brine disposal. This applies especially to the Burrton oil field in Reno county.
The presence of magnesium in oil field brines and the increasing usefulness of metallic magnesium as an essential strategic material for war purposes have prompted the State Geological Survey of Kansas to investigate the possibility of using the oil field brines of the state as a source of metallic magnesium. It has been known in a general way that some of the Kansas oil field brines have a magnesium content greater than that of sea water from which, in recent years, metallic magnesium has been commercially extracted at Freeport, Texas. Magnesium is also being extracted from oil field brines in Michigan and western Texas (Pawell, 1942, p. 331). Vast amounts of salt water or brine are produced annually along with the oil. At present, the brines are considered only a bothersome and expensive waste, the disposal of which constitutes one of the major problems of the oil operators. Oil field brine disposal is subject to regulations defined by statute. Brines must be disposed of without polluting surface streams, contaminating or mineralizing domestic underground water supplies, damaging soil, or killing vegetation.
Oil field brines in Kansas are disposed of legally in two general ways: (1) impounding it on the lease and (2) injecting the brine into subsurface formations at designated disposal wells. Occasionally, some brine is released directly on the surface, where it either penetrates into the soil, evaporates, or drains off into surface streams.
In any case, the disposal of the brine is bothersome. Furthermore, no mineral matter has as yet been salvaged from it; therefore, its disposal has been an expensive item in the cost of operating an oil lease or oil field. Because Kansas oil field brines contain mineral matter of economic wartime importance, the present investigation was inaugurated as part of the program of the State Geological Survey in the war effort.
The investigation for ascertaining the magnesium content of Kansas oil field brines and the possibility of extracting the mineral for wartime purposes was under the direction of R. C. Moore, State Geologist, and John C. Frye, Assistant State Geologist. The geological part of the project was assigned to Walter H. Schoewe, staff geologist. The chemical analyses were made by R. Q. Brewster, chemical consultant to the Geological Survey and professor of Chemistry at the University of Kansas, who was assisted by Calvin Vander Werf, assistant professor of Chemistry at the University of Kansas.
The present investigation covers the entire oil-producing sections of Kansas (fig. 1). It was impossible, however, because of limited time, to collect brine samples and other pertinent data from every oil pool in the state. Samples of brine were collected from practically all of the major and more important oil pools in western Kansas. Less time and effort were devoted to the eastern oil fields because of their declining production and also because of the greater difficulty in obtaining good and reliable brine samples. In all, 79 oil fields were visited. The names of the oil pools from which samples were obtained are indicated in table 1.
Figure 1—Map of Kansas showing location of oil and gas fields. After Raymond P. Kercher.
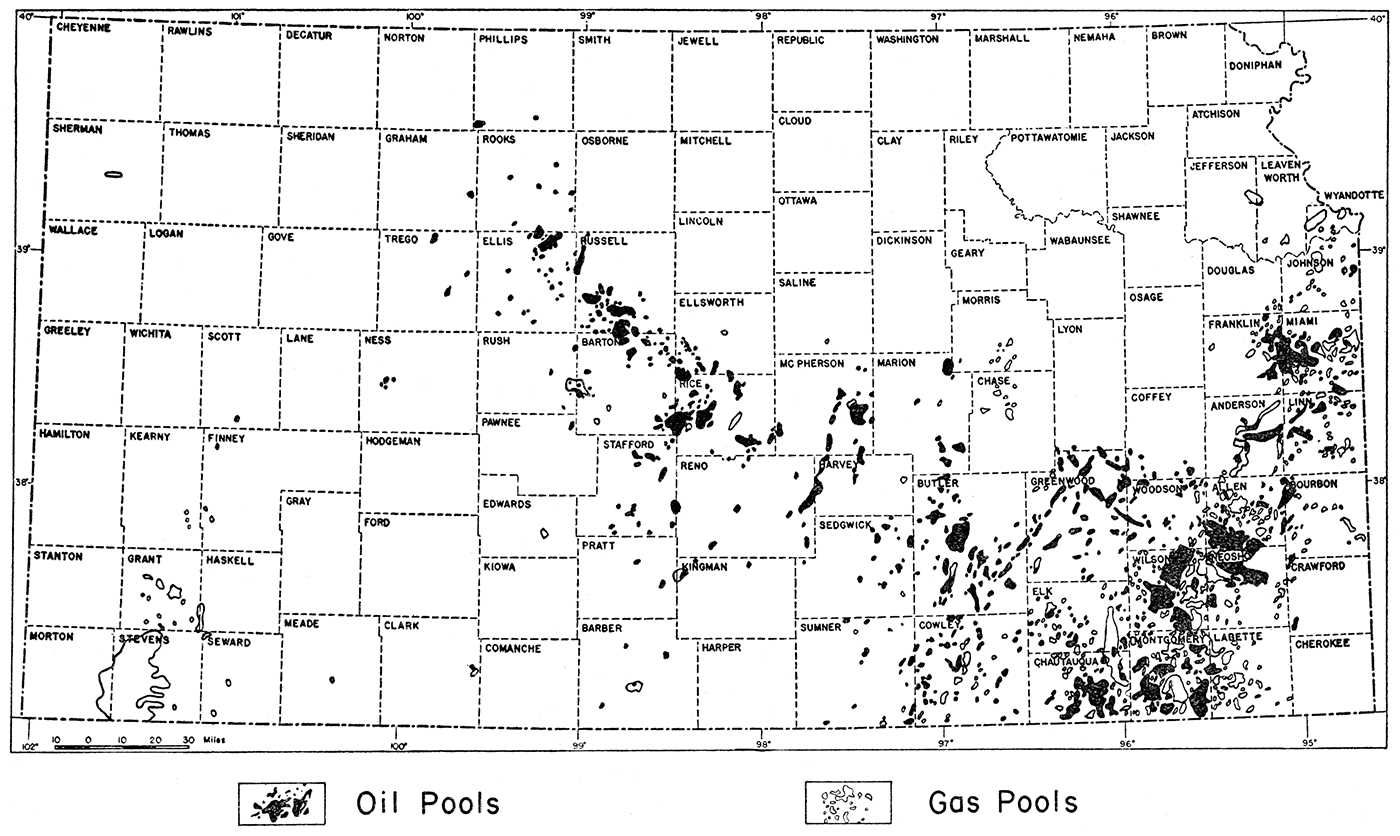
Table 1—Oil pools from which brine samples were collected
| Pool | County | Samples | Pool | County | Samples | |
|---|---|---|---|---|---|---|
| Agard | Greenwood | 1 | Hittle | Cowley | 2 | |
| Ainsworth | Barton | 2 | Johnson | McPherson | 1 | |
| Atherton | Russell | 1 | Keesling | Rice | 2 | |
| Augusta | Butler | 2 | Kraemer | Butler | 1 | |
| Augusta, North | Butler | 2 | Kraft-Prusa | Barton | 4 | |
| Beaumont | Greenwood | 2 | Lorraine | Ellsworth | 2 | |
| Bemis | Ellis | 2 | Madison | Greenwood | 2 | |
| Bloomer | Ellsworth, Barton | 4 | Miami | Miami | 5 | |
| New Albany | Elk | 1 | Oxford | Sumner | 5 | |
| Bornholdt | McPherson | 3 | Padgett | Sumner | 1 | |
| Browning | Greenwood | 1 | Peabody | Marion | 1 | |
| Burnett | Ellis | 1 | Peace Creek | Reno | 1 | |
| Burrton | Reno | 4 | Potwin | Butler | 2 | |
| Chase | Rice | 4 | Rainbow-Bend | Sumner | 1 | |
| Cunningham | Pratt | 4 | Ritz-Canton | McPherson | 8 | |
| Demalorie-Sowder | Greenwood | 1 | Russell | Russell | 2 | |
| Eastman | Cowley | 1 | Sallyards | Greenwood | 1 | |
| Edwards | Ellsworth | 2 | Sedan | Chautauqua | 1 | |
| Elbing | Butler | 4 | Seeley | Greenwood | 2 | |
| Eldorado | Butler | 6 | Sellens | Russell | 1 | |
| Eureka | Greenwood | 1 | Severy | Greenwood | 1 | |
| Fairport | Russell | 2 | Shutts | Ellis | 1 | |
| Fairport-Austin | Russell | 1 | Silica | Rice, Barton | 5 | |
| Fairport-North | Russell | 1 | Sperling | Harvey | 1 | |
| Florence | Marion | 1 | St. John | Stafford | 1 | |
| Foxbush | Butler | 1 | Stafford | Stafford | 1 | |
| Frog-Hollow | Cowley | 1 | State | Cowley | 1 | |
| Garden-Schaffer | Butler | 2 | Stoltenberg-Stratman | Ellsworth | 3 | |
| Geneseo | Rice | 3 | Trapp | Barton, Russell | 4 | |
| Goodrich | Sedgwick | 4 | Trapp, North | Russell | 1 | |
| Gorham-East | Russell | 5 | Valley Center | Sedgwick | 3 | |
| Gorham-West | Russell | 2 | Virgil-North | Coffey, Greenwood | 2 | |
| Graber | McPherson | 2 | Virgil-West | Greenwood | 1 | |
| Graham | Cowley | 5 | Voshell | McPherson | 2 | |
| Greenvale | Russell | 1 | Welch | Rice | 2 | |
| Greenwich | Sedgwick | 3 | Wellington | Sumner | 1 | |
| Hall-Gurney | Russell | 6 | Wherry | Rice | 2 | |
| Halstead | Harvey | 2 | Wherry-East | Rice | 2 | |
| Hinchman | Greenwood | 1 | Winterscheid | Woodson | 2 | |
| Zenith | Reno, Stafford | 4 |
Brine samples were collected from all of the important oil-bearing formations. Table 2 gives, in chronological sequence, the names of the oil-bearing formations or "pay zones" sampled, together with the number of samples obtained from each.
Table 2—Producing formations and number of samples collected
| Formation | Samples |
|---|---|
| Tarkio | 2 |
| Topeka | 4 |
| Hoover | 1 |
| Stalnaker | 1 |
| Kansas City-Lansing | 28 |
| Peru | 2 |
| Cherokee, top | 1 |
| Bartlesville (New Albany) | 14 |
| Gorham | 4 |
| "Basal conglomerate" | 4 |
| "Chat" | 18 |
| "Mississippi lime" | 8 |
| "Hunton" | 3 |
| Viola | 12 |
| Simpson ("Wilcox") | 16 |
| Arbuckle ("Siliceous lime, dolomite") | 51 |
Effort was made to collect brine samples representative of the entire oil field. The number of samples collected in any given field depended upon the size of the oil pool and the type of method used in disposing of the salt water. In the smaller fields, one or two samples were normally considered sufficient. In the larger fields, however, effort was made to obtain samples from various parts of the oil pool—from the center and both ends. Where the pool was sufficiently wide, samples were also collected at both sides of the field. Where the field was unusually long, samples were collected approximately every 2 miles along the trend of the field. In cases, however, where the salt water was disposed of in deep disposal wells, one sample Was collected at each disposal well, regardless of the length or breadth of the field.
Quantitatively, one quart of brine constituted the normal sample. The brine was collected in glass Mason jars provided with glass covers or tops. Samples were obtained at the wells, usually under the supervision of a farm boss, pumper, or other official. Because of the different methods employed in disposing of the brine waters, the samples were collected from various sources. Some came from the bleeder at the well. Others were obtained at the gun barrel at the siphon, gun barrel gauge, or at the place where the brine coming from the gun barrel siphon emptied into the settling tank, or where the brine, after leaving the siphon, emptied into a pond. In a few cases the valve of the gun barrel had to be opened in order to secure the sample. In oil pools where the salt water was disposed of by means of deep disposal wells, the samples were either collected at the bleeder of the well or at the place where the brine emptied into the settling tank or pit just previous to its entry into the well. In one or two cases, the sample was drawn off from the bottom of the oil storage tank.
The following oil companies and operators cooperated in the project: Aladdin Petroleum Corporation, Amerada Petroleum Corporation, Nate Appleman, Atlantic Refining Company, Aylward Production Company, Barnsdall Oil Company, Bay Petroleum Company, Beardmore Drilling Company, Inc., Beaumont Petroleum Company, H. C. Bennett, Bennett-Wolf, F. Berger, Big Brothers Oil Company, B. B. Blair, Bridgeport Machine Company, British-American Oil Producing Company, Carter Oil Company, Central Supply Company, Cities Service Oil Company, Colorado Petroleum, Inc., Conley and Cooper, Continental Oil Company, Darby Petroleum Company, Deep Rock Oil Corporation, Derby Oil Company, Dickey Oil Company, Drillers Gas Company, El Dorado Refining Company, Goldstein Company, Gulf Oil Corporation, J. J. Hall, Herndon Drilling Company, Carl Hipple, T. C. Johnson (Estate), Jones-Shelburne, A. Landon, Leader Oil Company, Leader Oil Company-Zephyr Drilling Company, Magnolia Petroleum Company, M. & L. Oil Company, Maxwell Petroleum Company, W. C. McBride, Inc., Ward McGinnis, McPherson Drilling Company, L. R. Mendenhall, Mid-Continent Petroleum Corporation, Mohawk Oil Company, National Refining Company, Ohio Oil Company, OKO Oil and Gas Company, Olson Drilling Company, Oskaloosa Oil and Gas Company, Palmer Oil Company, Phillips Petroleum Company, S. J. Polhamus (Estate), Prunty Production Company, Pryor and Lockhart, Inc., Santa Clara Drilling Company, Shaffer-Howell, E. B. Shawver, Shell Oil Company, Sinclair-Prairie Oil Company, Skelly Oil Company, Snowden and McSweeney, Solar Oil Corporation, L. Spencer, Stanolind Oil and Gas Company, State Training School, Sunray Oil Company, The Texas Company, Tidewater Oil Company, Transwestern Oil Company, White Star Oil, and York State Oil Company.
The present investigation for determining the magnesium content of the State's natural salt water or brines associated with the production of oil would have been impossible without the wholehearted and gracious cooperation of the various oil companies and operators. Although space does not permit the listing of the names of the many farm bosses, pumpers, and other employees of the various oil companies and operators, I wish to express my sincere thanks to them for their courteous and willing aid in helping me to obtain the brine samples and other pertinent data requested. The names of the oil companies and operators cooperating in this project are listed elsewhere in this bulletin. Special gratitude is due to the Cities Service Oil Company for providing me with office space at Oil Hill in the Eldorado district and also for permitting me to study certain data in the files.
I also wish to express my appreciation to Ogden S. Jones, geologist of the Oil Field Section, Division of Sanitation, Kansas State Board of Health, for making it possible for James Nelson, field geologist of the southern district of the same Oil Field Section, to accompany and assist me for several days in the field in collecting the first suite of samples and in obtaining the necessary information relative to them. Thanks are also due Paul Hollands, field geologist for the Great Bend district of the Conservation Division of the Kansas State Corporation Commission, for certain data relative to the project. I wish to express my thanks to John M. Jewett, geologist of the State Geological Survey, who collected five samples from Miami county. Lastly, I thank John C. Frye, Assistant State Geologist, under whose direct supervision the project was carried out, for his interest in the work and for his timely suggestions relative to the writing of this report; R. Q. Brewster for checking chemical data and for his suggestions relative to table 5 through 14; and Dorothea Weingartner for editorial assistance.
Chemical analyses of Kansas oil field brines show that the brines, irrespective of the oil fields and "pay zones" or horizons from which they come, are essentially alike in the dissolved chemical constituents that they contain, but that they differ materially as to their concentration. The analyses show that of all the dissolved chemical constituents contained in the brines, chloride, sodium, and calcium greatly predominate (table 3). Magnesium is next in importance quantitatively. Other elements present but occurring in much lesser amounts are bromine, iodine, aluminum, iron, and potassium. Magnesium, of all the chemical constituents contained in the brines, is of the greatest importance at the present time. It is the only one which occurs in sufficient amounts to offer possibilities of commercial extraction under present conditions. Other ingredients, such as sodium and calcium, occur in far greater amounts than does magnesium; but, since these substances may be obtained much more readily from other sources, their extraction from brines in Kansas is unwarranted and unprofitable. Studies show that certain formations are invariably higher in magnesium content than brines coming from other "pay zones" or oil-producing formations (table 4).
Table 3—Dissolved chemical constituents of Kansas oil field brines with range in milligrams per liter
| Chemical constituents |
Range |
|---|---|
| Total solids | 17,880-228,320 |
| Chloride | 10,729-142,547 |
| Sodium | 9,200-68,700 |
| Calcium | 246-7,900 |
| Magnesium | 153-3,960 |
| Bicarbonate | 12-869 |
| Bromide | 0-425 |
| Iodide | 0-8 |
| Sulphate | 0-2,800 |
| Aluminum | 0-781 |
| Iron | 0-15 |
Table 4—Magnesium content of Kansas oil field brines
| Formation | Magnesium in milligrams per liter | Samples represented |
|
|---|---|---|---|
| Range | Average | ||
| Layton | 3,960 | 1 | |
| Stalnaker | 2,620 | 1 | |
| Kansas City-Lansing | 972-3,910 | 2,525 | 31 |
| Hoover | 2,515 | 1 | |
| Topeka | 2,400 | 1 | |
| Peru | 1,840-2,610 | 2,225 | 2 |
| "Chat" | 704-2,460 | 1,461 | 19 |
| Bartlesville | 461-2,490 | 1,223 | 16 |
| "Conglomerate" | 750-1,455 | 1,184 | 4 |
| Simpson ("Wilcox") | 235-2,035 | 1,033 | 8 |
| "Mississippi lime" | 432-1,857 | 772 | 8 |
| Gorham | 450-1,030 | 701 | 3 |
| Viola | 185-1,547 | 584 | 14 |
| "Hunton" | 153-1,138 | 523 | 5 |
| Arbuckle | 152-3,110 | 523 | 52 |
| Sea Water | 1,045 (Jones, 1943, p. 18); 1,279 (Burnett, 1942, Table II); 1,400 (Frye, 1942, p. 105). | ||
According to the analyses, practically every oil-producing formation in Kansas yields, in some well or wells, brines containing magnesium in amounts greater than is contained in ordinary sea water. However, in most cases the magnesium content is much lower than that of sea water. The highest magnesium bearing brine is found in the Kansas City-Lansing zone. Not only does this zone produce the highest magnesium salt water, but it does so persistently in all wells and in all oil fields. The Arbuckle limestone, on the other hand, is prevailingly low in magnesium, with an average content less than 500 milligrams per liter. Even this formation, however, occasionally yields a brine high in magnesium. An Arbuckle brine obtained from a well in sec. 24, T. 11 S., R. 18 W., in the Burnett oil field, Ellis county, contained 3,110 milligrams of magnesium per liter.
Not only is there a difference in the magnesium content of the brines of the various oil-producing formations, but there is also a quantitative variation of this ingredient in anyone given formation. The magnesium concentration varies from one part of a field to another and also among the various oil fields. Variations among fields, as well as complete chemical analyses of the brines; are given in tables 5 through 14.
[Note: All chemical analyses in milligrams per liter. To convert to parts per million, divide by specific gravity of the brine. * denotes a concentration of less than 1 milligram per liter; ‡ denotes a concentration of less than 25 milligrams per liter; † denotes analyses supplied by private companies; all other analyses were made by R. Q. Brewster and Calvin Vander Werf at the University of Kansas.]
Table 5—Kansas City-Lansing brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Severy | Greenwood | 17 | 28 | 11E | 60 | 1225 | Pond | 1.098 | 164 | 2208 | 47600 | 6550 | 93905 | ‡ | 102 | 60 | 15 | ‡ | * | 150400 |
| Madison | Greenwood | 5 | 23 | 11E | - | 1060 | - | - | † | 1351 | 33812 | 3443 | 63333 | 76 | 109 | - | - | - | 12 | 103700 |
| Madison | Greenwood | 15 | 22 | 11E | - | 1310 | - | - | † | 1585 | 34366 | 3838 | 65333 | 67 | 113 | - | - | - | 12 | 106500 |
| Teeter | Greenwood | 16 | 23 | 9E | - | 1515 | - | - | † | 1785 | 40773 | 4739 | 78330 | 68 | 109 | - | - | - | 94 | 128200 |
| Teeter | Greenwood | 16 | 23 | 9E | - | 1915 | - | - | † | 1593 | 41852 | 5382 | 80330 | 70 | 106 | - | - | - | 84 | 131400 |
| Eldorado | Butler | 21 | 25 | 5E | - | 1743 | - | - | † | 2540 | 55690 | 8203 | 107783 | 41 | 48 | - | - | - | 42 | 174400 |
| Eldorado | Butler | 29 | 25 | 5E | - | 1970 | - | - | † | 3550 | 51483 | 14927 | 116032 | 50 | 130 | - | - | - | - | 186800 |
| Augusta | Butler | 35 | 27 | 4E | little | 2300 | Pond | 1.121 | 131 | 3410 | 53500 | 9050 | 131328 | ‡ | 72 | 105 | 5 | 210 | * | 197700 |
| Augusta | Butler | 17 | 28 | 4E | - | 2060 | - | - | † | 2974 | 58491 | 9533 | 115764 | 36 | 38 | - | - | - | 67 | 186900 |
| Augusta | Butler | 29 | 28 | 4E | - | 2140 | - | - | † | 2774 | 63020 | 8585 | 122500 | 128 | 38 | - | - | - | 17 | 199700 |
| North Augusta | Butler | 11 | 27 | 4E | 80 | 2200 | Pond | 1.106 | 130 | 2670 | 55200 | 8040 | 101534 | 150 | 108 | 50 | 8 | 320 | * | 177100 |
| Elbing | Butler | 17 | 23 | 4E | 20 | 2160 | Pond | 1.110 | 122 | 2262 | 55100 | 7200 | 107479 | 50 | 84 | 60 | 5 | ‡ | * | 172200 |
| Graham | Cowley | 10 | 33 | 3E | 40 | 2500 | Pond | 1.150 | 139 | 2940 | 77000 | 12480 | 145105 | ‡ | 12 | 100 | 3 | ‡ | * | 237600 |
| Hillsboro | Marion | 20 | 20 | 2E | - | 1980 | - | - | † | 2569 | 53564 | 6050 | 103000 | ‡ | 71 | - | - | - | 53 | 168000 |
| Goodrich | Sedgwick | 15 | 25 | 1E | 20 | 2626 | Pond | 1.150 | 30 | 3910 | 71380 | 10900 | 145860 | ‡ | 33 | 375 | 2 | 895 | 2 | 233400 |
| Silica | Rice | 27 | 19 | 10W | 30 | 3200 | Pond | 1.108 | 57 | 3492 | 52420 | 8085 | 109806 | ‡ | 82 | 130 | * | 864 | * | 174900 |
| Bloomer | Ellsworth | 31 | 17 | 10W | 30-40 | 3070 | Pond | 1.100 | 66 | 2705 | 49290 | 8965 | 98040 | ‡ | 113 | 155 | * | 461 | * | 161300 |
| Kraft-Prusa | Barton | 17 | 16 | 11W | 50 | - | Pond | 1.097 | 73 | 2690 | 42860 | 9610 | 92540 | 50 | 90 | 200 | 15 | 464 | 3 | 148200 |
| Cunningham | Pratt | 35 | 27 | 11W | 20 | 3450 | Pond | 1.158 | 174 | 3351 | 75600 | 14000 | 156749 | ‡ | 48 | 110 | 15 | 166 | 1 | 250000 |
| Cunningham | Pratt | 25 | 27 | 11W | little | 3450 | Pond | 1.150 | 176 | 2441 | 67400 | 16000 | 145855 | 175 | * | 115 | 10 | 84 | 80 | 232200 |
| Cunningham | Pratt | 24 | 27 | 11W | little | 3450 | Pond | 1.159 | 175 | 2810 | 75000 | 14730 | 156203 | 75 | 6 | 105 | 15 | 360 | 16 | 249300 |
| Greenvale | Russell | 4 | 15 | 12W | - | 3100 | Pond | 1.088 | 105 | 2685 | 41500 | 8440 | 89300 | ‡ | 110 | 30 | * | 93 | 2 | 142200 |
| Hall-Gurney | Russell | 31 | 14 | 13W | 5 | 2950 | pond | 1.114 | 92 | 3075 | 55300 | 11100 | 117825 | 200 | 48 | 70 | 10 | 51 | 1 | 187700 |
| Hall-Gurney | Russell | 26 | 14 | 13W | 8-30% | 2800 | Pond | 1.072 | 94 | 2420 | 38400 | 7000 | 80970 | 100 | 120 | 110 | * | ‡ | * | 129100 |
| Hall-Gurney | Russell | 22 | 14 | 14W | 32 | 2950 | Pond | 1.112 | 97 | 3240 | 53300 | 11200 | 117733 | 50 | 36 | 145 | 5 | 50 | 1 | 185800 |
| Gorham, East | Russell | 2 | 14 | 15W | - | 3050 | Deep well | 1.040 | 78 | 1015 | 16940 | 2540 | 34130 | 275 | 96 | 30 | 3 | 64 | 20 | 55000 |
| Gorham, West | Russell | 4 | 14 | 15W | 150 | 3050 | Pond | 1.040 | 81 | 972 | 17590 | 2560 | 34590 | 400 | 448 | 25 | * | ‡ | * | 56600 |
| Fairport-Austin | Russell | 31 | 12 | 15W | 75 | 3000 | Pond | 1.075 | 83 | 2200 | 34620 | 6420 | 73030 | 250 | 48 | 40 | 10 | 138 | 64 | 115200 |
| Fairport | Russell | 30 | 12 | 15W | little | 3000 | Pond | 1.095 | 84 | 2580 | 44675 | 9300 | 96660 | 75 | 48 | 95 | 5 | 402 | 2 | 152600 |
| Fairport | Russell | 8 | 12 | 15W | 5-10 | 3025 | Pond | 1.100 | 85 | 2240 | 50760 | 5670 | 94940 | 600 | 84 | 125 | 3 | 68 | * | 154200 |
| Fairport North | Russell | 32 | 11 | 15W | 30 | 3000 | Pond | 1.095 | 86 | 2760 | 43870 | 8620 | 92980 | 100 | 66 | 60 | 2 | 255 | 3 | 148700 |
Table 6—Bartlesville brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Shambaugh | Greenwood | 15 | 23 | 13E | 700 | 1565 | Deep well | 1.040 | 155 | 712 | 19900 | 1980 | 32059 | 50 | 232 | 25 | 2 | ‡ | * | 55000 |
| Seeley | Greenwood | 32 | 22 | 11E | 1000 | 2000 | Pond | 1.045 | 160 | 703 | 21675 | 2660 | 41172 | ‡ | 168 | 20 | 2 | ‡ | * | 66400 |
| Madison | Greenwood | 14 | 22 | 11E | 2 | 1900 | Pond | 1.052 | 156 | 900 | 25150 | 3065 | 49223 | ‡ | 180 | 40 | 2 | ‡ | * | 78600 |
| Madison | Greenwood | 14 | 22 | 11E | - | 1900 | - | - | † | 632 | 28629 | 3694 | 52830 | 491 | 88 | - | - | - | 13 | 87200 |
| Madison | Greenwood | 12 | 22 | 11E | 50 | 1800 | Pond | 1.060 | 157 | 1078 | 28800 | 3660 | 56876 | ‡ | 84 | 20 | 2 | ‡ | * | 90500 |
| Demalorie-Sowder | Greenwood | 12 | 22 | 10E | 2 | 2000 | Pond | 1.063 | 158 | 1093 | 29750 | 4400 | 61051 | ‡ | 6 | 75 | 2 | 81 | 62 | 96500 |
| Browning | Greenwood | 30 | 22 | 10E | 30 | 2300 | Pond | 1.068 | 159 | 1122 | 33150 | 4415 | 63875 | 100 | 54 | 60 | 5 | 129 | 10 | 102900 |
| Teeter | Greenwood | 16 | 23 | 9E | - | 2540 | - | - | † | 863 | 26715 | 3316 | 50000 | 14 | 61 | - | - | - | 99 | 81700 |
| Sallyards | Greenwood | 1 | 26 | 8E | little | 2400 | Deep well | 1.079 | 167 | 1187 | 34400 | 6160 | 77890 | 50 | 72 | 20 | 8 | 48 | 5 | 119800 |
| Eastman | Butler | 6 | 31 | 6E | 85 | 2850 | Pond | 1.100 | 133 | 1330 | 52000 | 5600 | 98098 | ‡ | 54 | 40 | 5 | 190 | 3 | 157300 |
| Garden-Schaffer | do. | 6 | 27 | 6E | 100 | 2750 | Pond | 1.102 | 126 | 1564 | 51100 | 7460 | 100184 | 75 | 96 | 150 | 3 | 87 | 1 | 160700 |
| Frog-Hollow | Cowley | 20 | 32 | 5E | 50 | 3000 | Pond | 1.140 | 134 | 2060 | 46000 | 14680 | 165494 | 50 | 18 | 60 | * | 230 | 8 | 228600 |
| Fox-Bush | Butler | 26 | 28 | 5E | 100 | 2800 | Pond | 1.095 | 132 | 1910 | 47400 | 6820 | 92396 | 400 | 36 | 20 | 2 | 293 | 3 | 149300 |
| Haverhill | Butler | 27 | 27 | 5E | - | 2774 | - | - | † | 1464 | 47095 | 7100 | 90000 | 33 | 84 | - | - | - | 36 | 146500 |
| Augusta | Butler | 16 | 28 | 4E | 400 | 2550 | Pond | 1.030 | 117 | 461 | 11900 | 1830 | 23659 | 750 | 210 | 110 | * | ‡ | * | 38900 |
| Rainbow-Bend | Sumner | 21 | 33 | 3E | 100 | 3200 | Pond | 1.141 | 141 | 2490 | 69600 | 12120 | 141167 | 50 | 12 | 130 | 2 | 302 | 7 | 225900 |
Table 7—"Conglomerate" brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Wherry | Rice | 6 | 21 | 7W | little | 3400 | Pond | 1.070 | 24 | 1190 | 34830 | 5100 | 66951 | ‡ | 74 | 175 | * | 33 | 4 | 108400 |
| Wherry | Rice | 12 | 21 | 7W | 40 | 3469 | Pond | 1.090 | 15 | 1455 | 46600 | 6050 | 89412 | ‡ | 27 | 425 | * | 440 | 10 | 144700 |
| Wherry | Rice | 16 | 21 | 7W | 20 | 3400 | Pond | 1.066 | 23 | 750 | 34520 | 5330 | 65570 | ‡ | 84 | 270 | * | 57 | 6 | 106700 |
| Wherry | Rice | 21 | 21 | 7W | - | - | Pond | 1.080 | 16 | 1340 | 40170 | 5700 | 67630 | ‡ | 120 | 385 | * | 294 | * | 125600 |
Table 8—"Chat" brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Greenwich | Sedgwick | 14 | 26 | 2E | 4000 | 2850 | Deep well | 1.072 | 37 | 1192 | 36420 | 4260 | 69000 | ‡ | 86 | 10 | 8 | 223 | * | 111200 |
| Goodrich | Sedgwick | 21 | 25 | 1E | 600 | 3000 | Pond | 1.075 | 29 | 1110 | 36020 | 4050 | 62588 | ‡ | 90 | 200 | 2 | 220 | * | 109200 |
| Wellington | Sumner | 4 | 32 | 1W | 20 | 3690 | Pond | 1.143 | 147 | 1950 | 66500 | 13480 | 137652 | 2200 | 30 | 190 | 2 | 781 | 15 | 222800 |
| Ritz-Canton | McPherson | 19 | 19 | 1W | 300 | 2900 | Pond | 1.079 | 44 | 704 | 40560 | 4070 | 72578 | 50 | 73 | 45 | 2 | ‡ | * | 117960 |
| Ritz-Canton | McPherson | 29 | 19 | 1W | 80 | 2960 | Pond | 1.071 | 41 | 1182 | 37270 | 3940 | 69010 | ‡ | 59 | 170 | * | 130 | * | 111760 |
| Ritz-Canton | McPherson | 25 | 19 | 2W | 360 | 2970 | Deep well | 1.075 | 42 | 1101 | 37470 | 3980 | 69560 | ‡ | 89 | 70 | 3 | 113 | 10 | 112160 |
| Ritz-Canton | McPherson | 36 | 19 | 2W | 120 | 3000 | Pond | 1.068 | 43 | 859 | 35380 | 3624 | 64610 | ‡ | 64 | 58 | * | 121 | * | 104720 |
| Halstead | Harvey | 2 | 23 | 2W | 3000 | 3000 | Deep well | 1.070 | 2 | 794 | 34770 | 3350 | 61700 | ‡ | 130 | 150 | * | 142 | 2 | 101250 |
| Halstead | Harvey | 12 | 23 | 2W | - | 2980 | Pond | 1.070 | 1 | 980 | 33110 | 3480 | 63310 | ‡ | 122 | 110 | 2 | ‡ | * | 101400 |
| Johnson | McPherson | 30 | 19 | 3W | 60-70 | 3100 | Pond | 1.087 | 48 | 1491 | 43980 | 5720 | 83450 | ‡ | 40 | 50 | * | 38 | * | 134720 |
| Burrton | Reno | 15 | 24 | 4W | 4000 | 3400 | Deep well | 1.115 | 8 | 2180 | 66652 | 9200 | 129015 | ‡ | 60 | 247 | * | 525 | 1 | 207880 |
| Burrton | Reno | 10 | 24 | 4W | - | - | Pond | 1.128 | 9 | 2460 | 75264 | 10600 | 143862 | ‡ | 84 | 110 | * | ‡ | * | 232380 |
| Burrton | Reno | 23 | 23 | 4W | - | - | Deep well | 1.110 | 10 | 2140 | 58720 | 8620 | 113200 | ‡ | 48 | 395 | * | ‡ | * | 183220 |
| Burrton | Reno | 1 | 23 | 4W | - | 3500 | Pond | 1.119 | 11 | 2270 | 67370 | 9510 | 128240 | 50 | 94 | 130 | * | ‡ | * | 207620 |
| Bornholdt | McPherson | 31 | 20 | 5W | - | 3333 | Deep well | 1.098 | 12 | 1510 | 57942 | 7370 | 109290 | 40 | 46 | 310 | * | 335 | 3 | 176850 |
| Bornholdt | McPherson | 29 | 20 | 5W | 550 | 3350 | Deep well | 1.090 | 13 | 1630 | 46400 | 7040 | 89316 | ‡ | 33 | 420 | * | ‡ | * | 146840 |
| Bornholdt | McPherson | 18 | 20 | 5W | 300 | - | Pond | 1.085 | 14 | 1410 | 38900 | 6100 | 91140 | ‡ | 40 | 90 | * | 208 | 4 | 137880 |
| Welch | Rice | 3 | 21 | 6W | 1700 | 3410 | Deep well | 1.105 | 6 | 1230 | 53568 | 7800 | 103324 | ‡ | 40 | 150 | * | 540 | 8 | 166660 |
| Welch | Rice | 2 | 21 | 6W | 1000 | 2350 | Deep well | 1.100 | 7 | 1560 | 51197 | 7450 | 101420 | ‡ | 46 | 100 | * | 900 | 7 | 162680 |
Table 9—Mississippi lime brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Stephenson | Woodson | 7 | 24 | 14E | 25 | 1700 | Pond | 1.037 | 152 | 514 | 16500 | 2340 | 32107 | 125 | 264 | 20 | * | 49 | 1 | 51900 |
| Winterschied | Woodson | 29 | 23 | 14E | 12 | 1700 | Pond | 1.050 | 153 | 458 | 24200 | 1890 | 43105 | ‡ | 104 | 10 | 2 | 20 | 11 | 69800 |
| Hinchman | Greenwood | 20 | 24 | 13E | 200 | 1600 | Pond | 1.036 | 150 | 641 | 16700 | 1765 | 29415 | 450 | 342 | * | 2 | 58 | 1 | 50000 |
| Blackwell | Greenwood | 10 | 24 | 13E | 100 | 1659 | Pond | 1.033 | 151 | 600 | 15050 | 1630 | 28484 | 550 | 396 | 10 | * | ‡ | * | 46700 |
| Virgil-North | Coffey | 14 | 23 | 13E | 25 | 1700 | Pond | 1.025 | 154 | 432 | 11050 | 1117 | 20677 | 150 | 444 | 10 | * | ‡ | * | 33900 |
| Virgil | Greenwood | 11 | 24 | 12E | 200 | 1750 | Deep well | 1.037 | 168 | 700 | 16700 | 2180 | 32441 | ‡ | 254 | 5 | * | ‡ | * | 52300 |
| Eureka | Greenwood | 36 | 25 | 10E | 20 | 2000 | Pond | 1.050 | 163 | 971 | 22500 | 2830 | 43673 | ‡ | 246 | 20 | * | ‡ | * | 70200 |
| Beaumont | Greenwood | 25 | 27 | 8E | 30 | 2510 | Pond | 1.091 | 165 | 1857 | 44550 | 6780 | 89009 | 175 | 96 | 10 | 3 | ‡ | * | 142500 |
Table 10—Hunton brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Peabody | Marion | 15 | 22 | 4E | 6097 | 2510 | Pond | 1.015 | 124 | 153 | 5760 | 710 | 10982 | 225 | 330 | * | * | ‡ | * | 18200 |
| Elbing | Butler | 20 | 23 | 4E | 3748 | 2370 | Pond | 1.020 | 120 | 320 | 8500 | 990 | 16195 | 725 | 300 | 10 | * | ‡ | * | 27000 |
| Graber | McPherson | 20 | 21 | 1W | 1200 | 3300 | Deep well | 1.044 | 38 | 751 | 21090 | 2327 | 40090 | ‡ | 96 | * | * | 133 | 3 | 64300 |
| Graber | McPherson | 32 | 21 | 1W | - | 3300 | Deep well | 1.075 | 39 | 1138 | 36590 | 4149 | 68430 | ‡ | 67 | * | 2 | 182 | * | 110600 |
| Sperling | Harvey | 13 | 22 | 2W | 60 | 3305 | Deep well | 1.031 | 3 | 253 | 14150 | 1260 | 25000 | 100 | 120 | 65 | * | ‡ | * | 41300 |
Table 11—Viola brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | |||||||||||||||||||
| Garden-Schaffer | Butler | 4 | 27 | 6E | 400 | 3100 | Pond | 1.031 | 127 | 516 | 13200 | 1800 | 25720 | 700 | 264 | * | * | ‡ | * | 42200 | |
| Eldorado | Butler | 4 | 26 | 5E | 15 | 2650 | Pond | 1.048 | 108 | 1170 | 22500 | 3140 | 44656 | 375 | 24 | 15 | * | ‡ | * | 71900 | |
| Eldorado | Butler | 17 | 26 | 5E | Much | 2600 | Pond | 1.040 | 112 | 864 | 18600 | 2640 | 36715 | 500 | 396 | 5 | * | ‡ | * | 59700 | |
| Florence | Marion | 18 | 21 | 5E | - | 2300 | Pond | 1.017 | 125 | 228 | 5670 | 616 | 10729 | 325 | 312 | * | * | ‡ | * | 17900 | |
| Elbing | Butler | 17 | 23 | 4E | - | 2350 | - | - | † | 371 | 5399 | 909 | 10000 | 1178 | 301 | - | - | - | 24 | 18100 | |
| Elbing | Butler | 17 | 23 | 4E | 150 | 2400 | Pond | 1.018 | 123 | 248 | 6600 | 870 | 12766 | 300 | 246 | 10 | * | ‡ | * | 21000 | |
| Greenwich | Sedgwick | 14 | 26 | 2E | 4000 | 3100 | Deep well | 1.020 | 36 | 204 | 7800 | 755 | 14320 | ‡ | 501 | * | * | ‡ | * | 23600 | |
| Hillsboro | Marion | 28 | 20 | 2E | - | 3037 | - | - | † | 271 | 5284 | 597 | 10000 | 15 | 79 | - | - | - | - | 16400 | |
| Ritz-Canton | McPherson | 20 | 19 | 1W | ** | 3000 | Deep well | 1.030 | 40 | 404 | 11980 | 1803 | 22990 | 75 | 233 | 15 | * | ‡ | * | 37400 | |
| Ritz-Canton | McPherson | 17 | 19 | 1W | 800 | 3387 | Pond | 1.030 | 46 | 353 | 11980 | 1619 | 22520 | 65 | 175 | 10 | * | ‡ | * | 36700 | |
| Ritz-Canton | McPherson | 11 | 19 | 2W | 35 | 3300 | Pond | 1.020 | 45 | 185 | 9280 | 1191 | 17111 | 55 | 322 | 15 | * | ‡ | * | 28200 | |
| Peace Creek | Reno | 22 | 23 | 10W | - | - | Pond | 1.035 | 22 | 640 | 16720 | 1670 | 30918 | ‡ | 650 | 15 | * | 65 | * | 50700 | |
| Cunningham | Pratt | 12 | 28 | 11W | 20 | 4250 | Pond | 1.095 | 177 | 1547 | 46500 | 7900 | 83852 | 175 | 12 | 90 | 8 | 110 | 6 | 140200 | |
| Stafford | Stafford | 15 | 24 | 12W | - | 3800 | - | 1.065 | 20 | 1140 | 30100 | 5540 | 60500 | ‡ | 210 | 65 | * | 91 | * | 97600 | |
| ** Several hundred. | |||||||||||||||||||||
Table 12—Simpson (Wilcox) brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Padgett | Sumner | 26 | 34 | 2E | 30 | 3500 | Pond | 1.140 | 140 | 2035 | 68500 | 14400 | 142547 | 300 | 18 | 105 | 3 | 407 | 5 | 228300 |
| Greenwich | Sedgwick | 15 | 26 | 2E | 300 | 3000 | Deep well | 1.020 | 35 | 288 | 8360 | 789 | 15990 | 90 | 530 | * | * | ‡ | * | 26000 |
| Valley Center | Sedgwick | 1 | 26 | 1E | 140 | 3390 | Deep well | 1.040 | 32 | 628 | 17830 | 1900 | 32965 | 90 | 250 | 80 | * | ‡ | * | 53600 |
| Ritz-Canton | McPherson | 21 | 19 | 1W | 75 | 3440 | Pond | 1.015 | 47 | 235 | 5560 | 892 | 10874 | 80 | 184 | * | * | ‡ | * | 17700 |
| Zenith | Reno | 7 | 24 | 10W | - | 3607 | Pond | 1.055 | 17 | 1150 | 26170 | 4150 | 51533 | ‡ | 230 | 45 | * | ‡ | * | 83300 |
| Zenith | Stafford | 14 | 24 | 11W | - | - | Deep well | 1.065 | 21 | 1450 | 31290 | 5260 | 63860 | ‡ | 204 | 75 | * | 350 | 1 | 102700 |
| Zenith | Stafford | 15 | 24 | 11W | 200 | 3600 | Pond | 1.075 | 18 | 1515 | 36080 | 5900 | 71000 | ‡ | 72 | 345 | * | ‡ | * | 114900 |
| Zenith | Stafford | 23 | 24 | 11W | - | 3600 | Pond | 1.050 | 19 | 985 | 20745 | 3220 | 29410 | ‡ | 335 | 70 | * | 47 | 1 | 76400 |
Table 13—Arbuckle brine data with chemical analyses
| Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | ||||||||||||||||||
| Beaumont | Greenwood | 25 | 27 | 8E | 100 | 2750 | Pond | 1.045 | 166 | 674 | 19000 | 3050 | 38034 | 325 | 312 | 5 | * | ‡ | * | 61400 |
| Eldorado | Butler | 5 | 26 | 5E | 400 | 2450 | Deep well | 1.028 | 109 | 408 | 10000 | 1635 | 20153 | 650 | 264 | 10 | * | ‡ | * | 33100 |
| Eldorado | Butler | 15 | 25 | 5E | - | 2500 | - | - | † | 313 | 11054 | 1398 | 19330 | 1357 | 322 | - | - | - | 25 | 33700 |
| Hittle | Cowley | 28 | 31 | 4E | - | 3300 | Pond | 1.043 | 115 | 519 | 15440 | 2070 | 30000 | 450 | 606 | 35 | * | ‡ | * | 49100 |
| State | Cowley | 15 | 32 | 4E | 300 | - | Pond | 1.055 | 114 | 835 | 24650 | 3720 | 48706 | 700 | 294 | 15 | * | ‡ | * | 78900 |
| Graham | Cowley | 9 | 33 | 3E | 375 | 3500 | Pond | 1.067 | 136 | 1050 | 30900 | 4130 | 59558 | 400 | 252 | 30 | * | ‡ | * | 96300 |
| Graham | Cowley | 10 | 33 | 3E | 400 | 3500 | Pond | 1.064 | 137 | 1263 | 29200 | 4130 | 57142 | 400 | 270 | 15 | * | ‡ | * | 92400 |
| Graham | Cowley | 10 | 33 | 3E | 800 | 3500 | Pond | 1.073 | 138 | 1263 | 34450 | 5170 | 67655 | 750 | 252 | 20 | * | ‡ | * | 109600 |
| Voshell | McPherson | 9 | 21 | 3W | 350 | 3395 | Pond | 1.028 | 5 | 380 | 12378 | 1550 | 22963 | 50 | 404 | 55 | * | ‡ | * | 37800 |
| Lorraine | Ellsworth | 23 | 17 | 3W | 500-1000 | 3260 | Deep well | 1.024 | 62 | 366 | 10320 | 1430 | 19350 | 275 | 522 | 15 | * | ‡ | * | 32300 |
| Geneseo | Rice | 19 | 18 | 7W | - | - | Deep well | 1.018 | 26 | 320 | 7150 | 1260 | 14220 | - | 245 | 10 | * | ‡ | * | 23200 |
| Geneseo | Rice | 6 | 19 | 7W | - | - | Pond | 1.014 | 25 | 237 | 6990 | 835 | 12832 | - | 576 | 10 | * | ‡ | * | 21500 |
| Geneseo | Rice | 12 | 18 | 8W | - | - | Pond | 1.025 | 27 | 340 | 10430 | 1760 | 20280 | - | 505 | 5 | * | ‡ | * | 33300 |
| Edwards | Ellsworth | 27 | 17 | 8W | 240 | 3200 | Pond | 1.033 | 60 | 505 | 13440 | 2205 | 25970 | 350 | 427 | 10 | * | ‡ | * | 43000 |
| Edwards | Ellsworth | 33 | 17 | 8W | 150-200 | 3250 | Deep well | 1.024 | 59 | 246 | 8460 | 1562 | 16413 | 400 | 512 | 5 | * | ‡ | * | 27200 |
| Lorraine | Ellsworth | 11 | 17 | 9W | - | 3216 | - | - | † | 152 | 6260 | 400 | 10660 | 35 | 476 | - | - | - | 1 | 18000 |
| Lorraine | Ellsworth | 23 | 17 | 9W | Much | 3200 | Pond | 1.024 | 61 | 188 | 6120 | 657 | 11070 | - | 632 | 20 | * | ‡ | * | 18700 |
| Chase | Rice | - | 19 | 9W | 800-1000 | 3250 | Deep well | 1.020 | 54 | 209 | 7360 | 647 | 14370 | - | 579 | 5 | * | ‡ | * | 21800 |
| Chase | Rice | 21 | 19 | 9W | 385 | 3250 | Deep well | 1.017 | 53 | 288 | 7520 | 994 | 14090 | - | 507 | 5 | * | ‡ | * | 23400 |
| Chase | Rice | 5 | 20 | 9W | 1500 | 3250 | Deep well | 1.020 | 52 | 299 | 7540 | 1304 | 14650 | 75 | 411 | 5 | * | ‡ | * | 24300 |
| Chase | Rice | 18 | 20 | 9W | 360 | 3380 | Pond | 1.020 | 51 | 227 | 6660 | 860 | 12310 | 70 | 510 | 5 | * | ‡ | * | 20600 |
| Chase | Rice | 19 | 20 | 9W | - | 3384 | - | - | † | 167 | 6484 | 917 | 11000 | 1340 | 351 | - | - | - | - | 20200 |
| Keesling | Rice | 10 | 20 | 9W | 100 | 3230 | Pond | 1.020 | 49 | 273 | 8910 | 788 | 15830 | 55 | 577 | 5 | * | ‡ | * | 26400 |
| Keesling | Rice | 16 | 20 | 9W | 900 | 3260 | Pond | 1.018 | 50 | 229 | 7000 | 761 | 12700 | - | 507 | - | * | ‡ | * | 21200 |
| Silica | Rice | 33 | 19 | 10W | 1224 | 3300 | Deep-well | 1.022 | 56 | 317 | 8690 | 1258 | 16550 | 80 | 344 | - | * | ‡ | * | 27200 |
| Silica | Rice | 34 | 19 | 10W | - | 3297 | Pond | 1.022 | 58 | 301 | 8180 | 1078 | 15280 | 500 | 519 | 5 | * | ‡ | * | 25400 |
| Stoltenberg | Ellsworth | 1 | 17 | 10W | 3000 | 3270 | Pond | 1.026 | 63 | 526 | 8750 | 2062 | 18820 | 625 | 394 | 25 | * | ‡ | * | 31200 |
| Bloomer | Ellsworth | 31 | 17 | 10W | 100 | 3240 | Pond | 1.020 | 65 | 281 | 7710 | 1190 | 14494 | 475 | 489 | - | * | ~ | * | 24600 |
| Bloomer | Ellsworth | 33 | 17 | 10W | 6 | 3800 | Pond | 1.024 | 71 | 445 | 7780 | 1370 | 15380 | 675 | 264 | 10 | * | ‡ | * | 26000 |
| Bloomer | Ellsworth | 36 | 17 | 11W | 50 | 3280 | Pond | 1.031 | 67 | 649 | 14100 | 2360 | 27500 | 550 | 581 | 30 | * | ‡ | * | 44800 |
| Silica | Barton | 1 | 20 | 11W | 900 | 3200 | Deep well | 1.020 | 55 | 287 | 7900 | 1041 | 14916 | - | 181 | - | * | ‡ | * | 24300 |
| Silica | Barton | 12 | 20 | 11W | 900 | 3300 | Deep well | 1.020 | 64 | 338 | 7980 | 1203 | 15030 | 525 | 497 | 25 | * | ‡ | * | 25600 |
| Kraft-Prusa | Barton | 17 | 16 | 11W | 240 | - | Pond | 1.020 | 72 | 310 | 6330 | 1180 | 12515 | - | 696 | 10 | " | ‡ | * | 21300 |
| Kraft-Prusa | Barton | 33 | 16 | 11W | 115 | 3350 | Pond | 1.020 | 70 | 296 | 6950 | 1270 | 13365 | 525 | 605 | 10 | * | ‡ | * | 23000 |
| Hall-Gurney | Russell | 24 | 14 | 13W | 120 | 3200 | Pond | 1.027 | 95 | 600 | 10900 | 2095 | 23031 | 650 | 269 | 15 | * | ‡ | * | 37600 |
| St. Johns | Stafford | 28 | 24 | 13W | 15 | 4200 | Pond | 1.035 | 178 | 500 | 10230 | 2335 | 32207 | 2200 | 378 | * | * | ‡ | * | 47900 |
| Ainsworth | Barton | 33 | 16 | 13W | 200 | 3400 | Deep well | 1.022 | 103 | 399 | 7790 | 1580 | 16537 | 450 | 444 | * | * | ‡ | * | 27200 |
| Ainsworth | Barton | 26 | 18 | 13W | - | 3380 | Pond | 1.022 | 102 | 340 | 8550 | 1595 | 17616 | 625 | 474 | * | * | ‡ | * | 29200 |
| Trapp | Barton | 8 | 16 | 13W | 30% | 3300 | Pond | 1.028 | 101 | 460 | 10850 | 1550 | 20360 | 250 | 690 | * | * | ‡ | * | 34200 |
| Trapp | Barton | 29 | 15 | 13W | 300 | 3300 | Pond | 1.032 | 99 | 618 | 13050 | 2370 | 27042 | 180 | 660 | * | * | ‡ | * | 43900 |
| Sellens | Russell | 26 | 15 | 13W | 350 | 3300 | Deep well | 1.029 | 100 | 618 | 11840 | 2260 | 26270 | 300 | 312 | * | * | ‡ | * | 41600 |
| Russell | Russell | 27 | 13 | 14W | 100 | 3290 | Deep well | 1.024 | 75 | 316 | 8750 | 715 | 15550 | 250 | 498 | 5 | * | ‡ | * | 26100 |
| Russell | Russell | 33 | 13 | 14W | 70 | 3290 | Pond | 1.022 | 106 | 479 | 8800 | 1910 | 19060 | 300 | 532 | - | * | - | * | 31100 |
| Ochs | Russell | 24 | 15 | 14W | - | 3350 | - | - | † | 425 | 12106 | 2142 | 22000 | 2227 | 501 | - | - | - | 31 | 39500 |
| Trapp | Russell | 25 | 15 | 14W | 100 | 3325 | Pond | 1.033 | 98 | 585 | 15050 | 2575 | 25763 | 160 | 588 | * | * | ‡ | * | 44700 |
| Trapp | Russell | 36 | 15 | 14W | - | 3400 | Pond | 1.028 | 104 | 545 | 12100 | 1690 | 23870 | 275 | 720 | * | * | ‡ | * | 39200 |
| Atherton | Russell | 30 | 13 | 14W | 100 | 3200 | Deep well | 1.023 | 107 | 420 | 7400 | 1780 | 16449 | 325 | 786 | * | * | ‡ | * | 27200 |
| North Trapp | Russell | 8 | 13 | 15W | 300 | 3000 | Pond | 1.032 | 96 | 708 | 12380 | 2470 | 26383 | 150 | 692 | 17 | * | ‡ | * | 42800 |
| Beemis | Ellis | 12 | 11 | 17W | 130 | 3350 | Deep well | 1.045 | 87 | 804 | 18400 | 3120 | 37430 | 125 | 294 | 35 | * | 107 | 5 | 60500 |
| Beemis | Ellis | 14 | 11 | 17W | 80 | 3396 | Pond | 1.044 | 88 | 766 | 17720 | 2660 | 35410 | 135 | 402 | 20 | * | ‡ | * | 57100 |
| Shutts | Ellis | 5 | 12 | 17W | 150 | 3600 | Pond | 1.037 | 90 | 615 | 15550 | 2500 | 31110 | 700 | 324 | * | * | ‡ | * | 50800 |
| Burnett | Ellis | 24 | 11 | 18W | little | 3150 | Pond | 1.115 | 89 | 3110 | 52000 | 10990 | 112670 | 75 | 144 | 77 | * | 248 | 6 | 179300 |
Table 14—Topeka, Hoover, Stalnaker, Layton, Peru, Gorham, and New Albany brine data with chemical analyses
| Zone | Pool | County | Location | Brine (bbls. day) |
Depth to horizon |
Disposal method |
Sp. Gr. (60° F.) |
Sample number |
Magnesium (Mg) |
Sodium (Na) |
Calcium (Ca) |
Chloride (Cl) |
Sulphate (SO4) |
Bicarbonate (HCO3) |
Bromide (Br) |
Iodide (I) |
Aluminum (Al) |
Iron (Fe) |
Total solids |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | |||||||||||||||||||
| Topeka | Oxford | Sumner | 14 | 32 | 2E | 75 | 1300 | Deep well | 1.140 | 145 | 2400 | 65000 | 9410 | 127530 | 2100 | 72 | 180 | 4 | 220 | 4 | 206900 |
| Hoover | Oxford | Sumner | 14 | 32 | 2E | 75 | 1700 | Deep well | 1.140 | 146 | 2515 | 67500 | 9415 | 131874 | 1400 | 36 | 280 | 2 | 290 | 8 | 213300 |
| Stalnaker | Oxford | Sumner | 14 | 32 | 2E | 870 | 2100 | Deep well | 1.155 | 142 | 2620 | 71100 | 11100 | 141072 | ‡ | 24 | 120 | 2 | 710 | 12 | 226800 |
| Layton | Oxford | Sumner | 14 | 32 | 2E | 100 | 2400 | Deep well | 1.158 | 144 | 3960 | 75000 | 11820 | 152162 | ‡ | 72 | 180 | 2 | 321 | 3 | 243500 |
| Peru | Sedan | Chautauqua | 12 | 33 | 10E | 50 | 1400 | Pond | 1.090 | 148 | 1840 | 40500 | 6910 | 82962 | ‡ | 54 | 90 | 4 | 115 | 5 | 132500 |
| Peru | Hittle | Cowley | 24 | 31 | 4E | little | 2400 | Pond | 1.135 | 116 | 2610 | 68700 | 11300 | 138884 | 50 | 24 | * | * | 371 | 5 | 221900 |
| Gorham | Hall-Gurney | Russell | 31 | 14 | 13W | 30-40% | 3300 | Pond | 1.024 | 93 | 450 | 10950 | 1150 | 19452 | 275 | 513 | 10 | * | ‡ | * | 32800 |
| Gorham | Gorham, East | Russell | 2 | 14 | 15W | - | 3250 | Deep well | 1.045 | 77 | 1030 | 17060 | 3960 | 35950 | 625 | 750 | 10 | * | ‡ | * | 59300 |
| Gorham | Gorham, East | Russell | 11 | 14 | 15W | Much | 3257 | Pond | 1.028 | 82 | 624 | 8300 | 1370 | 17075 | 50 | 841 | 5 | * | ‡ | * | 28400 |
| New Albany | New Albany | Elk | 3 | 29 | 13E | 25 | 550 | Creek | 1.055 | 149 | 1750 | 26800 | 2880 | 51164 | 50 | 186 | 30 | 8 | 170 | 2 | 83000 |
Metallic magnesium is a silvery white metal of high tensile strength. It is the lightest of all the known metals that are comparatively little altered under ordinary atmospheric conditions. Its specific gravity is 1.74; its melting point is 633° C.; and its boiling point is 1120° C.
Magnesium occurs in nature only in the combined state and is one of the most common metals associated with rocks. It constitutes approximately 2.1 percent of the earth's crust; which makes it the eighth most abundant element or sixth most abundant metal.
Common magnesium-bearing minerals include magnesite, MgCO3; dolomite, CaMg(CO3)2; olivine, 2(MgFe)OSiO2; serpentine, H4Mg3Si2O9; epsomite (epsom salt), MgSO4 · 7H2O; brucite, and the Stassfurth salts of commercial importance—carnallite KMgCl3 · 6H2O; kainite, MgSO4 · KCl · 3H2O; kieserite, MgSO4 · H2O; and schoenite, K2SO4 · MgSO4 · 6H2O. In addition, other magnesium minerals are asbestos, meerschaum, spinel, tourmaline, chrondrodite, pyrope, biotite, phlogopite, chlorite, talc, sepiolite, garnierite, bradleyite, and some of the pyroxenes and amphiboles. Magnesium is also found in mineral waters, sea bitterns, sea water, and oil field brines.
At the present time metallic magnesium is derived from the following minerals: magnesite, dolomite, epsomite, olivine, and serpentine. It is also obtained from chemically produced magnesia, or magnesium oxide in its various forms, magnesium chloride, magnesium sulphate, magnesium hydroxide, and magnesium silicofluoride. Today it is also extracted from ordinary sea water, bitterns, saline deposits, and oil field brines.
Natural brines contain sodium chloride, calcium chloride, magnesium chloride, bromine, and other chemical constituents. The first step in obtaining metal magnesium, Mg; is to treat the brine with calcium hydroxide (calcined limestone or quicklime, CaO, or with calcined dolomite, CaO and MgO). As a result, magnesium hydroxide, Mg(OH)2, and calcium chloride, CaCl2, are formed. The magnesium hydroxide is then dissolved in hydrochloric acid, HCl, to form magnesium chloride, MgCl2, from which metallic magnesium and gaseous chlorine are derived by electrolysis. According to Gann (1930, p. 694), at the Dow Chemical Company's plant at Midland, Michigan, the bromine is first removed from the brine after which the brine is treated with a magnesium hydrate slurry to precipitate iron and any other impurities contained therein. After the sodium chloride has been removed by crystallization, the magnesium and calcium chlorides in the rotary-filter mother liquor are separated from each other by fractional crystallization, with addition of chlorine during the process. The purified magnesium chloride solution is concentrated further by crystallization. The crystals are melted in their water of crystallization, the fused mass flaked, and then air-dried until an almost anhydrous magnesium chloride, MgCl2, is produced. The magnesium chloride is then electrolyzed in a sodium chloride bath. The process is continuous, and the metal, which is of high purity, is periodically 'dipped from the rectangular cast-steel pots in which electrolysis occurs and cast into ingots of various sizes.
Although metallic magnesium is the end product sought, it may not be desirable, feasible, or practicable to extract the metal from the oil field brines at each recovery plant established. It may prove to be more advantageous merely to produce magnesium hydroxide, Mg(OH)2, dry it, and ship it elsewhere to be further processed. On the other hand, it may be desirable to further process the magnesium hydroxide to some specific magnesium product, such as "caustic," "calcined," or "dead-burned" magnesia, and then dispose of the product. Or the process may be carried on to completion until metallic magnesium is obtained. In the following pages a brief description is given concerning the processing of the more important magnesium compounds.
Regardless of what magnesium substance is desired at any oil field brine recovery plant, the first step consists in converting the contained magnesium chloride into magnesium hydroxide. This may be done by treating the magnesium chloride of the brine either with calcined limestone (quicklime, CaO), or with calcined dolomite, or with both. The chemical reactions represented by the first two processes are shown in the following equations:
1. MgCl2 + CaO + H2O = Mg(OH)2 + CaCl2.
magnesium chloride + lime + water = magnesium hydroxide + calcium chloride.
2. MgCl2 + CaO + MgO + 2H2O = 2Mg(OH)2 + CaCl2
magnesium chloride + lime + magnesia + water = magnesium hydroxide + calcium chloride
The magnesium hydroxide, Mg(OH)2, is an amorphous substance that is more than twice as heavy as metallic magnesium. It may be shipped to other plants for further processing or it may be further treated at the recovery plant, as outlined in the following pages.
The basic magnesium hydroxide when calcined or dehydrated is converted into magnesium oxide, MgO, which is a white powder, very soft and light. Commercially it is known as magnesia. This chemical substance may be had on the market in various forms which depend upon the temperatures at which the magnesium hydroxide was calcined (table 15).
Table 15—Forms of magnesium oxide or magnesia and the temperatures at which they are formed
| Product | Temperature (degrees C.) |
|---|---|
| "Caustic" magnesia | 700-1,200 |
| "Dead-burned" or sintered magnesia | 1,400-1,560 (in presence of iron) |
| 1,400-1,600 (absence of impurities) | |
| Artificial periclase | 1,700 and above |
| Magnesia brick | 1,700 and above |
The products in table 15 are also called "caustic" and "calcined magnesite." The chemical reaction involved in the formation of magnesium oxide from magnesium hydroxide is represented by the following chemical equation:
Mg(OH)2 + heat = MgO + H2O.
magnesium hydroxide + heat = magnesia + water
Although magnesium is present in the brine chiefly in the form of the chloride; nevertheless, in order to obtain it, it is necessary first to convert the magnesium chloride into either the hydroxide or oxide as mentioned above and then precipitate magnesium chloride by dissolving the hydroxide or oxide in hydrocloric acid. The chemical reactions are as follows:
Mg(OH)2 + 2HCl = MgCl2 + 2H2O
magnesium hydroxide + hydrochloric acid = magnesium chloride + water
Magnesium chloride is the substance from which metallic magnesium is derived by electrolysis.
Magnesium chloride may be had commercially in the form of a hydrous crystalline salt, MgCl2 · 6H2O, as a partially dehydrated flaky material, and also in the anhydrous state.
Magnesium hydroxide or magnesium oxide treated with sulphuric acid results in the formation of magnesium sulphate, according to the following chemical reactions:
Mg(OH)2 + H2SO4 = MgSO4 + 2H2O
magnesium hydroxide + sulphuric acid = magnesium sulphate + water
MgO + H2SO4 = MgSO4 + H2O
magnesium oxide + sulphuric acid = magnesium sulphate + water
Magnesium sulphate is found in salt beds and in a number of hydrous varieties, chief of which is Epsom salt, MgSO4 · 7H2O. Epsom salt is a soft, highly soluble, colorless to white mineral having a bitter salty taste. It occurs commonly as granular, fibrous, or earthy masses. Kieserite, MgSO · H2O, kainite, MgSO4 · KCl · 3H2O, and schoenite, K2SO4 · MgSO4 · 6H2O, are other hydrous magnesium sulphate salts of commercial importance. These are associated with the world-famous Stassfurt salt deposits in Germany.
Magnesium carbonate, MgCO3, which occurs in nature in the form of the mineral magnesite, is produced chemically by treating magnesium chloride or magnesium sulphate with sodium bicarbonate.
MgCl2 + 2NaHCO3 = MgCO3 + 2NaCl + H2O + CO2
magnesium chloride + sodium bicarbonate = magnesium carbonate + sodium chloride + water + carbon dioxide
MgSO4 + 2NaHCO3 = MgCO3 + Na2SO4 + H2O + CO2
magnesium sulphate + sodium bicarbonate = magnesium carbonate + sodium sulphate + water + carbon dioxide
Magnesium carbonate is an infusible, glassy to earthy substance, colorless to white, yellow, brown, or blackish. It is brittle and breaks with a conchoidal fracture. In the form of the mineral, it usually occurs as a granular, compact, or unglazed porcelain-like earthy mass.
Magnesium chloride or magnesium sulphate when treated with sodium silicate yields magnesium silicate, a substance in composition similar to that of the mineral talc, H2Mg3Si4O12. This substance is useful in various ways as indicated elsewhere in this report.
6MgCl2 + 8Na2SiO3 + 4H2O = 2H2Mg3Si4O12 + 12NaCl + 4NaOH
magnesium chloride + sodium silicate + water = magnesium silicate + sodium chloride + sodium hydroxide
6MgSO4 + 8Na2SiO3 + 4H2O = 2H2Mg3Si4O12 + 6Na2SO4 + 4NaOH
magnesium sulphate + sodium silicate + water = magnesium silicate + sodium sulphate + sodium hydroxide
Magnesium hydroxide or magnesium oxide treated with hydrosilicofluoric acid yields a water soluble, white, crystalline powder known as magnesium silicofluoride.
Mg(OH)2 + H2SiF6 = MgSiF6 + 2H2O
magnesium hydroxide + hydrosilicofluoride = magnesium silicofluoride + water
MgO + H2SiF6 = MgSiF6 + H2O
magnesium oxide + hydrosilicofluoride = magnesium silicofluoride + water
Metallic magnesium has been used largely as a deoxidizing and desulphurizing agent in the manufacture of alloys, especially aluminum. It is used in making castings for aircraft parts, such as crankcases, pistons, oil-pans, bearings, and control levers. It is used in the manufacture of motion picture machines, field glasses, microscopes, and surveying and other scientific instruments. Magnesium is an important ingredient in incendiary bombs, military flares, flashlight powders, pyrotechnics, tracer bullets, and shells. It is used in the making of optical mirrors, electric batteries, and numerous other articles.
Magnesium oxide or magnesia has been used extensively in making refractories and magnesia cements. It is also important in the manufacture of crucibles, furnace linings, and insulating and fireproofing compounds. Magnesia is an ingredient in face powder and toilet preparations, as well as in paints and varnishes. It is used in medicine and it is found in mineral waters.
Magnesium chloride is the substance from which, by electrolysis, metallic magnesium is derived. It is used in making hydrochloric acid, magnesia cements, stucco, flooring and fire-extinguishing compounds, and ceramic materials. It is also an ingredient in medicine. It has many other uses.
Like magnesium chloride, magnesium sulphate is used in ceramics, dyeing, and medicine. It is used in manufacturing printing ink, frosted paper, matches, motion picture snow, and explosives. It is used in fertilizers, tanning, sizing paper, and fireproofing and waterproofing textiles.
Magnesium carbonate is used largely in making refractories and, in general, fire-resisting materials. It goes into the making of cosmetics, tooth-paste, varnishes, paints, printing ink, fertilizers, linoleum, oilcloth, and many other substances. It is also used in medicine.
Magnesium silicate is used in paints, lacquers, and varnishes, in ceramics, refractories, rubber compounding, and as an oil-bleaching agent.
Magnesium silicofluoride is used in ceramics. It is also an ingredient in insecticides. It is employed in hardening and waterproofing concrete.
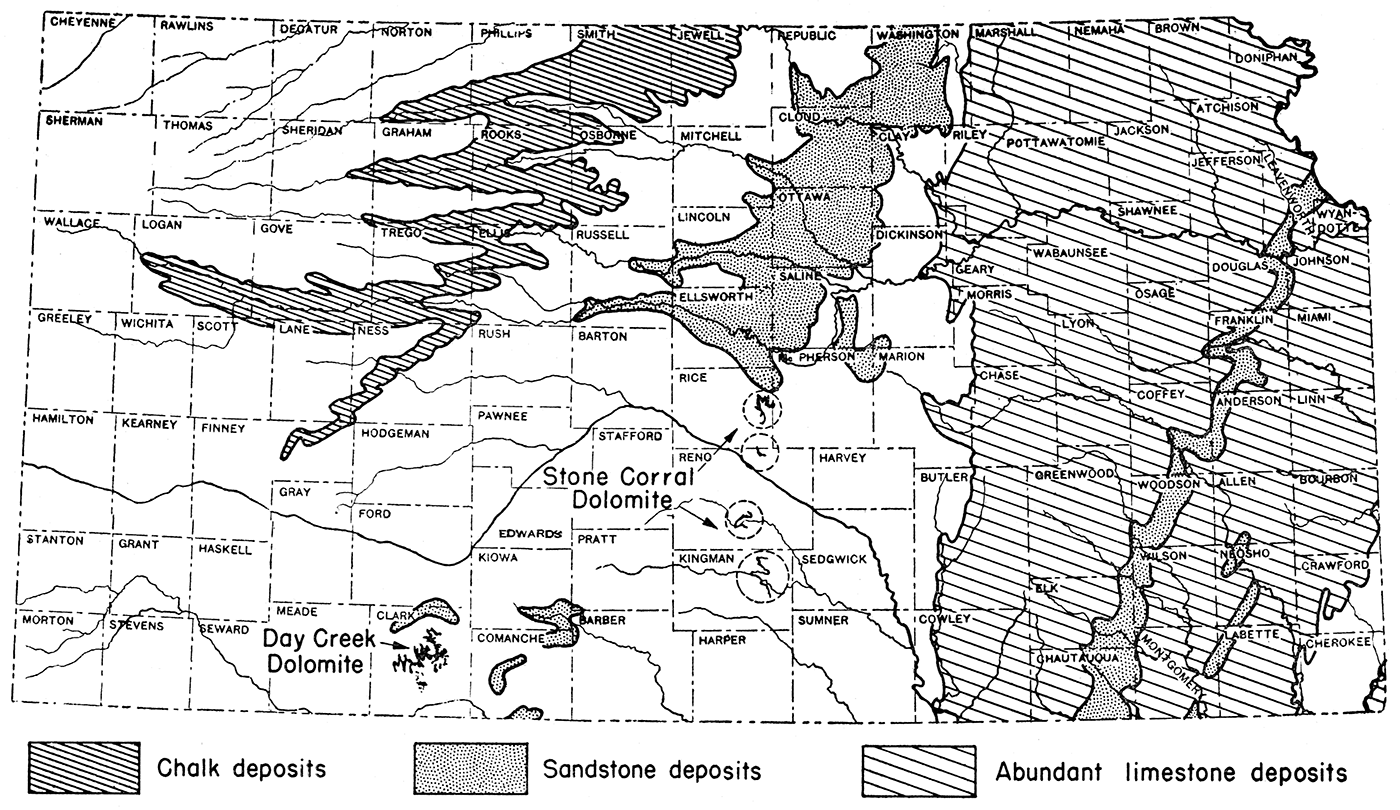
Calcined limestone (quicklime), or calcined dolomite, and fuel are the basic raw materials needed in the extraction of magnesium compounds from oil field brines. The practicability of magnesium extraction is, therefore, directly related to the availability of these basic raw materials or to the cost of the substance to the producer at the processing plant. Fortunately, limestone, including chalk, is abundant in Kansas and, in most cases, is close to the oil fields (fig. 2). Dolomite, which could be used instead of limestone, is available in limited quantities in Rice, Reno, and Kingman counties, where the Stone Corral dolomite crops out, and in Clark county where the Day Creek dolomite occurs.
Figure 2—Map of Kansas showing location of areas of abundant limestone, chalk, and dolomite, basic raw materials needed in magnesium recovery from brines. After J. M. Jewett.
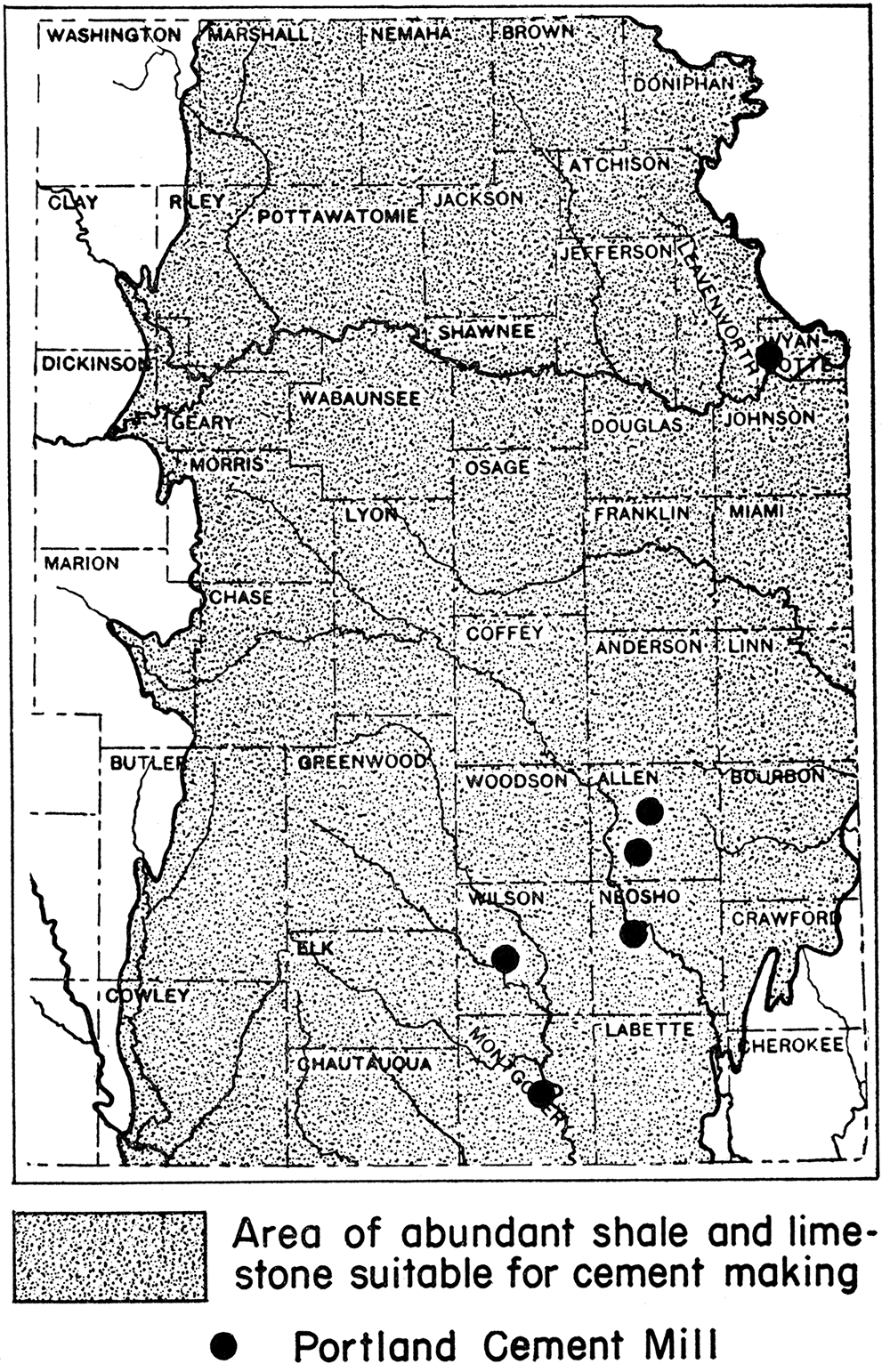
At the present time there are no lime kilns operating in the state, although in former years lime kilns were not uncommon. Calcined limestone or quicklime is manufactured, however, in connection with the making of Portland cement at six plants now operating in Kansas. The Portland cement plants are located mainly in eastern Kansas, not too distant from the oil fields, at Bonner Springs, Wyandotte county; lola and Humboldt, Allen county; Chanute, Neosho county; Fredonia, Wilson county; and Independence, Montgomery county (fig. 3). It is conceivable and not unreasonable that quicklime plants might easily be established in the chalk area of western Kansas close to the important oil fields of that part of the state.
Figure 3—Map of eastern Kansas showing location of Portland cement mills, possible sources for calcined limestone needed in the recovery of magnesium from brines. After J. M. Jewett.
Sufficient cheap fuel in the form of natural gas or fuel oil is available in most of the oil fields, or is within easy reach. In eastern Kansas, sufficient coal resources are at hand to replace the gas or oil for fuel if necessary. Apparently there is no necessity for shipping in from great distances the required processing materials, and certainly there is no need for shipping from outside of the state.
It is impossible to calculate the actual cost involved in establishing a plant designed to process the oil field brines for magnesium. In general, the cost of the brine should not be charged against the recovery of the magnesium when the brine is a waste product pumped up with the oil and when the extraction plant is operated in connection with oil production. The only legitimate cost involved, so far as the brine is concerned, is for pipe lines to be used in transporting the brine from a number of leases to a central recovery plant. In cases where brine is specifically produced as a raw material and is not related to oil production, the cost of drilling both brine well and disposal well, as well as producing and disposing of the brine, will necessarily have to be charged against the cost of recovering the magnesium. Burwell (1942) summarized the cost of a brine-treating plant as follows:
Other items involved are the cost of the quicklime or calcined dolomite-s-costs that are variable with location of the recovery plant and source of raw materials.
In 1942, metallic magnesium sold at 22% cents per pound, or at 450 dollars per ton. The price of other magnesium compounds in 1942, according to Burwell, are listed in table 16.
Table 16—Prices per ton of magnesium compounds in 1942
| Magnesium oxide, powdered | $58.75 |
| Magnesium oxide, U. S. P. light | $400.00 |
| Magnesium oxide, heavy | $500.00 |
| Magnesium chloride, flake, 97% MgCl2 · 6H,O | $32.00 |
| Magnesium chloride, anhydrous | $260.00 |
| Magnesium sulphate, technical | $36.00 |
| Magnesium sulphate, U. S. P. | $40.00 |
| Magnesium carbonate, precipitated, technical | $125.00 |
| Magnesium carbonate, precipitated, U. S. P. | $180.00 |
| Magnesium silicofluoride, technical | $400.00 |
The commercial practicability of recovering magnesium from oil field brines, aside from the cost of erecting and maintaining a recovery plant, is dependent upon several factors: (1) the magnesium content of the brine, (2) the amount of brine produced per well, (3) the total number of wells producing brine in the field, (4) the number of days per year the wells are producing, (5) the presence or absence of deep disposal wells, (6) the selling price of the recovered magnesium or its compounds, and (7) special chemical characteristics of the brine that would either inhibit or aid magnesium recovery.
At present, magnesium is being profitably extracted from ordinary sea water which has a magnesium content varying from 1,040 to approximately 1,400 milligrams per liter. Many of the Kansas oil field brines (tables 5 to 14) not only have a magnesium content equal to that of ocean water, but some of them have a much greater content, in some cases as much as double or triple the amount. So far as the magnesium content is concerned, many of the Kansas oil field brines qualify for magnesium recovery.
Important as the magnesium content of a brine may be, it is, nevertheless, of little value for commercial magnesium recovery unless sufficient brine is produced. This relationship of high magnesium content to volume of brine produced is well illustrated by the brines coming from the Kansas City-Lansing formations. Brines produced from this pay zone have practically the highest magnesium content of any of the Kansas oil field brines (table 4). Analyses of the samples collected (table 5) show a magnesium content varying from 972 to 3,910 milligrams per liter, with an average of 2,525 milligrams. So far as the magnesium content is concerned, the Kansas City-Lansing brines are very favorable for magnesium extraction. The amount of brine produced from wells drilled into this formation, however, is insignificant. Many of them produce practically no brine and most yield less than 20 barrels per day. In a few cases some of the wells furnish from 60 to 150 barrels of brine per day. Such wells, however, are exceptional. Table 17 has been prepared to show the minimum number of barrels of brine required to produce 2,000 pounds of metallic magnesium (or 6,840 pounds of magnesium chloride) from brines containing from 1,000 to 3,500 milligrams of magnesium per liter.
Table 17—Number of barrels of brine required to produce 2,000 pounds of metallic magnesium
| Mg. (milligrams per liter) |
Barrels |
|---|---|
| 1,000 | 5,700 |
| 1,100 | 5,181 |
| 1,200 | 4,755 |
| 1,300 | 4,381 |
| 1,400 | 4,065 |
| 1,500 | 3,802 |
| 1,600 | 3,559 |
| 1,700 | 3,350 |
| 1,800 | 3,165 |
| 1,900 | 3,000 |
| 2,000 | 2,850 |
| 2,100 | 2,714 |
| 2,200 | 2,587 |
| 2,300 | 2,478 |
| 2,400 | 2,372 |
| 2,500 | 2,278 |
| 2,600 | 2,191 |
| 2,700 | 2,110 |
| 2,800 | 2,033 |
| 2,900 | 1,967 |
| 3,000 | 1,900 |
| 3,100 | 1,838 |
| 3,200 | 1,781 |
| 3,300 | 1,727 |
| 3,400 | 1,676 |
| 3,500 | 1,629 |
The number of barrels of brine required to furnish 2,000 pounds of metallic magnesium varies inversely with the milligrams per liter magnesium content of the brine. In other words it takes a smaller number of barrels of brine to produce a given amount of metallic magnesium from a high magnesium brine that it does from one low in that constituent.
Since a large amount of brine is necessary to insure the success of a recovery plant, and since chloride and other constituents are not removed, it follows that provision must be at hand for the disposal of the brine after the magnesium has been extracted. The most satisfactory means of disposing of the processed brine is by means of deep disposal wells. In many of the Kansas oil fields, deep disposal wells are now in existence; especially is this true in the oil fields of western Kansas. In such cases there would be practically no added cost in disposing of the waste brine.
The selling price of magnesium or its compounds is another factor to be considered in establishing a magnesium recovery plant. The many uses to which magnesium and its compounds are adaptable, together with the growing demand for these substances, are certain to insure a fair selling price, not only during the war emergency but also in the future. Prices of magnesium and its compounds for 1942 are listed on page 67 of this report. It should be recognized that magnesium recovery from Kansas oil field brines is not on a competitive basis with larger establishments erected solely for the purpose of securing magnesium or its compounds. It is probable that Kansas oil field brines can never yield magnesium as more than a by-product of oil production. Such a by-product industry might pay the cost of brine disposal although showing little or no profit in itself. As an illustration, during the past five years, more than 6,863,100 barrels of brine having a magnesium content of 3,199 milligrams per liter have passed through a single disposal system in the Burrton oil field in Reno county. One barrel of such brine contains 1.123 pounds of metallic magnesium. On that basis, 7,707,261.3 pounds, or 3,853.6 tons, of metallic magnesium were returned to the underground strata during a five-year period. On the basis of the 1942 price, this amount of magensium has a value of $1,734,120.
High magnesium content, a large volume of brine, and the existence of deep disposal wells make several oil fields in Kansas worthy of study for possible establishment of magnesium recovery plants. Only those oil fields that theoretically could yield 1,000 tons of metallic magnesium annually are considered. The oil fields most worthy of consideration on the basis of the data at hand are: Burrton field, Reno county; Bornholdt field, McPherson county; Zenith field, Stafford and Reno counties; Welch field, Rice county; Hall-Gurney field, Russell county; and Oxford field, Sumner county. Data pertaining to these fields are summarized in table 18.
Table 18—Summary of data pertaining to Kansas oil fields worthy of further study for the possible establishment of magnesium recovery plants
| Field | County | Horizon | Producing wells |
Theoretical brine production per year (barrels) |
Disposal wells |
Average Mg. content (milligrams per liter) |
Average metallic Mg. content (per barrel) |
Theoretical yield (tons) | |
|---|---|---|---|---|---|---|---|---|---|
| Metallic Mg. | Anhydrous MgCl2 |
||||||||
| Burrton | Reno | "Chat" | 335 | 550,420,000 | 14 | 2,263 | 0.773 | 212,737.3 | 858,655.2 |
| Bornholdt | McPherson | "Chat" | 150 | 23,118,750 | 2 | 1,516 | 0.526 | 6,080.2 | 24,043.5 |
| Zenith | Stafford Reno |
Simpson Viola |
301 | 21,973,000 | 1,275 | 0.4.48 | 4,922.0 | 19,336.7 | |
| Welch | Rice | "Chat" | 24 | 11,826,000 | 1 | 1,395 | 0.492 | 2,909.2 | 11,471.2 |
| Oxford | Sumner | Topeka Hoover Stalnaker Layton |
19 | 4,401,900 | 1 | 2,874 | 1.000 | 2,200.9 | 8,759.7 |
| Hall-Gurney | Russell | Kansas City-Lansing | 396 | 2,168,100 | 2,912 | 1.010 | 1,094.8 | 4,368.7 | |
The calculated tonnage of metallic magnesium given in table 18 is only of relative and comparative value and is based upon two assumptions. In computing the total amount of brine produced in any given field during the year, it was assumed that the wells were pumped daily and at the same rate throughout the period. The amount of brine is, therefore, a variable factor and can be computed accurately only from actual pumping data. The amount of brine in barrels per year in the table is accordingly too high. In ascertaining the amount of metallic magnesium in a barrel of brine, as well as the potential yield, the specific gravity of the brine was taken as 1. On the basis of the chemical analyses, of the brine was taken as 1. On the bisis of the chemical analyses, the specific gravity ranged from 1.014 to 1.159. If the latter variable specific gravities had been used instead of 1, the metallic magnesium content per barrel would be higher than those indicated in the table. Consequently, the theoretical yield of metallic magnesium would have been increased correspondingly. It must also be pointed out that not all of the metallic magnesium as given in the table can be extracted from the brine in the recovery process.
The Burrton oil field is chiefly in Reno county east of Hutchinson in T. 22, 23, and 24 S., R. 4 W. The pool extends into Harvey county in T. 23 S., R. 3 W. Production is mainly from the Mississippian "chat" and from the "Hunton." Four brine samples were collected, each one of which showed on analysis a magnesium content of over 2,000 milligrams per liter (table 8). The brine originated in the "chat" at a depth varying between 3,300 and 3,400 feet. Data relative to the amount of brine produced were obtained from only one well, the Sinclair-Prairie well in sec. 15, T. 24 S., R. 4 W. This well is reported to yield 4,000 barrels of brine per day. Should the other "chat" wells, 335 in number, produce an equal volume of brine, the field would produce yearly 550,420,000 barrels of brine, exclusive of the salt water yielded by the 72 "Hunton" wells in the field. On the basis that the magnesium content of the 550,420,000 barrels of brine is the same as the average of the four samples collected and analyzed (2,263 milligrams per liter) and that each barrel of brine contains 0.773 pounds of metallic magnesium (table 19), the Burrton "chat" well brines capable of being produced during the year would contain 21,273.7 tons of metallic magnesium. At the 1942 price, 21,273.7 tons of metallic magnesium are worth $9,573,165. At the present time, there are 14 disposal wells now in use in the Burrton field (table 20) with two disposal associations operating. The North Burrton Disposal Association, operated by the SinclairPrairie Company, takes care of much of the brine in the north part of the field; whereas, the South Burrton Disposal Association, operated by the Sinclair-Prairie and Barnsdall companies, disposes of the brines in the south end of the field. The disposal wells are distributed in twelve different sections, six in T. 23 S. and six in T. 24 S.
Table 19—Metallic and other magnesium content of one barrel of brine (42 gal.)
| Mg. (milligrams per liter) |
Metallic Mg. (lbs.) |
MgCl2 (Anhydrous) (lbs.) |
Mg(OH)2 (lbs.) |
MgO (lbs.) |
|---|---|---|---|---|
| 1,000 | 0.351 | 1.39 | 0.848 | 0.582 |
| 1,100 | 0.386 | 1.52 | 0.926 | 0.640 |
| 1,200 | 0.421 | 1.66 | 1.010 | 0.698 |
| 1,300 | 0.456 | 1.80 | 1.094 | 0.756 |
| 1,400 | 0.492 | 1.94 | 1.180 | 0.816 |
| 1,500 | 0.526 | 2.08 | 1.262 | 0.873 |
| 1,600 | 0.562 | 2.22 | 1.348 | 0.932 |
| 1,700 | 0.597 | 2.36 | 1.432 | 0.932 |
| 1,800 | 0.632 | 2.50 | 1.516 | 0.991 |
| 1;900 | 0.667 | 2.64 | 1.600 | 1.049 |
| 2,000 | 0.702 | 2.78 | 1.684 | 1,107 |
| 2,100 | 0.737 | 2.92 | 1.768 | 1.165 |
| 2,200 | 0.773 | 3.05 | 1.855 | 1.223 |
| 2,300 | 0.807 | 3.19 | 1.936 | 1.283 |
| 2,400 | 0.843 | 3.33 | 2.023 | 1.339 |
| 2,500 | 0.878 | 3.47 | 2.107 | 1.457 |
| 2,600 | 0.913 | 3.61 | 2.191 | 1.515 |
| 2,700 | 0.948 | 3.75 | 2.275 | 1.573 |
| 2,800 | 0.984 | 3.86 | 2.361 | 1.633 |
| 2,900 | 1.017 | 4.02 | 2.440 | 1.688 |
| 3,000 | 1.053 | 4.16 | 2.527 | 1.747 |
| 3,100 | 1.088 | 4.30 | 2.611 | 1.806 |
| 3,200 | 1.123 | 4.43 | 2.695 | 1.864 |
| 3,300 | 1.158 | 4.57 | 2.779 | 1.922 |
| 3,400 | 1.193 | 4.75 | 2.863 | 1.980 |
| 3,500 | 1.228 | 4.85 | 2.947 | 2.038 |
Table 20—Disposal wells now in use in the Burrton oil field
| Company or operator | Location | |
|---|---|---|
| Lloyd, Frost, and Study | SE | 2-23-4W. |
| Ledo and Wilcox | W2 SW NW | 12-23-4W. |
| Barnsdall | NW SE SE | 11-23-4W. |
| Sinclair Prairie | SE NE SE | 14-23-4W. |
| North Burrton Disposal Association (Sinclair-Prairie) |
NW NW NE | 14-23-4W. |
| NE NE SE | 23-23-4W. | |
| NE NE SW | 26-23-4W. | |
| NW NW SE | 26-23-4W. | |
| Skelly | S2 SW NW | 2-24-4W. |
| Gulf | NW SE NW | 3-24-W. |
| South Burrton Disposal Association (Sinclair-Prairie) |
C NE NW | 15-24-4W. |
| Texas | NW NW SE | 17-24-4W. |
| South Burrton Disposal Association (Barnsdall) |
NW SW | 20-24-4W. |
| Nadel and Gussman | NW NE NW | 30-24-4W. |
The available data suggest that the Burrton oil field offers the best possibilities for magnesium recovery of any field studied. The other oil fields studied are listed, however, because further study may reveal the practicability of using their brines for magnesium recovery, at least to the extent of offsetting the cost of present brine disposal. Any fields theoretically capable of producing approximately 1,000 tons of metallic magnesium annually are considered.
The Bornholdt oil field is in McPherson and Rice counties. The major part of the field is in T. 20 S., R. 5 W., McPherson county. In 1941, there were 150 wells in the field, all producing from the "chat" at a depth of around 3,340 feet. Three brine samples were collected from this field (table 8). The average magnesium content of the brines analyzed is 1,517 milligrams per liter, somewhat higher than average sea water. Data as to the amount of brine produced were obtained from two of the wells. The average volume produced was 425 barrels per day. At that rate the 150 wells in the field would produce annually 23,118,750 barrels of brine. Since one barrel of brine having a magnesium content of 1,500 milligrams per liter (table 19) contains 0.526 pounds of metallic magnesium, the total potential volume of brine capable of being produced per year would contain 6,080.2 tons of metallic magnesium having a value of $2,736,090, based on the 1942 prices. There are at least two disposal wells in the field, one in sec. 25, T. 20 S., R. 6 W., and one in sec. 29, T. 20 S., R. 5 W.
The Zenith oil field is in stafford and Reno counties and had, in 1941, 301 producing wells. Production is mainly in the Mississippian Misener formation and Viola limestone 'of Ordovician age. Four samples of brine were collected, one in Reno county and three in Stafford county (table 12). According to information received, production was from the Simpson formation at a depth of approximately 3,600 feet. Analyses showed that the brines had an average magnesium content of 1,275 milligrams per liter, or approximately the same as that of ordinary sea water. Two hundred barrels of brine were produced per day from the one well on which data were obtained (table 12). Should all the wells in the field produce at the rate of 200 barrels of brine per day, a total of 21,973,000 barrels of brine would be produced during the year. Even at the low magnesium content of this brine, it contains 4,922 tons of metallic magnesium which, on the 1942 basis of prices, would be worth $2,214,900. Four well-distributed disposal wells in the field now provide for disposal of much of the brine produced.
The Welch oil field is in Rice county. The 24 wells producing from the "chat" are theoretically capable of producing 11,826,000 barrels of brine annually. This volume of brine, on the basis of analyses (table 8), contains 2,909.2 tons of metallic magnesium. In 1942, 2,909 tons of metallic magnesium were valued at $1,309,050. At least one deep disposal well is located in the field.
The Hall-Gurney oil field is in Russell county. In 1941, 396 wells were producing from the Kansas CityLansing rocks. On the basis of three samples collected (table 5), the brine contains, on the average, 2,912 milligrams of magnesium per liter. In spite of the low production of brine per well per day, the calculated total brine production for the year amounts to 2,168,100 barrels. This volume of brine, because of the high metallic magnesium content per barrel (table 19), contains 1,094.8 tons of metallic magnesium, worth approximately half a million dollars.
The Oxford oil field is in secs. 14 and 23, T. 32 S., R. 2 E., Sumner county. In 1941, 13 wells were producing from the Stalnaker formation, 6 from the Layton, and 21 from the Arbuckle. Four brine samples were collected, one each from the Topeka, Hoover, Stalnaker, and Layton pay zones (table 14). The samples came from the Amerada Petroleum Corporation wells, all of which were located in an area less than 10 acres in extent. The analyses showed an average magnesium content of 2,874 milligrams per liter. All of the non-Arbuckle wells in the field, if pumped daily, would produce, per year, 4,401,900 barrels of brine containing 2,200.9 tons of metallic magnesium worth approximately one million dollars. The volume of brine and, therefore, the metallic magnesium content could be increased materially if all or part of these wells penetrated all four of the pay zones, a possibility suggested by the offset wells from which some of the samples were collected. All of the brine of the sampled wells is returned into subsurface strata by means of a deep disposal well.
Burwell, A. L., 1942, The possibility of magnesia from Oklahoma oil field brines: Oklahoma Geol. Survey, Mineral Report no. 14, pages unnumbered, fig. 1, tables 1-6.
Frye, John C., and Jewett, J. M., 1492, Magnesium; in, Kansas mineral resources for wartime industries, by John M. Jewett and W. H. Schoewe: State Geol. Survey of Kansas, Bull. 41, pt. 3, pp. 69-180, figs. 1-13, tables 1-26. [available online]
Gann, John A., 1930, The magnesium industry: Industrial and Engineering Chemistry, vol. 22, no. 7, pp. 694-700, figs. 1-6, tables 1-4.
Jones, Odgen S., 1942, The state's responsibility in oil and brine pollution originating in oil fields: Kansas State Board of Health, pp. 1-20, tables unnumbered.
Pawel, George W., 1942, Olivine: potential source of magnesia: Mining and Metallurgy, vol. 23, no. 426, pp. 331-333, figs. unnumbered.
Ver Wiebe, Walter A., 1942, Exploration for oil and gas in, western Kansas during 1941: State Geological Survey of Kansas, Bull. 42, pp. 1-123, figs. 1-42, tables 1-28.
Kansas Geological Survey
Placed on web Jan. 15, 2019; originally published July 30, 1943.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/47_2/index.html