
Kansas Geological Survey, Bulletin 41, Part 10, originally published in 1942

Originally published in 1942 as Kansas Geological Survey Bulletin 41, Part 10. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (4 MB) is also available.
Extensive deposits of bentonite of various types exist in Kansas. Uses for this product are steadily expanding. It is now employed as a bleaching agent for oil, as a bond in foundry sand, as a thickener for drilling mud in oil fields, as a bonding material in ceramics and refractories, as a filler for paper, in soap and cosmetics because of its fineness and freedom from grit, for de-inking newsprint because of its absorptive properties, and for many other purposes. Laboratory tests indicate that bentonite is especially valuable in bleaching oil and in acting as a bonding agent in foundry sand. This report covers the use of Kansas bentonite in the more important applications only.
Kansas should well be able to compete in the relatively new and growing bentonite industry because of the favorable mining and shipping conditions in the state, together with the fact that Kansas is nearer to eastern markets than certain other western producing states.
The investigations described in this report are intended to provide information concerning the nature and possible uses of Kansas bentonite. Bentonite is a very fine-grained claylike substance, derived from volcanic ash. The clay mineral montmorillinite, a hydrous aluminum silicate, is its chief constituent. Several varieties have the property of swelling when immersed in water. The chemical formula Al20a • 4SiO2 X H20 has been assigned to the mineral, but its exact composition is a matter of controversy. Alumina in the above formula may be replaced, in certain varieties, by iron, lime, or magnesia, and, to a limited extent, by alkalies. The water of combination may vary from 1 to 15 parts, depending on the dryness of the sample. Bentonite has a great physical avidity for water, but the amount chemically combined does not ordinarily exceed two parts. When dry, bentonites are generally somewhat brittle and crush easily with conchoidal fracture. When wet, they are plastic. All forms of bentonite are quite soft, the degree of softness increasing with the water content. and decreasing with increasing silica. The softer varieties, especially, have a soapy feel and waxy luster. The color is often a pale green, but sometimes it may be white, pale yellow, or gray.
Special thanks are due to the persons who gave valuable aid in connection with this study. Roy E. McDowell, County Engineer, Phillips County, gave the location of bentonite deposits in Phillips County. Norman Plummer had charge of churn-drilling of bentonite deposits in Phillips County, in 1938, and furnished much valued information. Hugh F. Crain did the laboratory work in connection with the use of bentonite in molding sands. Ray F. Thompson made all the included analyses of the Kansas bentonite. Owen De Woody, of the Socony Vacuum Oil Company, furnished certain samples of oil and bentonite. K. K. Landes contributed a photograph of a bentonite deposit in Wallace County. The manuscript was edited and the illustrations prepared by Dorothea M. Weingartner.
Bentonite deposits occur in beds from a few inches to several feet in thickness, mainly in deposits of Tertiary and upper Cretaceous ages, but, to some extent, in strata of Paleozoic age, too. The beds are found in many parts of the United States, Canada, and foreign countries. In this country the production comes mostly from Wyoming, South Dakota, California, Arizona, New Mexico, Kentucky, Tennessee, Texas, Arkansas, and Mississippi. Bentonite beds are often lenticular and vary considerably in thickness. Thin beds of fairly uniform thickness and great lateral extent, such as would occur from the settling of volcanic ash in an extensive body of quiet water, also occur.
Although differences of opinion exist, many deposits of bentonite, including those of Kansas, are generally believed to have resulted from the devitrification and partial decomposition of volcanic ash. The strongest evidence is in the presence of volcanic ash, decomposition products, and bentonite, all in the same deposit. Bentonite, like volcanic ash, contains small amounts of feldspar, but no free quartz. It has the composition expected to result from the decomposition of volcanic ash, with subsequent leaching by water of some of the contained alkalies. Various field relationships, such as the nearby presence of alkaline salts, also tend to confirm the theory that, in the plains area at least, bentonite is derived from volcanic ash.
Bentonite may be divided into two classes: (1) that which absorbs large quantities of water, swelling greatly in the process, and having the property of remaining in thin water dispersions and (2) that which absorbs practically no more water than ordinary plastic clay or fuller's earth, not swelling noticeably, and settling rapidly in thin water dispersions. There are, of course, some intermediate gradations. Bentonite has been divided into four groups mineralogically: alkaline bentonites, alkaline sub-bentonites, alkaline-earth bentonites, and alkaline-earth subbentonites.
Bentonite of the first class, which is found mostly in eastern Wyoming and western South Dakota, is an alkali bentonite. It has a wide variety of commercial applications because of its peculiar property of swelling in water and forming gelatinous mixtures that are unique in an inorganic substance. Its principle uses are as an ingredient of molding sand and for thickening oil field drilling mud. Bentonite of the second class is produced in larger quantities and is used extensively as a bleaching agent. It comes mostly from Texas, Arkansas, Mississippi, Kentucky, and Tennessee. It is often an alkaline earth sub-bentonite. Some clay of this class has natural bleaching properties such as are in fuller's earth, but usually it requires chemical activation before it can be used as a bleaching agent.
The use of bentonite is increasing. U. S. production in 1939 was 219,720 short tons valued at $1,702,393. compared to the previous. high record of 194,768 tons valued at $1,500,758. in 1937. The principal uses in 1939 are shown in Table 1.
Table 1—Principal uses of U. S. bentonite during 1939.
| Use | Per cent of total | Type of bentonite |
|---|---|---|
| Filtering and bleaching oils | 43 | Non-swelling |
| Conditioner for foundry sands | 25 | Swelling type, mostly |
| Petroleum and natural gas industries, for drilling mud |
16 | Swelling type, mostly |
| Miscellaneous uses | 15 | Both types |
Bentonite is used: as a bond for molding sand, for oil drilling mud, for bleaching petroleum products, in the manufacture of cement and ceramic products, soaps, refractory materials, paper, cosmetics, water softeners, sealing agents, paints, medicinal emulsions, acid proofing, for de-inking newsprint, for clarifying dry cleaner fluids, as the core of earth-fill dams, as a lining for irrigation ditches, and for many other purposes.
No quotations can be given on Kansas bentonite as a market is yet to be established. Bentonite is sold in two forms, crude and processed. The latter is material ground to fine size. For 1939, the Bureau of Mines reported crude sales ranging from $4. to $8. a short ton, f.o.b.; the average returns on all sales ranged from less than $7. in South Dakota, to nearly $12. a ton in California.
The most widely used bentonite is 200 mesh powder, which, in 1939, was worth $10.25 per ton, f.o.b., Black Hills shipping points, in 100 pound bags, carload lots. A Wyoming-type bentonite, dried and crushed (mostly 4 to 20 mesh), is sold in carload lots at $7. a ton in bulk and $8.75 a ton in bags.
Kansas bentonite appears to have properties midway between the high-swelling and the so-called non-swelling type. All forms swell in water, and most of them have good bleaching properties after activation. With few exceptions, they compare favorably with other typical kinds of commercial bentonite in not having excessive amounts of objectionable impurities. The full extent of the different bentonite deposits in Kansas is not completely known. The maps in figure 1 show the location of known bentonite deposits in Phillips and Wallace counties.
Figure 1—Maps showing location of bentonite deposits in Phillips and Wallace counties.
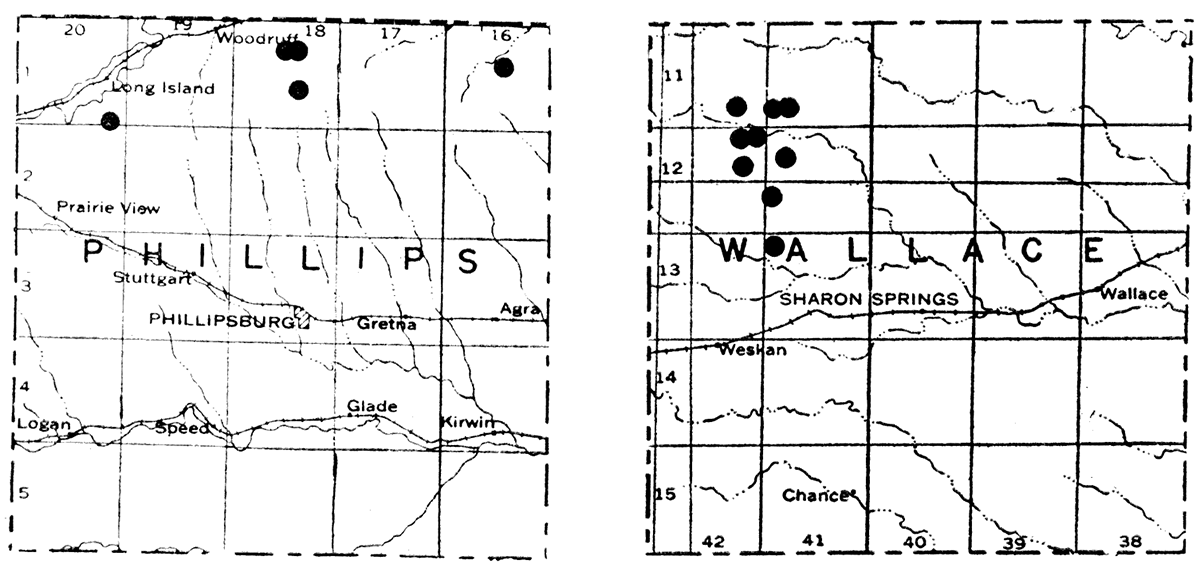
The only detailed information of the Geological Survey comes from work in Phillips County, where, in 1938, a considerable number of auger holes were drilled in sec. 35, T. 1 S., R. 20 W., and in sec. 10, T. 1 S., R. 18 W. Bentonite was found in most of the holes, in some cases to a reported thickness of 25 feet, although the thick beds are not of uniform quality. This bentonite, presumably, occurs between the Pierre shale and the underlying Niobrara chalk. The overburden varies from 3 to 20 feet. Numerous outcrops at widely separated points indicate many deposits. In Wallace County long narrow mounds of bentonite, often 150 yards long and 5 yards in thickness, have been isolated by erosion. In Wallace County the bentonite occurs in the Ogallala formation of Tertiary age. Where the deposits have not been isolated by erosion, the overburden appears to range from 10 to 40 feet. In both Wallace and Phillips counties good and poor quality 'bentonite often occurs in close proximity, a condition calling for selective mining to assure a uniformly good product.
With one exception all of the deposits studied are within half a mile of good roads. The deposit represented by sample 13 is one mile distant. The distance from railroad shipping points in Phillips County is from 1 1/2 miles to 6 miles, and, in Wallace County, it is 15 miles.
Table 2 gives the location and description of deposits sampled.
Table 2—Location and description of analyzed bentonite samples.
| Sample no. |
County | Location | Thickness sampled (ft.) |
Remarks |
|---|---|---|---|---|
| 1 | Phillips | Sec. 10, T. 1 S., R. 18 W. | 1 1/2 | Upper part of deposit, 1/2 mile west Kan. Highway 1. Color: blue-gray, Overburden 15'. |
| 2 | Phillips | Sec. 10, T. 1 S., R. 18 W. | 1 | Same as above, middle formation. Color: very light gray. |
| 3 | Phillips | Sec. 10, T. 1 S., R. 18 W. | 1 1/2 | Same as above, lower formation. Color: green-gray. |
| 4 | Phillips | SE sec. 35, T. 1 S., R. 20 W. | 1 | Upper part of deposit. From roadside drainage ditch, 1 1/2 miles south of Long Island. Color: green-gray. Overburden 5'. |
| 5 | Phillips | SE sec. 35, T. 1 S., R. 20 W. | 2 | Same as above, middle formation. Color: dark green- gray. |
| 6 | Phillips | SE sec. 35, T. 1 S., R. 20 W. | 1 | Same as above, lower formation. Color: light gray. |
| 7 | Phillips | SE sec. 35, T. 1 S., R. 20 W. | 66/100 | 1/3 mile west of highway. Bottom of deposit. Color: very light yellow-gray. |
| 8 | Phillips | SE sec. 35, T. 1 S., R. 20 W. | 3 1/2 | Upper part of deposit from which no. 7 came. Traces of volcanic ash. Color: light greenish gray. Overburden 5'. |
| 9 | Wallace | SW sec. 19, T. 12 S., R. 41 W. | 10 | Residual mounds exposed at head of large circular draw, west side of center. Color: pale olive-green. Overburden 0-20'. |
| 10a | Wallace | SW sec. 19, T. 12 S., R. 41 W. | 10 | Taken from mound 150' north of no. 9. Color: pale olive-green, streaked with light calcium carbonate. Overburden 0-20'. |
| 10b | Wallace | SW sec. 19, T. 12 S., R. 41 W. | 1/2 | A 6" deposit at bottom of mound from which no. 9 came. Color: pale greenish white. |
| 10c | Wallace | SW sec. 19, T. 12 S., R. 41 W. | Grab sample |
100 yds. east of sample where no. 9 lay. From a mound deposit showing white streaks. Color: greenish white. |
| 11 | Wallace | NW sec. 29. T. 12 S., R. 41 W. | 5 | From mound of bentonite 50' long, 5' thick. Color: light brown. No overburden. |
| 13 | Wallace | NE sec. 12, T. 12 S., R. 42 W. | 4 | From draw 1/2 mile west and 3/4 mile south of Woodhouse ranch. Somewhat shaley. Color: brownish gray. Overburden 40'. |
| 14 | Wallace | SE sec. 2, T. 12 S., R. 42 W. | 5 | From draw 1/4 mile south of Roy C. Johnson ranch. Color: pale greenish white. Overburden 2'. |
| 15 | Wallace | SE sec. 2, T. 12 S., R. 42 W. | 5 | 200 yds. southwest of where sample no. 14 lay. Color: pale gray-green. Overburden 20'. |
| 16 | Wallace | SE sec. 2, T. 12 S., R. 42 W. | 5 | 150 yds. west of where sample 14 lay. A mound 5' thick. Color: pale gray-green. Sandy. |
Although chemical analyses are of some value, especially in showing the impurities present, they are not so useful in predicting the physical properties of commercial bentonite. Table 3 shows the chemical composition of 17 samples and associated rocks; table 4 shows the chemical composition of bentonite from producing localities in other states. From experience it has been found that in the case of bleaching clays the silica and alumina contents should range from 55 to 65 per cent and from 12 to 22 per cent, respectively, on a dry weight basis. Of the 17 samples shown analyzed in table 3, eight were within these limits (numbers 3, 4, 5, 7, 8, 9, 13, 16). Number 16 was not tested; 7 and 8 were poor, barely coming within the above limits; but all the others showed good bleaching power. Five samples (1, 2, 6, 11, 15) were slightly outside the limits. Samples 2 and 15 bleached well, number 6 rather poorly, number 1 very poorly. Sample 11 was not tested. Four samples (10a, 10b, 10c, 14) were completely outside the limits, being high in calcium and not suited for bleaching.
Table 3—Chemical analyses1 of Kansas bentonite and associated formations.
| No. | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O K2O |
TiO2 | H2O 105°C |
Loss 900°C |
SO3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51.92 | 20.52 | 2.10 | 2.17 | 0.02 | 0.62 | 0.02 | 12.2 | 22.67 | 0.84 | 100.9 |
| 2 | 51.60 | 20.46 | 1.86 | 2.25 | 0.04 | 0.19 | 0.02 | 13.6 | 22.95 | 1.10 | 100.5 |
| 3 | 57.25 | 15.34 | 2.76 | 4.67 | 0.10 | 0.52 | 0.03 | 8.4 | 14.70 | 0.99 | 96.4 |
| 4 | 61.35 | 17.47 | 2.90 | 1.87 | 0.09 | 1.54 | 0.02 | 6.0 | 14.18 | 0.93 | 100.3 |
| 52 | 59.34 | 16.45 | 4.16 | 2.59 | 0.05 | 0.03 | 6.0 | 14.65 | 1.87 | 99.1 | |
| 6 | 66.44 | 17.26 | 2.30 | 2.34 | 0.60 | 0.35 | 0.01 | 6.1 | 10.01 | 1.00 | 100.3 |
| 7 | 52.36 | 18.82 | 3.16 | 1.98 | 0.45 | 0.02 | 12.9 | 22.48 | 0.90 | 100.2 | |
| 8 | 53.39 | 18.15 | 4.05 | 5.15 | 0.13 | 1.80 | 0.03 | 9.0 | 16.02 | 0.34 | 99.1 |
| 9 | 52.28 | 20.20 | 3.20 | 2.04 | 0.49 | 0.27 | 0.01 | 6.4 | 20.06 | 1.57 | 100.1 |
| 10a | 44.91 | 16.07 | 2.14 | 11.26 | 1.77 | 2.95 | 0.01 | 5.4 | 16.20 | 0.26 | 95.6 |
| 10b2 | 11.68 | 4.17 | 0.99 | 38.12 | 0.83 | 0.02 | 0.85 | 37.69 | 2.60 | 96.1 | |
| 10c2 | 21.93 | 8.22 | 1.22 | 25.0 | 1.63 | 0.04 | 3.9 | 30.69 | 2.35 | 91.1 | |
| 112 | 51.06 | 17.09 | 1.81 | 6.15 | 2.51 | Tr. | 6.7 | 20.61 | 1.05 | 100.3 | |
| 132 | 62.57 | 19.31 | 2.61 | 0.94 | 1.80 | 0.01 | 4.1 | 8.51 | 0.49 | 96.2 | |
| 14 | 33.14 | 10.94 | 1.54 | 21.05 | 0.77 | 0.04 | 3.9 | 25.07 | 1.00 | 93.6 | |
| 15 | 55.71 | 21.55 | 3.31 | 1.32 | 0.16 | 0.02 | 9.0 | 11.78 | 2.13 | 96.0 | |
| 162 | 60.94 | 18.79 | 2.52 | 1.39 | 0.71 | 0.06 | 5.5 | 9.74 | 1.73 | 95.9 | |
| 1 Analyses by Ray Thompson. Chemist State Geological Survey of Kansas. 2 Not bentonite. Samples 5, 11, and 16 are ordinary clays. or clay mixed with bentonite. Samples 10b and 10c are clays high in calcium carbonate. Sample 13 is a clay-shale. All others are bentonites. |
|||||||||||
Table 4—Chemical analyses of bentonite from other states.1
| No. | Description and location |
SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O K2O |
TiO2 | H2O 105°C |
Loss 900°C |
SO3 | CO2 | Cl | P2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yellow, colloidal, Belle Fourche, S. Dak. |
60.64 | 23.26 | 3.92 | 0.59 | 2.19 | 4.70 | 0.12 | 2.83 | 98.25 | |||||
| 2 | Yellow, colloidal, Medicine Bow, Wyo. |
57.98 | 22.46 | 3.80 | 1.92 | 3.24 | 1.35 | 7.93 | 0.75 | 99.43 | |||||
| 3 | White, colloidal, Barstow, Calif. |
58.68 | 25.91 | 3.97 | 1.45 | 1.49 | 1.39 | 6.84 | 0.11 | Tr. | 0.10 | 0.06 | 100.0 | ||
| 4 | White, fine grained, Otay, Calif |
59.84 | 11.84 | 3.26 | 2.90 | 2.32 | 4.47 | 10.50 | 95.13 | ||||||
| 5 | Type material, Rock Creek, Wyo. |
60.18 | 26.58 | 0.23 | 1.01 | 1.23 | 10.26 | 99.49 | |||||||
| 6 | Big Horn Basin, Wyo. |
63.20 | 12.90 | 2.46 | 0.82 | 2.09 | 0.92 | 0.11 | 13.80 | 3.502 | 0.203 | 100.00 | |||
| 7 | Supposed bentonite, Shelbyville, Tenn. |
54.00 | 24.48 | 3.00 | 2.08 | 2.75 | 1.74 | 9.12 | 0.71 | 97.88 | |||||
| 8 | "Ardmorite," Ardmore, S. Dak. |
55.22 | 21.00 | 3.61 | 4.94 | 3.04 | 1.56 | 10.28 | 0.43 | Tr. | 100.08 | ||||
| 1. Laddo. 1925. 2. Sand and insoluble material. 3. Water soluble. |
|||||||||||||||
Objectionable impurities in bentonites are lime, iron oxide, and sulfates. Lime in the form of carbonate consumes acid where the bentonite is activated; as the sulfate, lime forms an insoluble compound. Iron oxide consumes acid in activation; it is objectionable In bentonite which might be used in ceramics or for refractory ware.
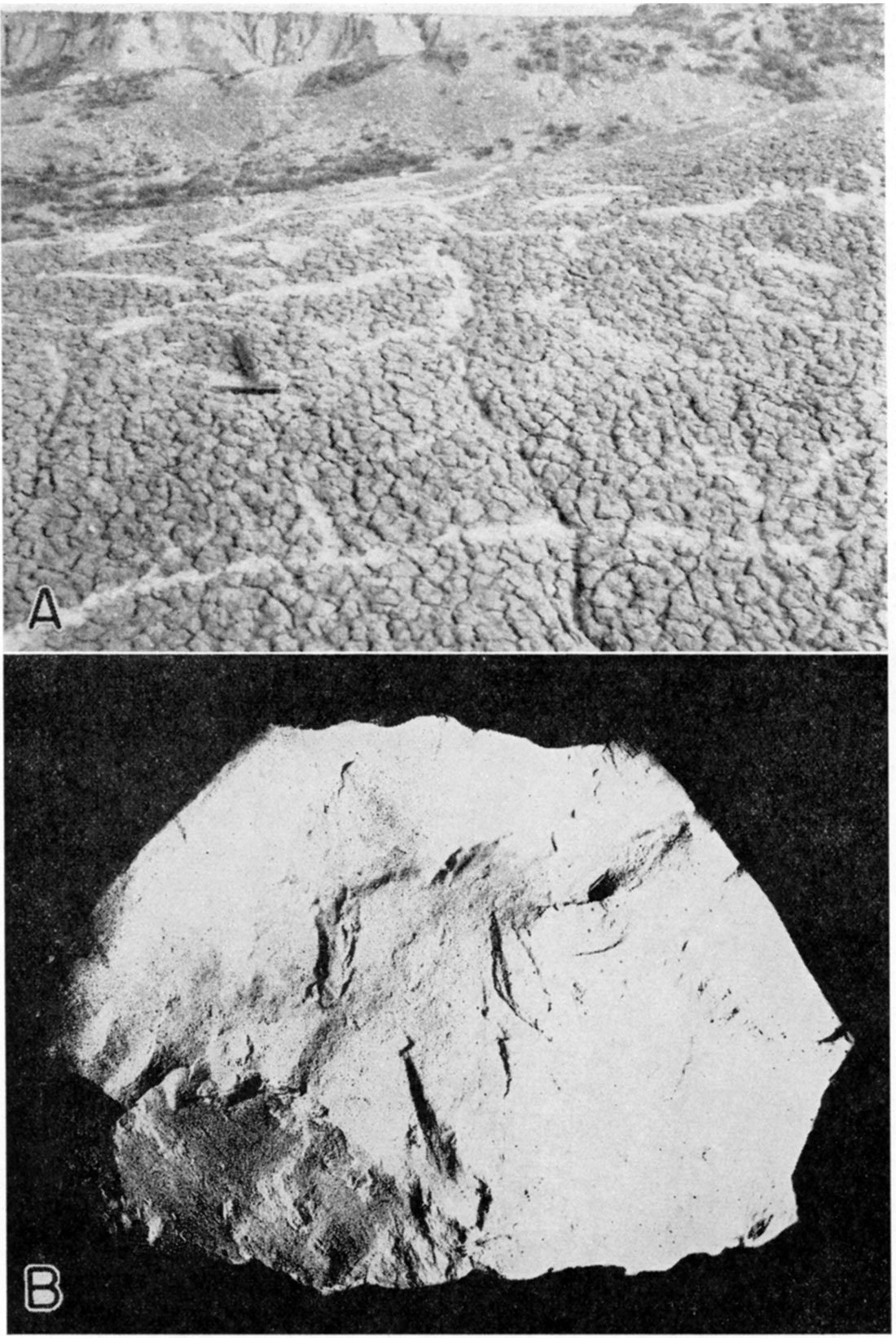
With the exceptions mentioned, the samples have only normal amounts of impurities. It should be noted, however, that impure bentonite is often located very near the pure material. The deposits represented by samples 10a, 10b, and 10c are very close to the relatively high grade material represented by sample 9. Where the bentonites are highly impure, gypsum appears more generally in the upper part of the deposit, while calcium carbonate is precipitated in channels throughout. Sample 10b is from such a channel. Plate 1A shows the deposit from which sample 10a was taken.
Plate 1—A. Bentonite clay deposit, Wallace County (photo by K. K. Landes). B. Alkali bentonite, Wallace County (sample 9, actual size).
The role of magnesia in bentonite may be a factor of considerable importance. Most naturally absorptive clays are relatively high in this constituent. In the Kansas bentonite, magnesia is rather low.
The classification of the samples of Kansas bentonite tested is given in table 5. The procedure of the U.S. Bureau of Mines was followed in identifying Kansas bentonite (Davis, Vacher, and Conley, 1940). The Bureau classifies bentonite according to its properties into four groups termed "alkali bentonites," "alkali sub-bentonites," "alkali-earth bentonites," and "alkali-earth sub-bentonites."
"A bentonite containing easily replaceable alkali bases and having original properties that are not permanently destroyed by the action of sulphuric acid, but can be restored by treatment with an alkali salt followed by regulated dialysis. This group includes Wyoming type bentonite and others similar to it."
Four of the samples were in this class although their swelling power in water is much less than Wyoming bentonite (samples 2, 8, 9, 10a.)
Plate 2—A. Alkali bentonite, Phillips County (sample 2, actual size). B. Alkali sub-bentonite, Wallace County (sample 15, actual size).
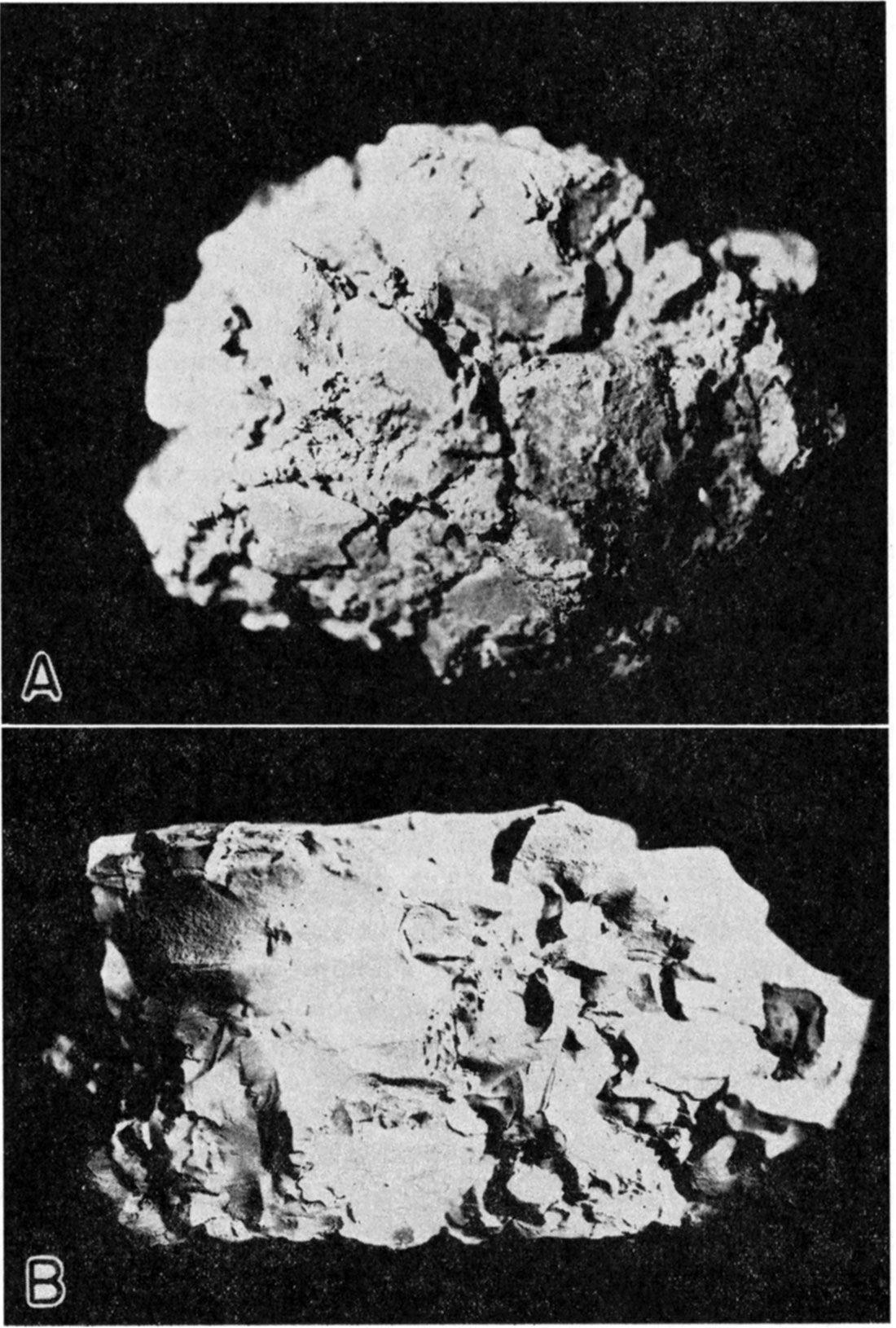
"A bentonite containing easily replaceable alkali bases but having original properties that are destroyed by acid treatment." (Samples 7, 14, 15.)
"A bentonite containing easily replaceable alkali-earth bases and, either before or after acid treatment, capable of being made to assume the properties of an alkali bentonite." (Sample 6.)
"A bentonite, containing easily replaceable alkali-earth bases, which, after treatment with acid, is not capable of being made to assume the properties of an alkali bentonite. Most bleaching clays are in this class." (Samples 1, 3, 4.)
This classification depends on the temporary osmotic pressure produced by bentonite having exchangeable alkali basis and on the absence of temporary osmotic pressure produced by bentonites with alkali-earth bases. .
Table 5—Classification of types of bentonite samples.
| Sample | Name | Sample | Name | |
|---|---|---|---|---|
| 1 | Alkali-earth sub-bentonite | 10a | Alkali bentonite | |
| 2 | Alkali bentonite | 10b | Impure clay, high in calcium carbonate | |
| 3 | Alkali-earth sub-bentonite | 10c | Impure clay, high in calcium carbonate | |
| 4 | Alkali-earth sub-bentonite | 11 | Clay | |
| 5 | Clay | 13 | Shale-clay | |
| 6 | Alkali-earth bentonite | 14 | Alkali sub-bentonite | |
| 7 | Alkali sub-bentonite | 15 | Alkali sub-bentonite | |
| 8 | Alkali bentonite | 16 | Clay | |
| 9 | Alkali bentonite | |||
| Photographs of bentonite showing different textures are shown in plates 1 and 2. | ||||
In drilling deep oil wells various mixtures of clay, shale, and other substances known as drilling muds are used in the holes to lubricate and cool the rotary bits, carry away rock cuttings, and act as a seal against the escape of gas. Frequently local clay is made into a thick slurry and used, but the tendency is to employ high-swelling bentonite. Where the latter is desired, Kansas cannot compete with the Wyoming product, which swells as much as fifteen times its original volume in water as compared to a little over three times for the best observed Kansas bentonite (tables 6 and 7). Many forms of Kansas bentonite, notably samples 2, 7, 9, and 10a, with moderate amounts of water, form thick colloidal pastes; and, on account of their cheapness and accessibility, such material should be found useful among local drillers. The bentonite mentioned is also very low in grit (table 16).
Table 6—Effect of immersion in water on solid crude samples.
| Sample no. | Reaction | Disintegration | Diffusion | Swelling |
|---|---|---|---|---|
| 1 | Slow | Partial | Partial | Little |
| 2 | Fast | Complete | Considerable | Moderate |
| 3 | Slow | Complete | None | Little |
| 4 | Slow | None | None | Little |
| 5 | Slow | None | None | Little |
| 6 | Slow | Partial | None | Little |
| 7 | Fast | Complete | Yes | Much |
| 8 | Fast | Complete | Partial | Little |
| 9 | Fast | Complete | Moderate | Much |
| 10a | Fast | Complete | Moderate | Much |
| 10b | Fast | Complete | Yes | Some |
| 10c | Fast | Complete | Moderate | Some |
| 11 | Fast | Complete | None | Much |
| 13 | Fast | Broke, into scaly pieces | None | Little |
| 14 | Fast | Complete, into fine pieces | Moderate | Some |
| 15 | Fast | Complete | Yes | Some |
| 16 | Fast | Complete, into small pieces | Moderate | Some |
Table 7—Swelling properties in water. (2.5 gram samples of crude bentonite—10 mesh in 15 cc distilled water)
| Sample no. | Volume without water (cc) |
Volume with water (cc) |
Increase (per cent) |
|---|---|---|---|
| 1 | 2.5 | 5.7 | 230 |
| 2 | 2.4 | 6.25 | 260 |
| 3 | 2.5 | 5.0 | 200 |
| 4 | 2.1 | 5.8 | 280 |
| 5 | 2.5 | 6.5 | 260 |
| 6 | 2.5 | 5.8 | 230 |
| 7 | 2.4 | 5.5 | 230 |
| 8 | 2.4 | 5.7 | 240 |
| 9 | 2.1 | 7.0 | 330 |
| 10a | 2.2 | 7.0 | 320 |
| 10b | 2.2 | 3.0 | 140 |
| 10c | 2.2 | 6.0 | 270 |
| 11 | 2.1 | 4.25 | 200 |
| 13 | 2.0 | 5.0 | 250 |
| 14 | 2.5 | 4.0 | 160 |
| 15 | 2.2 | 5.5 | 250 |
| 16 | 2.6 | 6.5 | 250 |
| Sample Wyoming alkali bentonite |
2.5 | 13.0 | 520 |
Table 8—Results of gelation test as applied to samples of Kansas and Wyoming bentonite.
| Sample | Supernatant liquid (cc) | Result |
|---|---|---|
| Wyoming | 10 | Stiff gel |
| 1 Kansas | 85 | No gel |
| 2 Kansas | 80 | Gelatinized slightly |
| 3 Kansas | 83 | Gelatinized slightly |
| 4 Kansas | 85 | Gelatinized slightly |
| 5 Kansas | 85 | Gelatinized slightly |
| 6 Kansas | 90 | No gel |
| 7 Kansas | 85 | Gelatinized slightly |
| 8 Kansas | 85 | Gelatinized slightly |
| 9 Kansas | 85 | Gelatinized slightly |
| 10a Kansas | 85 | Gelatinized slightly |
| 10b Kansas | 85 | Gelatinized slightly |
| 10c Kansas | 90 | Gelatinized slightly |
| 11 Kansas | 90 | No gel |
| 13 Kansas | 90 | Gelatinized slightly |
| 14 Kansas | 90 | Gelatinized slightly |
| 15 Kansas | 90 | Gelatinized slightly |
| 16 Kansas | 90 | No gel |
A specific test, obviously best adapted to the evaluation of Wyoming bentonite, follows:
"Dry 4 grams of 200 mesh material, to which 5 per cent MgO has been added, at 220°F. Agitate in stoppered bottle with 100cc water for one hour. After standing 24 hours, a high grade Wyoming bentonite will yield a stiff gel that does not break on inverting." [Cross Engineering Co. Bentonite Handbook. Kansas City, rev. ed .. 1934, p. 9.]
Qualitatively, the sample yielding the least clear supernatant liquid is the best (table 8). Alkaline Wyoming bentonite will suspend itself uniformly in thin water dispersions; Kansas bentonite will not.
In these tests 50 cubic centimeters of a dirty black crank case oil was heated to about 150° centigrade and at the same time agitated for half an hour with various amounts of bentonite, both raw and activated, and finally filtered. The results are shown in table 9.
Table 9—Bleaching tests with Kansas bentonite by contact method.
| Sample | Amount bentonite 100-mesh (grams) |
Upper part: clear oil (per cent) |
Lower part: cloudy oil (per cent) |
|---|---|---|---|
| blank | 0 | 0 | 100, black |
| 1 | 10, activated | 0, opaque dark olive | 100, dark olive |
| 1 | 2, activated | 0, opaque dark olive | 100, dark olive |
| 2 | 10, activated | 66, amber | 34, dark amber, cloudy |
| 2 | 2, activated | 32, amber | 68, dark amber, cloudy |
| 3 | 10, activated | 50, amber | 50, dark amber, cloudy |
| 3 | 2, activated | 35, amber | 65, dark amber, cloudy |
| 3 | 10,raw | 0, opaque dark olive | 100, dark olive, cloudy |
| 4 | 10, activated | 81, amber | 19, dark amber, cloudy |
| 4 | 2, activated | 32, amber | 68, dark amber, cloudy |
| 5 | 10, activated | 75, amber | 25, dark amber, cloudy |
| 5 | 2, activated | 20, amber | 80, dark amber, cloudy |
| 6 | 10, activated | 50, amber | 50, dark amber, cloudy |
| 6 | 2, activated | 13, amber | 87, dark amber, cloudy |
| 7 | 10, activated | 20, amber | SO, dark amber, cloudy |
| 7 | 2, activated | 2O, amber | 80, dark amber, cloudy |
| 8 | 10, activated | 50, amber | 50, dark amber, cloudy |
| 8 | 2, activated | 0, opaque dark olive | 100, dark olive, cloudy |
| 9 | 10, activated | 87,amber | 13, dark amber, cloudy |
| 9 | 2, activated | 35, amber | 65, dark amber,cloudy |
| 9 | 5, activated | 45, amber | 55, dark amber, cloudy |
| 9 | 10, raw | 25, amber | 75, dark amber, cloudy |
| 10 | 10, activated | 0, opaque dark olive | 100, dark olive, cloudy |
| 10 | 2, activated | 0, opaque dark olive | 100, dark olive, cloudy |
| 13 | 10, activated | 63,amber | 37, dark amber, cloudy |
| 13 | 2, activated | 35, amber | 65, dark amber, cloudy |
| 15 | 10, activated | 56, amber | 44, dark amber, cloudy |
| 15 | 2, activated | 35, amber | 65, dark amber, cloudy |
| Wyoming | 5, raw | 0, opaque dark olive | 100, dark olive, cloudy |
| Wyoming | 5, activated | 0, opaque dark olive | 100, dark olive, cloudy |
The results show many of the forms of Kansas bentonite have excellent bleaching properties. In comparison, the Wyoming sample has no bleaching power. The activated bentonite proved to be far superior to the raw product. A comparison of results shows a wide variation in the bleaching efficiency of the different samples. Sample 9 proved to be the best, or equal to the best, using either ten-gram or two-gram samples. Samples 1 and 10 had no bleaching power. Ranging from highest in efficiency to lowest in efficiency the samples may be listed: 9, 4, (2, 5), 13, 15, 3, 6, 8, 7, (1, 10), Wyoming.
The contact process is the same as the previous test except that only half of the bentonite was added at the start of the test. After agitating for half an hour, the oil was filtered, the second half of the bentonite added, and the test finished as usual. This process occupied one hour instead of half an hour.
The efficiency of the two-stage addition of bentonite is shown by the results summarized in table 10. Four grams added intermittently gave better results than ten grams added at one time; the oil was completely bleached.
Table 10—Bleaching tests with Kansas bentonite, by intermittent contact method.
| Sample | Amount bentonite 100-mesh (grams) |
Upper part: clear oil (per cent) |
Lower part: cloudy oil (per cent) |
|---|---|---|---|
| 9 | 2, activated | 50, amber | 50, dark amber, cloudy |
| 9 | 4, activated | 100, amber | 0 |
Where all the bentonite is added at one time, samples agitated for one hour apparently showed no greater decolorization than the same samples agitated half an hour.
Bentonite in these tests was activated by boiling half an hour with sulphuric acid (25 per cent acid by weight, in the ratio of 1 gr. of bentonite to 3 cc of acid). Clays that may be activated are characterized by a waxy or soapy luster. Samples 2 and 7 show this luster strongly, 9 and 10 only faintly; the other bentonite samples show a luster in intermediate degrees.
The importance of oil retention among competitive types of bentonite used for bleaching in the contact process is second only to the decolorizing value, since the oil held in the clay after use is usually not recovered. The standard test is to determine the increase in weight of a given quantity of clay after contacting with oil and blowing the cake produced with air at 40 pounds per square inch pressure, and at a temperature of 375° F. The results given in table 11 were obtained in this manner except for the substitution of mechanical pressure for air pressure. The test is subject to error because of the small amount of sample taken—5 grams of bentonite to 50 cubic centimeters of oil.
Table 11—Oil retained in bentonite after contact process of bleaching.
| Sample | Oil (cc) |
Bentonite (grams) |
Oil retained (per pound) |
|---|---|---|---|
| 4 | 50 | 5 | 0.58 |
| 6 | 50 | 5 | 0.90 |
| 9 | 50 | 5 | 0.70 |
| 15 | 50 | 5 | 0.73 |
The results of bleaching with bentonite by percolation were unsatisfactory. The raw samples filtered well but showed little decolorizmg power; the activated samples decolorized satisfactorily but filtered too slowly, probably because of too much fine material. Particle size of the bentonite was represented by a mixture that would pass a 10 mesh screen and be caught on 100 mesh. The sample activated from this material was still finer in size. In testing, 50 cubic centimeters of crank case oil, heated first to 125° centigrade, was passed through 40 grams of bentonite placed in a one-inch glass cylinder to act as a filter. Several days were required for this oil to filter through the activated bentonite. Results are shown in table 12.
Table 12—Bleaching tests with Kansas bentonite by percolation method.
| Sample | Bentonite (grams) |
Crank-case oil (cc) |
Clear oil in filtrate (per cent) |
Cloudy oil in filtrate (per cent) |
|---|---|---|---|---|
| 9 | 40, crude | 50 | 0 | 100.0 |
| 9 | 40, activated | 50 | 98 | 2.0 |
As a bleaching agent activated bentonite is much more efficient than either raw bentonite or fuller's earth. Activated bentonite is commonly used pulverized and applied by the contact method, because of the difficulty in obtaining a satisfactory granular product for percolation. Crude bentonite applied by the contact method is sometimes used for gasolines and for oils that have been acid treated because it neutralizes the acid.
Where activated bentonite is used on light oil or gasoline, it can be rejuvenated by igniting and burning off carbonaceous matter. On heavy oils it can be used but once; the discard can be burned under boilers or used as road surfacing material.
For the percolation method of bleaching, the fuller's earth type of clay is used. It requires no activation, can be used again after burning, and is less expensive than activated bentonite. These advantages, however, are apparently more than balanced by the greater efficiency of activated bentonite. During the last few years the use of activated bentonite has doubled; there has been no increase in the use of other bleaching clays.
Large percentages of bentonite are not employed in ceramic mixtures on account of shrinkage. In limited degree bentonite adds to the bonding power and strength of clays. The results of tests in which bentonite was added to high grade Georgia kaolin, both raw and calcined, are shown in table 13.
Table 13—Bentonite in ceramic mixtures.
| Sample | Koalin (per cent) |
Bentonite (per cent) |
Modulus of rupture (lbs. per sq. m.) | |
|---|---|---|---|---|
| Raw koalin | Calcined koalin | |||
| Georgia kaolin | 100 | 0 | 159 | too low to record |
| Kaolin plus bentonite no. 2 |
95 | 5 | 185 | 155 |
| Kaolin plus bentonite no. 8 |
95 | 5 | 320 | 146 |
| Kaolin plus bentonite no. 9 |
95 | 5 | 297 | 275 |
| Kaolin plus bentonite no. 2 |
90 | 10 | 391 | 522 |
| Kaolin plus bentonite no. 8 |
90 | 10 | 479 | 567 |
| Kaolin plus bentonite no. 9 |
90 | 10 | 243 | 568 |
| Formula for modulus of rupture for table 13. Modulus of rupture: M = 3 PI / 2bd2 Where M = modulus of rupture in pounds per sq. in. P = breaking load in pounds I = distance between knife edges in inches b = breadth of bar in inches d = depth of bar in inches |
The test samples were 1 square inch in cross section and 3 inches long. They were prepared by tempering the clay mixtures and firing at 2200° F.
It is apparent that the breaking strength is increased by the use of bentonite. Also, except in the case of sample no. 9 and raw kaolin, the strength is greater with 10 than with 5 per cent bentonite. It is possible that the no. 9 sample with 10 per cent had too much bonding agent and suffered from shrinkage strains after firing.
Table 14—Molding sand test values.
| Kind of casting |
Type of ramming |
Moisture (approximate per cent) |
Strength (lbs. per sq. in.) | Permeability | |||
|---|---|---|---|---|---|---|---|
| Sheer | Compressive | ||||||
| Green | Dry | Green | Dry | ||||
| Stove plate | Squeeze | 7.5-8.5 | 1.3 | 6 | 7 | 19 | 9 |
| 8 lb. plate | Jolt squeeze | 7-8 | 1.4 | 6 | 7 | 33 | 15 |
| 15 lb. plate | Jolt Squeeze | 6-7.7 | 1.4 | 6.5 | 7 | 40 | 25 |
| 20 lb. jobbing | Hand | 7-8 | 1.8 | 7 | 8 | 40 | 35 |
| Radiator | Hand | 6-7 | 1.2 | 7 | 6.5 | 52 | 35 |
| Radiator | Machine | 6-7 | 1.4 | 7 | 7 | 52 | 35 |
| Bath tub | Sand slinger | 5-6 | 1.1 | 7 | 6 | 46 | 70 |
| Cylinder block | Sand slinger | 5.5-6.5 | 1.5 | 7 | 7.5 | 42 | 80 |
| Cylinder block | Jolt | 6-7 | 1.5 | 7 | 8.5 | 44 | 80 |
| Car wheel | Jolt | 7.5-8.5 | 1.4 | 7 | 6.2 | 40 | 130 |
| Boiler section | Jolt | 6-7 | 1.3 | 7 | 7 | 40 | 80 |
| Boiler section | Sand slinger | 5.5-6.5 | 1.2 | 7 | 6.5 | 40 | 80 |
| Pipe | Jolt | 8-9 | 1.3 | 8 | 7 | 48 | 300 |
| Pipe | Pneumatic | 6-7 | 1.3 | 8 | 7 | 48 | 300 |
| Plow | Jolt | 6-7 | 1.4 | 8 | 7.5 | 47 | 30 |
| Steel | Jolt | 3-4 | 1.5 | 10 | 7.5 | 52 | 160 |
| Steel plate | Jolt squeeze | 3-4 | 1.5 | 10 | 7.5 | 50 | 110 |
| Fly wheel | Jolt | 6.3-7.3 | 1.3 | 7 | 7.5 | 40 | 90 |
| Fly wheel | Sand slinger | 6-7 | 1.2 | 7 | 7 | 40 | 100 |
| Bronze bushing | Jolt | 6-7 | 1.2 | 6 | 7.5 | 39 | 35 |
| Aluminum plate | Jolt squeeze | 6-7 | 1.2 | 6 | 7 | 20 | 20 |
| Aluminum large | Jolt | 6-7 | 1.2 | 6 | 7.5 | 32 | 27 |
The use of bentonitic clay as a constituent of molding sand is increasing for the following reasons.
The physical requirements of the usual molding sand for different purposes are shown in table 14. The sand contains a clay bond, not bentonite, and is relatively high in moisture.
The tests to determine the effect of bentonite in molding sand mixtures were carried but using apparatus and methods in accordance with the specifications of the American Foundrymen's Association [American Foundrymen's Association. Testing and Grading Foundry sands and clays—Standards and Tentative Standards. Chicago. 4th ed., 1938. pp. 1-61]. The results are shown in table 15. Pure quartz sand, -50 + 70 mesh was used in all cases to mix with the bentonite which was ground to pass 270 mesh. A number of tests were necessary in order to determine the optimum amounts of sand, bentonite, and water, to give best results. A sample of, an advertised brand of bentonite (not native Kansas) used in foundry work was tested for purposes of comparison.
Table 15—Molding sand test values on mixtures containing Kansas bentonite.
| Sample | Composition of sand mixture |
Strength (lbs. per sq. in.) | Permeability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bentonite | Moisture | Shear | Compressive | ||||||
| Type | (Pct.) | (Pct.) | Green | Dry | Green | Dry | Green | Dry | |
| Advertised brand |
Alkaline | 4 | 1.7 | 1.1 | 5.4 | 4.4 | 25.6 | 359 | 318 |
| Kansas 9 | Alkaline | 4 | 1.7 | 1.4 | 1.7 | 5.6 | 6.2 | 355 | 272 |
| Kansas 8 | Alkaline | 4 | 1.7 | 1.1 | 4.6 | 4.1 | 18.8 | 193 | 315 |
| Kansas 6 | Alk.-earth 4 | 1.7 | 1.0 | 4.2 | 4.1 | 25.4 | 279 | 343 | |
| Kansas 15 | Alk.-sub | 4 | 1.7 | 1.3 | 3.9 | 5.1 | 19.6 | 295 | 285 |
| Kansas 3 | Alk.-earth sub. |
4 | 1.7 | 0.8 | 7.0 | 2.9 | 38.0 | 315 | 300 |
| Advertised brand |
Alkaline | 8 | 1.7 | 2.9 | 4.3 | 14.4 | 14.3 | 310 | 290 |
| Kansas 9 | Alkaline | 8 | 1.7 | 2.4 | 4.7 | 15.5 | 21.3 | 358 | 270 |
| Kansas 8 | Alkaline | 8 | 1.7 | 2.6 | 5.4 | 13.9 | 24.7 | 322 | 225 |
| Kansas 6 | Alk.-earth | 8 | 1.7 | 3.2 | 5.6 | 15.2 | 25.3 | 285 | 276 |
| Kansas 15 | Alk.-sub | 8 | 1.7 | 4.7 | 6.9 | 17.5 | 23.4 | 338 | 348 |
| Kansas 3 | Alk.-earth sub. |
8 | 1.7 | 1.6 | 2.8 | 8.6 | 8.0 | 270 | 253 |
| Advertised brand |
Alkaline | 8 | 2 | 2.9 | 6.5 | 10.7 | 26.3 | 500 | |
| Kansas 9 | Alkaline | 8 | 2 | 2.9 | 3.4 | 13.1 | 15.9 | 470 | |
| Kansas 8 | Alkaline | 8 | 2 | 5.5 | 9.1 | 17.1 | 40.0 | 310 | |
| Kansas 15 | Alk.-suh. | 8 | 2 | 3.6 | 5.8 | 18.3 | 26.9 | 355 | |
| Advertised brand |
Alkaline | 8 | 3· | 3.1 | 16.0 | 9.6 | 75.5 | 355 | |
| Kansas 9 | Alkaline | 8 | 3 | 2.2 | 10.5 | 7.9 | 36.8 | 300 | |
| Kansas 8 | Alkaline | 8 | 3 | 3.3 | 16.3 | 11.5 | 72.2 | 310 | |
| Kansas 15 | Alk.-sub. | 8 | 3 | 3.0 | 11.6 | 9.9 | 56.0 | 325 | |
| K.U. natural molding sand |
3 | 2.7 | 6.7 | 11.0 | 33.7 | 24 | |||
| K.U. natural molding sand |
4 | 2.3 | 12.7 | 9.5 | 61.0 | 60 | |||
| Clay bonded molding sand |
12 (clay) 2 | 2.8 | 6.9 | 9.8 | 19.3 | 220 | |||
| Clay bonded molding sand |
12 (clay) 3 | 2.1 | 10.9 | 7.1 | 43.5 | 270 | |||
The mixture containing 4 per cent bentonite and 1.7 per cent moisture has rather poor physical properties compared with those given in table 14. The mixture containing 8 per cent bentonite and 1.7 per cent moisture shows an improvement, but both the dry shear and dry compressive strengths are relatively low. Note that Kansas bentonite, with the exception of sample 3, shows belter properties than the advertised product. The results with 8 per cent bentonite and 2 per cent moisture are more consistent, except for sample 9, with the normal results as shown in table 14. Sample 9 gave better results with less moisture. Note that samples 8 and 15 gave the best results. With 8 per cent bentonite and 3 per cent moisture, the dry strength values are higher than required. Lowering the moisture 0.5 per cent or more would make for general improvement. For purposes of comparison, results of tests on natural molding sand, used at the University of Kansas in Fowler Shops, and on a mixture of quartz sand bonded with ordinary clay from Cloud County, Kansas, are given in table 15.
A study of the tests shown leads to the conclusion that, using selected Kansas bentonite in place of clay, with proper regard for moisture content, any reasonable combination of physical properties desirable in a molding sand can be obtained (table 14) . Furthermore, these results will be obtained by using less of both bentonite and moisture, less moisture being a decided advantage. The permeability in all cases was relatively high.
Table 16—Miscellaneous tests using Kansas bentonite.
| Sample | Density1 (lbs. per sq. ft.) Activated |
Grit on 250 mesh Pct. Raw |
Acidity3 (Mgs. KOH per gr.) Activated |
Color on heating to 900°C. (oxidizing Raw |
|---|---|---|---|---|
| 1 | 69 | 0.55 | 7.5 | Gray |
| 2 | 69 | 0.50 | 5.6 | Very light gray |
| 3 | 76 | 15.80 | 4.8 | Medium red-brown |
| 4 | 68 | 2.4 | 10.7 | Light red |
| 5 | 68 | 16.7 | 15.7 | Dark red |
| 6 | 67 | 9.2 | 6.3 | Light red |
| 7 | 62 | 1.1 | 4.4 | Light gray |
| 8 | 67 | 0.65 | 7.2 | Grayish brown |
| 9 | 61 | 13.4 | Medium grayish brown | |
| 9 | 83 (raw) | 0.80 | Neutral (raw) | |
| 10a | 62 | 0.40 | 13.1 | Grayish brown |
| 13 | 58 | 4.2 | Light brick red | |
| 14 | 19.8 | Light gray | ||
| 15 | 63 | 0.3 | 5.0 | Medium gray-brown |
| 1. After 5 minutes mechanical tamping in graduated cylinder. 2. After dispersing 100 grams in 2 liters of water, washing out clay, drying, and weighing. 3. 1 gram activated bentonite leached in distilled water. Extract titrated with KOH using phenolphthalein as indicator. |
||||
The results indicate that by employing selected samples of bentonite, the correct amount for general work is 8 per cent bentonite and possibly 2.25 or 2.50 per cent moisture. Two per cent moisture gave dry compression values that are somewhat low, except in the case of sample 8, while those obtained with 3 per cent are, in most cases, unnecessarily high.
Although the samples of bentonite taken for these tests were selected somewhat at random, all proved to be suitable except number 3. With the exception of the results from using 4 per cent bentonite and 1.7 per cent moisture, all of which were unsatisfactory, some of the Kansas samples in every test showed properties superior to those of the advertised sample from out of state. Samples 8 and 15 gave the best general results.
Table 16 shows results of miscellaneous tests with Kansas bentonite.
In this investigation many of the samples of Kansas bentonite have been found to have properties highly desirable for certain applications. Most of the bentonite produced in this country is used for bleaching of oils, for conditioning of .foundry sand, and for use in drilling mud. For bleaching of oils and conditioning of foundry sand, choice Kansas material proved highly efficient and gave better performance than competitive bentonite from other states. For use in drilling mud, Kansas bentonite, so far as the samples tested are concerned, is inferior to the Wyoming product. This use, however, is being further investigated by actual tests in the field. Considering the accessibility of the deposits, the nearness to railroad shipping points, and the shorter haul to Eastern industrial centers (compared with certain other Western producing states), Kansas is in position to compete in the sale of bentonite, which does around a two million dollar business annually.
In connection with the physical properties and usefulness of Kansas bentonite for certain purposes some distinctions can be made. All types are represented in the state: alkaline bentonite, alkaline sub-bentonite, alkaline earth bentonite, and alkaline earth sub-bentonite. These may have somewhat different properties from bentonites of the same general classification found elsewhere. In general, in this state as elsewhere, the alkaline or alkaline sub-bentonite has the greatest swelling power in water, making it valuable in drilling muds. In bleaching power the best material in Kansas is alkaline bentonite or alkaline earth sub-bentonite. Some samples which did not bleach effectively, however, were found in both classes. The tests, indicate that bonding power for molding sands is distributed among all classes, with the possible exception of the alkaline earth sub-bentonite. Table 17 lists some of the uses of bentonite together with the numbers of the samples thought to be best adapted to these special uses. Samples 15, 10a, 2, 8, and 9 were low in grit content.
Samples 2, 14, 7, and 15 were light in color. The locations of the Kansas deposits, of which these samples are representative, are given on page 355.
Table 17—Uses for Kansas bentonite.| Use | Bentonite samples giving best results, left to right. |
|---|---|
| Bleaching agent for oil | 9, 4, (2, 5) 13, 15 |
| Bond for foundry sand | 8, 15 |
| Oil field drilling mud | 9, 10a, 2, 7, 15, 8 |
| To strengthen ceramic products | 8, 9, 2, 15 |
| Refractory ware | 2, 15 |
| Filler for paper | 2 (nearly white, little grit) |
| Soap (toilet) | 2 (nearly white), 9, 15 |
| Soap (mechanics) | 7 (some volcanic ash) |
| Cosmetics | 2 (nearly white, fine grain, little grit) |
| De-inking newsprint | 9, 4, (2,5) 13, 15 |
| Clarifying dry cleaner fluids | 9, 4, (2, 5) 13, 15 |
| Core for earth fill dams (to prevent leaking of ponds and ditches) |
9, 10a, 2, 7, 15 |
| Cement mixtures | 8, 15 |
American Foundrymen's Association, 1938, Testing and grading foundry sands and clays-standards and tentative standards, 4th ed.: Chicago, Ill., pp. 1-61.
Cross Engineering Co., 1934, Bentonite handbook, rev. ed.: Kansas City, Mo., pp. 1-40.
Davis, C. W., Vocher, H. C., and Conley, John E., 1940, Bentonite: its properties, mining, preparation, and utilization: U.S. Bur. Mines, Tec. paper 609, pp. 1-83.
Elias, M. K., 1931, The geology of Wallace County, Kansas: Kansas Geol. Survey Bull. 18, pp. 1-252, figs. 1-7, pls. 1-42. [available online]
Ladoo, R. B., New York, 1925, Nonmetallic minerals: McGraw Hill, N.Y., pp. 1-93.
Minerals Yearbook, 1941, U.S. Bur. Mines.
Kansas Geological Survey
Placed on web Nov. 10, 2017; originally published Dec. 14, 1942.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/41_10/index.html