Kansas Geological Survey, Bulletin 233, p. 123-137
by
Robert L. Brenner1, Greg A. Ludvigson2, Roland Scal3, and A. Umran Dogan4
1. University of Iowa
2. Iowa Geological Survey Bureau
3. University of New Hampshire
4. Princeton University
Basin analysis (the reconstruction of the dynamics and history of sedimentary basins) has entered a quantitative stage that requires analytical lithologic data. These data must include geologic parameters that describe the characteristics of sediments and the diagenetic changes that they undergo through time. Diagenesis is controlled by eight geologic parameters: sediment composition, temperature history, rate of accommodation (subsidence + sea-level changes + sediment compaction), rate of sediment accumulation, age (time that sediments have been exposed to other variables), internal sediment-body architecture (sedimentary texture and structure), sediment-body external geometry, and fluid chemistry and flow history. Tectonic and paleogeographic settings determine the primary compositions of both chemical and siliciclastic sediments. Siliciclastic provenances are reflected by the mineralogy of sandstones. The source or sources of sediment in sandstone units within genetic sequences and the contribution of each source need to be evaluated in terms of quantitative effects on the various diagenetic styles observed. With the use of modern settings as partial analogues, stratigraphic, sedimentologic, and petrographic data can be used to reconstruct sandstone architecture and to draw inferences about original pore fluid chemistry. Subsidence histories, isotopic signatures, trace element compositions, and fluid inclusion studies combined with petrographic observations can be used to set constraints on the geologic parameters for sandstone bodies within a time-temperature-basin setting framework. As more insight is gained into the reaction kinetics within specific paleotectonic and depositional settings, diagenetic modeling will become increasingly more quantitative and precise.
An Acrobat PDF file containing the complete paper is available (1.4 MB).
Basin analysis has its roots in the first half of the twentieth century when tectonic settings were shown to control the compositions of rock associations. Since that time basin analysis has evolved into a catchall term for integrative studies involving the dynamics and history of sedimentary basins. Selection of data types and methodologies employed depends on the specific goals of a project and the areas of expertise covered by the researchers involved. One manifestation of basin analysis that has been receiving increasing emphasis is the integration of depositional architecture and diagenetic processes in a synthesis of basin history.
The importance of diagenesis became apparent when petroleum exploration efforts expanded into deep basins and into mineralogically unstable potential reservoir rock facies. Simple temperature-dependent models, such as the porosity basement concept of the early 1970's, were unreliable predictors of reservoir characteristics and petroleum accumulations in many basins. The presence of porous and permeable sandstone petroleum reservoirs of varying ages at great depths and of tight sandstone units at depths of only a few hundred meters demonstrates that temperature, pressure, and time are not the only parameters that determine diagenetic style and that the importance of these and other parameters varies from basin to basin.
With the advent of quantitative basin modeling [e.g., Cross (1990)], quantitative evaluations of diagenetic processes must be taken into account to construct quantitative forward models that can be used to make reliable predictions. To understand diagenetic effects, researchers must determine what diagenetic reactions take place, where they take place, what the rates of reaction are under a variety of conditions, and when these reactions take place during the history of a basin. In addition, we must be able to determine whether or when the rocks we are modeling were within an open fluid-dominated system versus a closed rock-component-dominated system with respect to the cycling of fluids and dissolved chemical constituents.
In this article we review the geologic parameters that must be considered when constructing a diagenetic model and evaluate the current state of the art in regard to these parameters. Published papers and our own research are used to review important diagenetic reactions in siliciclastic rocks and to illustrate diagenetic studies, constraints placed on geologic parameters, and attempts at diagenetic modeling. Finally, we examine those processes for which there are no readily available techniques for quantitative evaluation and suggest ways in which these processes and parameters might be developed and estimated.
As sediments pass through changing temperature, pressure, and fluid regimes in a subsiding basin, they undergo diagenetic changes that alter their lithologic characteristics. Diagenetic processes include compaction, cementation, mineral replacement and dissolution, plastic deformation of detrital grains, and grain fracturing. The combined effects of these processes on the original sediments are referred to as the diagenetic style.
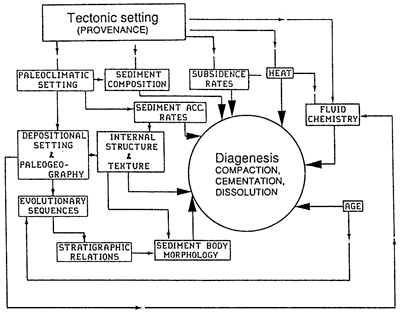
Diagenetic style is controlled by eight interactive parameters: (1) sediment composition, (2) temperature history, (3) rate of accommodation (subsidence + sea-level change), (4) rate of sediment accumulation, (5) age (the time that sediments have been exposed to other variables), (6) internal sediment-body architecture (sedimentary texture and structure), (7) sediment-body external geometry, and (8) fluid chemistry and flow history (fig. 1). Each of these variables is considered to be independent, and each should be an integral part of any basin analysis. In addition, other important dependent variables are determined by combinations of the listed parameters. For example, pressure gradients within a sediment package are determined by the comparative rates of accommodation and sediment accumulation and by internal sediment-body architecture (i.e., sedimentary texture and structure determine porosity and interconnectiveness of pores) and sediment-body external geometry (which determines potential flow paths). Pressure gradient history is a critical factor in diagenetic modeling because it determines the direction and timing of fluid flow pathways.
Figure 1--Relationships among tectonic settings, paleoclimates, depositional settings, and the eight geologic parameters that directly control diagenesis.

Tectonic and paleogeographic settings determine the primary compositions of both chemical and siliciclastic sediments. Siliciclastic provenances are reflected by the mineralogy of sandstones and pelitic sedimentary rocks. The source or sources of sediment in sandstone units within genetic sequences and the contribution of each need to be evaluated because the original sediment composition places quantitative limits on some diagenetic processes. This is important because for the most part diagenetic products are formed from the dissolution or alteration of original detrital grains. Therefore large contributions by source areas covered with unstable rocks, such as andesitic volcanic rocks, would result in an unstable primary sediment package compared to sediment packages derived from granitic sources. The postdepositional hydrochemical regimes that sediment packages are subjected to also contribute to the diagenetic style because they control the generation of authigenic materials derived from sources outside sandstone bodies, such as carbonate cements from the degradation of organic matter [e.g., Surdam et al. (1989)]. These hydrochemical regimes depend to some extent on the depositional environments of the sediment packages being studied.
Heat flow, subsidence rates, and sediment accumulation rates are related to a basin's tectonic setting and fill history. Relative changes in sea level (i.e., eustasy + subsidence) affect rates of accumulation. The resulting burial history determines the thermal and pressure gradients within a basin. Geohistory analysis and backstripping are procedures that are used to trace the burial history of reservoir units as they move through time-temperature-pressure domains [e.g., Guidish et al. (1985), Sclater and Christie (1980), Siever (1983), Surdam et al. (1989), Thorne and Watts (1989), and Van Hinte (1978)]. As mineral grains move through these domains, the chemistry and sense of movement of fluids around them vary in response to changing temperature and pressure conditions. Chemical changes involve such parameters as pH, Eli, partial pressure of carbon dioxide (PCO2), salinity, and composition, which in turn control the relative solubilities of aluminosilicates and carbonates. In hydrocarbon-generating basins the interaction of chemical by-products from organic maturation with mineral grains and authigenic cements may play a major role in diagenesis and resulting reservoir qualities (Curtis, 1978; Surdam et al., 1989).
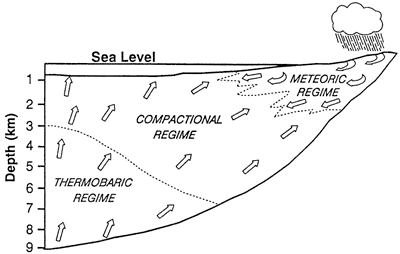
Galloway (1984) suggested that the hydrologic systems of large sediment-filled basins can be divided into three hydrologic regimes: meteoric, compactional, and thermobaric (fig. 2). In the meteoric regime sediments are in contact with waters that are flowing down topographic gradients from surface recharge areas. Flow rates are relatively high and are controlled by the permeability and homogeneity of sand bodies. In depositional systems with high sand body connectivities, we can consider this regime to be an open system in that waters are continuously replaced as they are discharged. The compactional regime is characterized by upward and outward expulsion of pore waters from compacting sediments. Pressures can be greatly increased as a result of lithostatic loading or the application of compressive tectonic stress (Galloway, 1984). Because there is a finite amount of fluid in this regime, we can consider the sediments and fluids to be in a closed system. Likewise the thermobaric regime can be considered to constitute a closed system. Fluids in this regime include waters released by mineral dehydration reactions, such as those that take place during clay mineral transformations (e.g., smectite to mixed-layer clays). Fluids move in response to pressure gradients generated by inorganic and organic phase changes (including hydrocarbon generation) and by ongoing lithostatic loading (Galloway, 1984). Harrison (1990) suggests that each regime can be distinguished by characteristic diagenetic processes. We consider regimes and characteristic diagenetic products in our discussion of diagenetic reactions.
Figure 2--Hydrologic regimes that sediments pass through in a subsiding basin. Arrows show fluid flow directions. Modified from Galloway (1984) and Harrison (1990).
The internal architecture and external geometry of sandstone bodies form the primary plumbing system for the movement of formation fluids. Rates of movement of chemically active fluids are determined by pressure differentials and porosity-permeability pathways. The primary plumbing system establishes the original pathways along which reactive fluids move and some diagenetic reactions take place. Distributions of diagenetic products and rates of diagenetic reactions depend to a large extent on rock heterogeneities on many scales. At the largest scale sand body continuity and connectivity between porous bodies are important parameters affecting both fluid flow and the degree to which the system remains open. Within a sand body the presence and distribution of permeability baffles and permeability pathways are critical factors for distributing diagenetic products. Stratification, especially cross -stratification involving fine-grained clay-rich layers, plays a major role in heterogeneous fluid movement on a microscopic scale (Van de Graaff and Ealey, 1989).
Most diagenetic reactions take place within fluid media. These reactions include ionic exchanges between sediment particles and interstitial fluids and between chemically different fluids in pore spaces. To model diagenetic processes, we must be able to identify potential reactions and the stability fields that control them. To determine these parameters, the starting chemical ingredients and their temporal flux must be established. In addition to establishing the compositions of original mineral and matrix materials, the initial fluid chemistry must be known or discerned. Changes in fluid composition throughout the geologic history of the sediment package must be reconstructed, and thermodynamic and kinetic equations of state must be defined.
The original pore fluids in a sediment can be reconstructed through analogy to modern sedimentary environments. These interpretations can be enhanced or modified in some cases by analyzing fluid inclusions in endogenic crystals (e.g., carbonate grains) or early diagenetic cements.
The relative importance of diagenetic reactions varies between rock packages because variations occur in the eight controlling parameters. To determine constraints for diagenetic models, we must understand the mechanisms and burial conditions for these reactions. In this section we discuss some of the reactions that occur most commonly in the rocks that we studied and in those studied in recent literature.
Precipitation and dissolution of carbonate cements in sandstones play major roles in the destruction and enhancement of reservoir porosity and permeability. These processes are controlled primarily by the relative concentrations of carbon dioxide and hydrogen ions in formation waters. The following simplified equations for calcite illustrate these relationships:
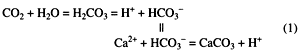
Because seawater is saturated with respect to calcium carbonate (aragonite or calcite), early calcite cements often are observed in sandstones deposited in open marine environments [e.g., Bjørlykke (1988) and Brenner (1989)]. These cements can be derived directly from saturated seawater as it is heated or from partial dissolution and reprecipitation of carbonate grains (e.g., aragonite skeletal fragments). Taylor (1990) suggests that calcite cements in Miocene sandstones of the northern Gulf of Mexico were derived from degradation of basic volcanic rock fragments or previously deposited marine carbonates. These conclusions were based on the oxygen, carbon, and strontium isotopic signatures of the calcite cements. On the other hand, ankerite cements in the same sandstones have isotopic signatures that indicate that they were precipitated from waters expelled from feldspars and clays in shales undergoing diagenesis at great depth (thermobaric regime) (Taylor, 1990). If cements such as these are exposed to acidic waters from terrestrial sources (e.g., from decaying organic materials) in the meteoric regime, then the reactions in Eqs. (1) can be reversed, resulting in carbonate dissolution and the creation of secondary porosity.
Carbonate precipitation and dissolution reactions continue to be important in the compactional regime. Organic matter appears to be a major source for H+ and CO2 in many sedimentary systems. Acids derived from the maturation of kerogens appear to be important in many diagenetic reactions involving siliceous and carbonate grains and cements [e.g., Bjørlykke (1988), Crossey et al. (1986), Curtis (1978), Moncure et al. (1984), and Surdam et al. (1984, 1989)]. For example, Surdam et al. (1984) suggested that carbonate cements are generated under low-temperature conditions through the bacterial destruction of short-chain carboxylic acids. This reaction occurs under low SO42- concentrations (e.g., <25 mg/L), which increase PCO2 and buffer pH. A similar carbonate-cement-producing condition is created at high temperatures through thermal degradation of short-chain carboxylic acids (Surdam et al., 1984).
In petroliferous basins there is available a large volume of organic material whose reaction products may be adequate to account for secondary porosity created by carbonate cement dissolution [e.g., Crossey et al. (1986), Curtis (1978), Irwin et al. (1977), and Wood (1986)]. Estimates made by Bjørlykke (1988) based on many sources indicate that the production of CO2 varies between 0.5% and 15% by weight of various kerogens. Assuming that each mole of CO2 has the potential to dissolve 1 mol of calcite, kerogen-derived CO2 alone could account for significant volumes of calcite dissolution. However, Bjørlykke (1988) points out that this CO2 is released over a long period of time during maturation of kerogens and that much of it is neutralized by other reactions, leaving only a small fraction available for calcite dissolution in sandstones.
Milliken and Land (1991) suggest that acid generated through organic maturation may be inadequate to account for secondary porosity and other petrographic characteristics of the Oligocene Frio formation along the Texas Gulf Coast. They suggest that most of the porosity-modifying acids were derived from a reverse weathering reaction involving illitization of smectite in surrounding mudrocks. These acids dissolved carbonate grains in the mudrocks, leading to the movement of H+, dissolved Ca2+, and CO2 to the sandstones. Reactive feldspars in the sandstones reacted with these fluids, creating an effective buffer and allowing authigenic carbonate to form and maintain a low PCO2. Secondary porosity in the Frio formed when the supply of reactive feldspar was depleted and the mudrock-derived H+ could dissolve carbonate in sandstones (Milliken and Land, 1991).
Other researchers have also suggested that, in the lower (geopressured) part of the compactional regime and in the thermobaric regime, inorganic reactions involving clay minerals and carbonates are sources for H+ ions and CO2. For example, Hutcheon et al. (1980) suggested the following reaction for temperatures between 180°C and 250°C:
Al2Si2O5(OH)4 + 5MgCa(CaCO3)2 + SiO2 + 2H2O → Mg-chlorite + 5CaCO3 + 5CO2. (2)
Thus there are many sources for carbonate cements and the acids required to dissolve them. To properly constrain models involving carbonate precipitation and dissolution, we must be able to evaluate the potential mechanisms, such as those mentioned earlier. Each acid source has characteristic types of reactions on which volumetric and kinetic constraints must be established.
Sources of authigenic quartz include (1) dissolution of quartz grain margins by pressure solution, (2) silica dissolved by undersaturated waters circulating around quartz grains, (3) silica released during shale compaction, and (4) silica liberated during alteration of feldspars, volcanic rock fragments, micas, and clay minerals (Bjørlykke, 1988; Leder and Park, 1986). The last two sources are important in the compaction and thermobaric regimes. For example, hydrolysis of 1 cm3 of potassium feldspar yields 0.46 cm3 of kaolinite and 0.43 cm3 of quartz according to the following reaction (Leder and Park, 1986):
2KAlSi3O8 + 2H+ + H2O Al2Si2O5(OH)4 + 4SiO2 (aq) + 2K+. (3)
Similar reactions can be written for the formation of illite (see later discussion).
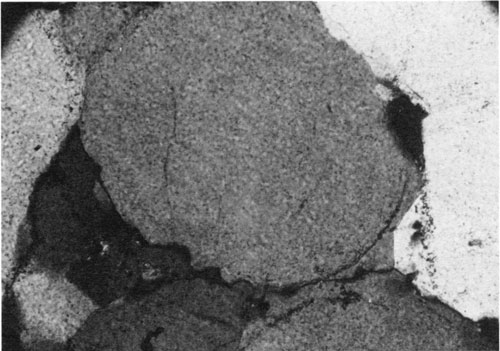
Experimental work and petrographic observations made by Weyl (1959) indicate that pressure solution is an important source of silica for quartz cementation. Weyl's hypothesis is that material dissolved by compressive stresses inside grain-to-grain contacts diffuses through an intergranular solution into adjacent pores, where it precipitates (fig. 3). In chemical environments in which the formation waters are saturated with respect to silica, the quartz is precipitated as overgrowths on nearby detrital quartz grains. If the waters are undersaturated, then the silica can be moved in solution and precipitated elsewhere. This may account for the early quartz cementation observed in sandstones that do not show evidence of aluminosilicate dissolution or pressure solution.
Figure 3--Photomicrograph showing pressure solution effects on quartz grains. Sutured grain boundaries (e.g., lower left boundary of central grain) with adjacent quartz overgrowths (lower right). Cambrian sandstone, Wasatch Range, Utah. Cross-polarized light; width of field = 0.6 mm.
The solubility of quartz under varying temperature-pressure conditions is an important aspect of diagenetic modeling of systems that have potential for silica cementation or dissolution. Thorough treatments of this subject have been presented by, among others, Siever (1962) and Fournier and Potter (1982), and these works should be consulted when setting modeling constraints.
One of the most commonly observed diagenetic alterations of quartz is replacement by calcite (fig. 4). In some cases quartz dissolution and calcite precipitation are two separate events, reflecting two temporally distinct fluid regimes. However, in many cases the two processes must have been nearly simultaneous to prevent the collapse of the remnant quartz grains after partial dissolution. For the same reason quartz grain and calcite cement must also be in constant contact with each other during replacement. Weyl (1959) suggested that many dissolution and precipitation reactions occurred within a thin film, of the order of a micron or less in thickness, that allowed relatively rapid movement of dissolved silica away from the reacting surfaces while bringing solutes for the precipitating calcite to the surfaces from the open pore system. Pettijohn et al. (1987) suggested that SiO2 dissolves from the quartz surface into an undersaturated solution and hydrates to H2SiO4. In dilute solutions at earth surface conditions with pH values less than 9, the following reaction and equilibrium constant (Keq) could result:
SiO2 + 2H2O → H4SiO4; Keq = 10-4. (4)
Figure 4--Photomicrograph showing quartz grains (dark) that are embayed and partially replaced by calcite (light). Middle Pennsylvanian Cherokee Group sandstone, Forest City basin, northeastern Kansas. Cross-polarized light; width of field = 0.6 mm.
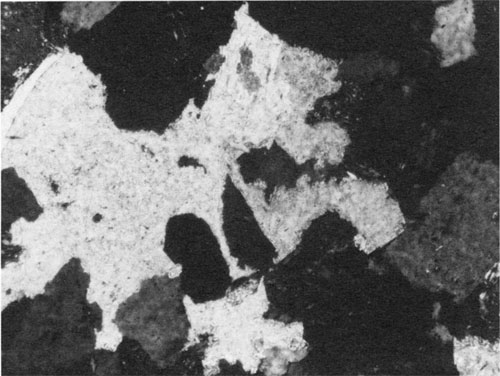
In order for dissolution to proceed, the concentration of H4SiO4 must be higher in the film than in the pore fluid, resulting in the diffusion of H4SiO4 out into the pore system. On the other hand, the concentrations of Ca2+ and HCO3- must be higher in the pore system fluids, allowing these two ions to diffuse into the film. The product of the activities of these two ions yields a Keq greater than 10-8.35 because calcite precipitates according to the equation
Ca2+ + 2HCO3- = CaCO3 + H+ + HCO3- (5)
H+ must also diffuse out of the film into the open pore system (Pettijohn et al., 1987, pp. 335-336). The problem that many researchers have with this mechanism is the rate of diffusion through this thin film, which controls the kinetics of the replacement (dissolution and precipitation) reactions. Ultra thin films, such as those described by Weyl (1959) and Pettijohn et at. (1987), must transport a large volume of materials in a relatively limited period of geologic time. Without constraints on possible diffusion rates, we cannot predict the extent of quartz replacement by calcite or any similar replacement process. This remains a problem for modeling a system in which calcite replacement of quartz has been observed.
In our studies of Pennsylvanian sandstones in the North American midcontinent and of Cretaceous sandstones in the North American Western Interior, feldspar alteration and dissolution play major roles in sandstone composition and reservoir properties. One phenomenon, partial albitization of plagioclase feldspar, has little volumetric significance, but it provides insight into the history of feldspar alteration in some of the Pennsylvanian sandstones studied. Figure 5 shows a hollow albite grain. X-ray microanalysis shows that the shell and shelflike structures within the grain are pure albite (NaAlSi3O8). Unaltered plagioclase grains in other Pennsylvanian sandstones in the region contain calcium and appear to be predominantly oligoclase. A reaction for albitization of oligoclase involves the addition of sodium and silica and the release of calcium and alumina ions and water (Boles, 1982):
0.8NaAlSi3O3 - 0.2CaAl2Si3O8 + 0.203H4SiO4 + 0.201Na+ + 0.796H+ → 1.001NaAlSi3O8 + Ca2+ + 0.199Al3+ + 0.804H2O. (5)
Figure 5--SEM photomicrograph of albitized plagioclase grain shell and shelves. Upper Pennsylvanian sandstone, Forest City basin, Iowa.
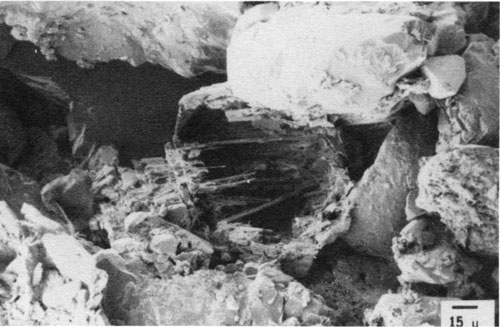
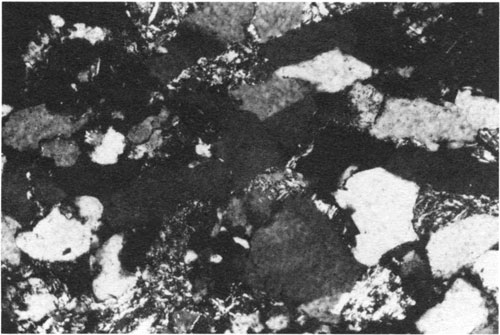
Figure 6--Collapsed feldspar grain partially altered to clay (center of photomicrograph). Partially sericitized rock fragments squeezed between quartz grains are scattered throughout. Middle Pennsylvanian Cherokee Group, Forest City basin, northeastern Kansas. Cross-polarized light; width of field = 0.6 mm.
Alteration must have penetrated grains along cleavage traces, accounting for the shelflike projections in fig. 5. However, before complete albitization the process was terminated. At a later time acidic conditions caused differential dissolution of calcium-bearing feldspar. Boles (1984) observed a similar phenomenon in the Miocene Stevens sandstone in the San Joaquin Valley of California. He suggested the following equation for the dissolution of plagioclase and calcite:
CaCO3 + Na0.7Ca0.3Al1.3Si2.7O8 + 3.45H2O + 2.3H+ → 1.3Ca2+ + HCO3- + 0.65Al2Si2O5(OH)4 + 0.7Na+ + 1.4H4SiO4- (6)
In both the Miocene and Pennsylvanian examples the dynamics of this reaction was such that only the less stable oligoclase was dissolved, leaving the pure albite shell and shelves as remnants. Other plagioclase grains that either had less competent albite shells or were not partially albitized before dissolution collapsed under lithostatic pressure (fig. 6).
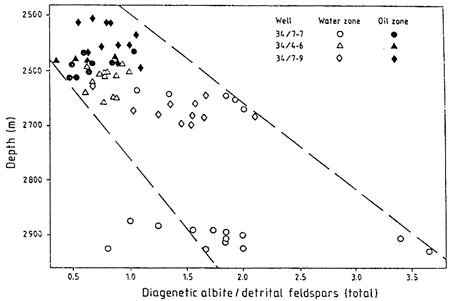
While studying Triassic sandstone reservoirs in the Norwegian North Sea, Morad et al. (1990) observed that detrital plagioclase with compositions between An12 and An28 had been partly to completely albitized, whereas detrital albite (An2-An11) and potassium feldspar grains were fresh and relatively unaltered. By relating sample depths, oil and water zones, and degree of feldspar alteration, Morad et al. concluded that albitization was strongly restricted by oil emplacement (fig. 7). Albitization was strongest in poorly cemented sandstones and in more deeply buried sandstones, indicating that this process is related to residence time of sandstones in a zone of albitization and increases in burial temperature.
Figure 7--Distribution of diagenetic albite in water-filled reservoirs and oil-bearing reservoirs. From Morad et al. (1990, fig. 10).
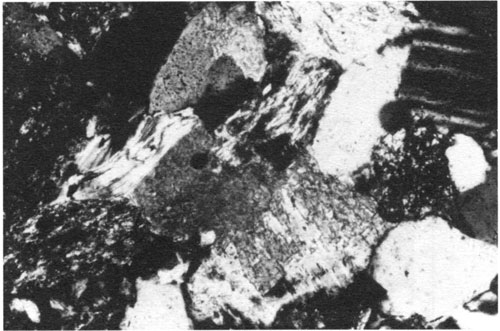
The chemical equations used by Morad et al. (1990) yield kaolinite, quartz, and calcite as by-products of albitization. Their SEM analyses showed that albitization started along cleavage traces and pits. It is conceivable that calcite replacement of plagioclase feldspar, a relationship observed in many sandstones [e.g., Brenner (1989) and Dogan (1984)], took place in a similar manner (fig. 8). Perhaps as pH in pore fluids rises, calcite replaces plagioclase in place of albite through a mechanism similar to the one described for calcite replacement of quartz.
Figure 8--Photomicrograph showing calcite (dark gray) replacing feldspar (lighter gray) along cleavage traces. Middle Pennsylvanian Cherokee Group sandstone, Cherokee shelf, eastern Kansas. Cross-polarized light; width of field = 0.6 mm.
Clay mineral diagenesis In addition to grain dissolution and alteration processes, silica and carbonate cements are also generated during diagenesis of clay minerals, especially in the compactional and thermobaric regimes [e.g., Bjørlykke (1988), Galloway (1984), Molenaar (1989), Moncure et al. (1984), and Surdam et al. (1989)].
As expanded-lattice clay minerals, such as montmorillonite, are subjected to higher temperatures during burial, they alter to illite and kaolinite. The following two equations illustrate these reactions (Siever, 1962):
Kaolinite formation:
1.2Na0.66Al3.34Mg0.66Si8O20(OH)4nH2O + 3.2H+ → 2Al2Si2O5(OH)4 + 5.6SiO2(aq) + 0.79Na+ + 0.79Mg2+ + 1.2nH2O (7)
Illite formation:
1.78Na0.66Al3.34Mg0.66Si8O20(OH)4nH2O + 3.8H+ + 1.33K+ + 4.82Mg2+ → K1.33Al5.95Mg6Si6.61O20(OH)4 + 7.63SiO2(aq) + 1.18Na+ + (1.78n + 3.46)H2). (8)
This clay conversion necessitates the release of iron from octahedral sites (Boles and Franks, 1979) and a resulting electron transfer. Oxidation of organic matter in shales provides an ideal electron donor (Crossey et al., 1986). Crossey et al. (1986) observed that there is a coincidence between the ordering of mixed-layer smectite-illite and the peak abundance of organic acids at approximately 28% expandability. Thus organic acids and iron compounds released at the same point in the burial history of a siliciclastic package would be expected to produce diagenetic alterations indicative of this process, such as the dissolution of grains or earlier precipitated cements and the precipitation of iron-bearing cements.
This relationship was observed in a sandstone-over-shale core from 10,221 ft (3,115.4 m) to 10,226.5 ft (3,117.0 m) in the Oligocene Frio formation of south Texas, where Moncure et al. (1984) observed that the sandstone had zoned reservoir qualities that Moncure and colleagues related to clay conversions and organic acid generation. Siliceous grains were dissolved and iron-rich ankerite and chlorite cements were precipitated in the zone nearest the sandstone-shale contact. This zone is characterized by enhanced porosity because grain dissolution created more porosity than authigenic cements destroyed. This zone is overlain by a zone of kaolinite enrichment, possibly representing aluminosilicates resulting from dissolution of reactive detrital grains (fig. 9). However, in evaluating this mechanism, we should keep in mind the reverse weathering mechanism proposed by Milliken and Land (1991), discussed earlier.
Figure 9--Porosity-diagenetic zones in a Frio core showing distributions of porosity, quartz overgrowths, chlorite, and kaolinite. Modified from Moncure et al. (1984) and Crossey et al. (1986).
Clay conversion is but one example of the effects of clay mineral diagenesis on sandstone diagenesis. In addition, we must keep in mind that movements of aqueous mineral species with waters of expulsion provide important sources of authigenic cements within the compactional regime.
An important constraint on any diagenetic model is the assumptions made about the original mineral composition of sandstones. When we have petrographic samples to study, we tend to use these samples to help set this constraint. However, how closely does the present-day composition of a sandstone represent the composition of the original sand? We can took at this problem from the perspective of petrologists trying to interpret provenance from sandstone mineralogy.
During the past 20 years, numerous researchers have used petrographic techniques to interpret the provenance of sandstones [e.g., Dickinson (1985, 1988), Dickinson and Suczek (1979), Dickinson and Valloni (1980), Graham et al. (1984), Ingersoll (1983), Mack et al. (1981), and Thornburg and Kulm (1987)]. Most of these researchers report varying affects of diagenesis on sandstone mineralogy that fog some provenance interpretations. In some cases these diagenetic effects take place early or are preburial, occurring during weathering, erosion, and transportation of the siliciclastic sediment. These effects may be related directly to climatic influences [e.g., Johnsson (1990)]. However, some petrologists working primarily in petroliferous areas (e.g., the northern shelf of the Gulf of Mexico) claim that postburial diagenesis has completely destroyed provenance information [e.g., Milliken (1988) and McBride (1985)].
This is an important issue not only because we tend to use petrographic data to set constraints on original sand mineralogy but also because (1) provenance interpretations may be critical in reconstructing paleotectonic settings needed to set reasonable constraints on several other model parameters and (2) grain preservation, ranging from no destruction to complete dissolution of nonquartz grains, reflects the intensity of diagenetic processes and thus the kinetics of dissolution and replacement reactions [e.g., Shanmugam (1985)]. All these factors must be evaluated when constructing any diagenetic model.
Many (but not all) of the successful provenance studies were done primarily in nonpetroliferous areas, whereas those researchers who observed "diagenetic" quartzarenites worked primarily in petroliferous areas and therefore studied rocks that may have been penetrated by waters enriched in organic acids or in H' derived in some other manner. These differences illustrate the importance of relating fluid chemistry and the hydrologic regime to the geohistory of the basin when considering constraints for quantitative diagenetic models.
The 1980's was a decade of proliferating sedimentary geochemical research and gave us some of the first significant attempts to model diagenetic systems. Because of the large amount of interest and available data, the northern margin of the Gulf of Mexico has served as a natural laboratory for developing and testing diagenetic models. For example, in analyzing the Oligocene-Miocene Frio and Catahoula formations of the Texas Gulf coastal plain, Galloway (1984) suggested that diagenetic models can be constructed by studying existing hydrologic settings. He suggested that generalized hydrologic histories be constructed using diagenetic features that are observed in depositional sequences. In the Frio and Catahoula study he showed that hydrologic regimes and the fluid mixing zones between those regimes coexist with associations of diagenetic features.
Harrison (1990) reviewed three commonly used modeling methods: empirical porosity decline curves, facies and burial history diagenetic models, and theoretical fluid-rock interaction models. The first two methods have been used for many years, and the third has recently gained momentum through the use of computers to simplify operations and carry out required repetitive calculations. Harrison (1990) showed that a theoretical reaction-path approach provides detailed models of fluid-rock interactions. An important element in building this kind of model is an understanding of the paleohydrologic evolution of the sedimentary basin being modeled [e.g., Galloway (1984), Harrison (1990), and Siever (1986)]. Several of the eight key geologic parameters discussed earlier must be considered in reconstructing paleohydrologic conditions, including temperature, pressure, subsidence history, sedimentary characteristics, and fluid chemistry.
In this article the term "diagenetic model" is used in a general sense for all attempts to reconstruct or predict diagenetic effects during the burial history of a rock package. This includes theoretical models, empirically derived function models, and hybrid models that contain elements of both theoretical and empirically derived models. Most of these models have been designed to predict the porosity and permeability of potential petroleum-bearing sandstones. Although definitive quantitative models have not yet been published, a few papers have reported results of semiquantitative diagenetic models as applied in frontier petroleum exploration areas. An approach used by some researchers is to construct a model that is based on all known geologic parameters and that is widely applicable. For example, Scherer (1987) constructed an empirically derived model for sandstone porosity prediction based on 428 cases from which he calculated function coefficients for the relationship between geologic parameters and porosity. Despite the use of a large, varied database, Scherer's model is valid only for sandstones older than 3 Ma with little or no cement, no leaching, depth of burial in excess of 500 m (1,600 ft), and little or no shear stresses (Scherer, 1987). Therefore this model may be applicable in a variety of basins, but it may not be useful for many sandstones within any one basin.
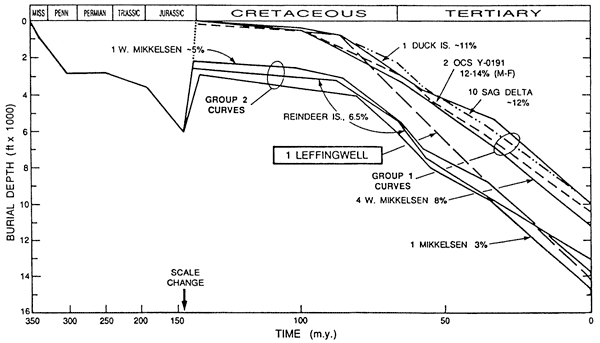
Another approach to quantitative diagenetic modeling combines elements of theoretical and empirically derived models by relating geologic parameters for the sediments in one particular basin or type of basin to the burial history of that basin or basin type. Bloch et al. (1990) provide us with on sandstone porosity. From Bloch et al. (1990). an example of how this type of modeling technique is used to predict reservoir properties in the sandstones of the Mississippian Kekiktuk Conglomerate on the north slope of Alaska. They used sedimentologic and petrographic analyses of outcrop and core samples and geohistory analysis using formation tops interpreted from seismic data to establish a calibration data base in areas of well-data control. The calibration involved a systematic evaluation of the relationship between reservoir quality and data base parameters (fig. 10). The calibration data set was then used to estimate porosity in a wildcat well outside their control area. The resulting model was used to correctly predict the sedimentary facies that the wildcat well penetrated, but, more significantly, Bloch et al. (1990) were able to predict potential reservoir porosities within 2 porosity percent. The key step in their study was relating the kinetics of silica cementation, the process most responsible for porosity reduction in their data base, to the geohistory of the area.
Figure 10--Geohistory diagram of the Kekiktuk Conglomerate showing influence of burial history
The modeling approaches just discussed could be used to make predictions about the reservoir qualities of sandstones. However, in most exploration situations the constraints mandated by Scherer's (1987) model cannot be anticipated. The approach used by Bloch et al. (1990) establishes a calibration data base that can be applied in a four-dimensional sense by iteratively calculating the simultaneous influences of several processes on a sedimentary system during successive time increments. Once this is done successfully in a basin, then perhaps some modification of the approach used by Scherer (1987) can be used to analyze other basins. Regardless of the approach used, we believe that useful, reliable quantitative diagenetic models will result from integrative studies involving all geologic parameters and the geohistories of the basins involved.
To build quantitative diagenetic models to make predictions in unexplored areas, we must be able to set reasonable constraints on parameters that we cannot measure directly. As the burial histories (geohistories) and diagenetic styles become more complicated, the success of models will increasingly hinge on setting correct constraints on geologic parameters. The calibration data base approach of Bloch et al. (1990) is one way in which model constraints can be set and used on depositional systems and basins from which the data bases were generated.
Provenance studies and paleotectonic reconstructions are used to predict the characteristics and distributions of siliciclastic sediments [e.g., Dickinson (1988)]. Provenance tectonics is the prime factor controlling siliciclastic source and the primary nature of sandstone grains. However, grain characteristics are also affected by weathering, transportation, and diagenesis. These processes tend to reduce chemically and physically less resistant grains, and, as their intensities or rates increase, sandstones become more quartzose.
One approach to constraining sedimentary parameters is to study petrographic characteristics of samples from areas of control (e.g., outcrops and drill cores and cuttings) and to use these results along with stratigraphic and sedimentologic characteristics (also derived from outcrops and wells plus seismic lines where available) to (1) reconstruct sediment dispersal paths between source areas and depositional basins and (2) delineate depositional systems within these basins.
We illustrate this approach with a study of stratigraphic variations in the petrographic characteristics of Pennsylvanian sandstones in the Forest City basin in Iowa. We found that sandstones from the Lower Pennsylvanian to the lower part of the Middle Pennsylvanian in well core samples were derived from older sedimentary sources and were deposited in fluvial systems under wet climatic conditions. These sandstones are associated with coals, show extensive rooting in some horizons, and probably were often exposed to acid weathering conditions [as suggested, for example, by Cecil et al. (1985)]. Kaolinite cements dominate, with minor authigenic quartz and late-stage siderite nodular cement engulfing both framework grains and early formed kaolinite. Detrital apatite is conspicuously missing from these sandstones, although it is present in both marine-deposited units and the Upper Pennsylvanian units. Garnet grains are extensively etched, and both the absence of apatite and the relatively shallow burial depths attained [around 1 km (0.6 mi) maximum; Bunker et al. (1988)] suggest that etching occurred under acid weathering conditions. Skeletal feldspar grains and feldspar showing extensive coalescing columnar etch pits (Berner and Holdren, 1979) also suggest extensive weathering (fig. 5). Dissolution of unstable grains probably took place in an open meteoric flow system as a result of multiple incursions of fluid over moderately long periods of time. Stacked, incised sandstone channel geometries observed in many Lower and Middle Pennsylvanian sandstone-rich complexes support this interpretation.
Upper Desmoinesian and Virgilian units (Middle to Late Pennsylvanian) were deposited during periods of gradually increasing marine influence within eustatically controlled cyclic sedimentary packages (Heckel, 1977, 1983, 1984). Some of these units, although probably exposed to some meteoric waters, were covered quickly by relatively impermeable marine shales and limestones and possibly became saturated by modified marine fluids whose pH's were buffered by carbonate equilibria. Because Late Pennsylvanian climates were drier, the amount of acidic meteoric water would have been reduced, leading to preservation of detrital apatite, reduced etching of garnet, and better preservation of feldspars. Feldspars in the Virgilian strata are much fresher, showing an increase in well-preserved grains and an overall reduction in the intensity of etching solutions.
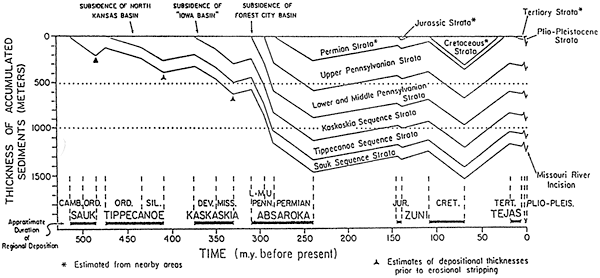
Using sedimentologic and tectonic reconstructions and geohistory diagrams (e.g., fig. 11) derived from the petrographic and sedimentologic data [from Bunker et al. (1988)], we can set constraints for most of the parameters required to construct a quantitative forward diagenetic model.
Figure 11--Geohistory diagram of the Iowa portion of the Forest City basin near the Nebraska-Kansas border. From Bunker et al. (1988).
Almost all diagenetic reactions take place at temperatures less than 200°C, and therefore rarely go to completion except after long periods of time (Siever, 1986). Predicting the amount of authigenic material that is formed in a sediment requires the use of reaction kinetic equations that relate time and temperature to reaction yields. The Arrhenius equation, which relates reaction rate changes to temperature increases, is commonly used (Siever, 1986):
δ(ln k) / δT = Ea / RT2, (9)
where Ea is the activity energy, k is the reaction rate constant, R is the gas constant per mole, and T is temperature in kelvins. An integrated form of this equation has been used when Ea is not temperature dependent:
k = A exp[-Ea / RT], (10)
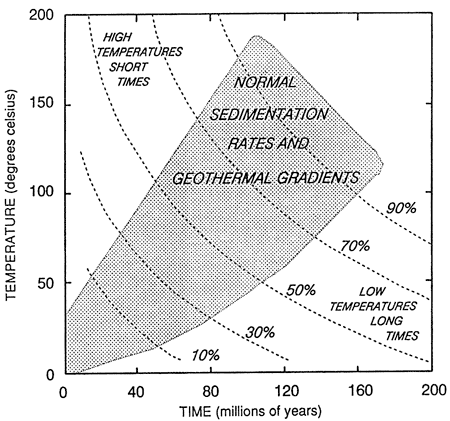
where A is an integration constant (or frequency factor). This equation may be applicable in the laboratory but not under varying diagenetic regimes where reaction mechanisms differ with temperature changes. Siever (1986) suggests that the general form of this equation is useful and that the integrated equation may be useful for making approximations in specific cases. Siever (1983) constructed a time-temperature diagram to show generalized yield curves for a hypothetical slow reaction (fig. 12). Diagrams such as this one can be used to help set constraints on authigenic mineral yields for specific temperature and time domains.
Figure 12--Generalized yield curve for a hypothetical slow reaction. Modified from Siever (1983).
Experiments by Walter (1986) were used along with previously delineated equilibrium constraints to evaluate carbonate dissolution and precipitation rates during diagenesis. One result of this study was that aragonite precipitation may be favored in high-sulfate low-phosphate solutions, whereas magnesium-calcite precipitation may be favored in low-sulfate high-phosphate solutions. This happens because SO42- selectively inhibits calcite precipitation and PO43- selectively inhibits aragonite precipitation. Experiments such as those carried out on carbonate sediments by Walter (1986) show that parameters can be constrained for modeling purposes through the use of carefully controlled kinetics experiments.
The Jurassic of the North Sea exemplifies the major role that diagenetic reaction kinetics play in determining the quality of sandstones in petroleum reservoirs. For instance, the Middle Jurassic Fangst Group has porosities over 30% and permeabilities in excess of 1,000 md despite containing most of the diagenetic features that one would predict in a subarkose to lithic subarkose deposited in a fault-bounded basin. Harris (1989) observed dissolution of feldspar (mostly plagioclase), which accounts for 2-4% of rock volume, minor amounts of quartz cement (about 2%), alteration of micas to kaolinite, formation of pore-filling kaolinite, localized discontinuous layers of polkilotopic calcite cement, siderite replacement of mud and pore fillings, and pyrite pore fillings. When the porosities and permeabilities in these sandstones are compared with the diagenetic styles and primary sedimentary characteristics, they show a direct relationship with depositional setting and not with diagenetic effects (Harris, 1989). It appears that diagenetic processes were not extensive enough to significantly overprint original sedimentary characteristics. The reason for this must be that the reaction kinetics in this setting, for the amounts of time that the sediments were in meteoric and compactional domains, were not rapid enough to either destroy large portions of effective pores or create more than 4% secondary porosity. Similar rocks under different time and temperature conditions and resulting reaction kinetics could have either little effective primary porosity preserved (e.g., Leder and Park, 1986) or secondary porosities that are much greater than preserved primary porosities (e.g., Frio sandstone of south Texas; Land, 1984).
The chemistries of fluids that pass through sediments at or near the earth's surface can be estimated in a general way by using modern sedimentary systems as partial analogues. Table 1 lists the compositions of some natural waters. The molality values of these waters could be used to set constraints on meteoric waters responsible for some early diagenetic processes. We cannot use surface analogies to set realistic constraints when considering a wedge of sediments subsiding through various hydrologic and thermal regimes. Formation waters leave their fingerprints in diagenetic cements in the form of trace element composition, distinctive isotope ratios, and trapped fluid inclusions. If these characteristics can be related to formation fluid type, then they can be used to set constraints on chemical parameters in a quantitative diagenetic model.
Table 1--Composition of some natural surface waters (in mmol/L). From Curtis (1990, table 1).
| Species | Seawater | River Water | Rain Water |
|---|---|---|---|
| Cl- | 535.2 | 0.22 | 0.11 |
| Na+ | 456.2 | 0.27 | 0.09 |
| Mg2+ | 54.2 | 0.17 | 0.01 |
| SO2- | 27.6 | 0.12 | 0.00 |
| K+ | 9.7 | 0.06 | 0.01 |
| Ca2+ | 10.0 | 0.38 | 0.002 |
| HCO3- | 2.3 | 0.95 | 0.002 |
| SiO2 | 0.1 | 0.21 | <<0.001 |
| Al | <<O.1 | <<0.01 | <<0.001 |
Carbon, oxygen, and sulfur isotopes have been used to relate carbonate, sulfate, and sulfide cements to the temperature and sources of formation waters since the 1970's [e.g., Irwin et al. (1977)]. More recently, these types of data have been integrated with strontium isotopes to trace the movement of fluids through a sandstone reservoir. Sullivan et al. (1990) showed that a linear correlation exists between δ13C and 87Sr/86Sr in diagenetic dolomite and ankerite in the Permian Rotliegend Sandstone beneath the North Sea. They used this relationship to trace the movement of formation waters through the Rotliegend relative to organic maturation, dissolution of feldspars, and other diagenetic events. In studying Miocene sandstones in the northern Gulf of Mexico, Taylor (1990) used oxygen, carbon, and strontium isotopic signatures of carbonate cements to trace each cement to its most likely source. In the process of making these interpretations, Taylor established temperature, depth, and source constraints.
The stable oxygen isotope ratios of authigenic minerals, in particular, offer the potential to evaluate both fluid sources and temperatures of precipitation. The calcite paleotemperature equation, as given by Anderson and Arthur (1983) and Hudson and Anderson (1989), is
T°C = 16.0 - 4.14(δC - δW) + 0. 13(δC - δW)2, (11)
where δC = δ18O of calcite relative to PDB, and δW = δ18O of water relative to SMOW. Other paleotemperature equations for sedimentary minerals of interest have been given by Friedman and O'Neil (1977).
Because temperatures of precipitation cannot be calculated without knowing the oxygen isotope composition of both the mineral and the pore fluid, independent evidence on fluid compositions or temperatures must be obtained to exploit paleotemperature equations. Assumptions concerning the isotopic compositions of ancient seawaters or derivative pore fluids must be viewed with skepticism because of uncertainties regarding the thermal and isotopic history of the Phanerozoic ocean system (Hudson and Anderson, 1989). Girard et al. (1989) and Lee et al. (1989) used radiometric dating and δ18O values of authigenic silicate minerals in sandstones along with temperature estimates from burial history curves to calculate pore fluid δ18O values and to interpret sources of ancient formation fluids. Likewise, Girard et al. (1988) and Ludvigson and Spry (1990) integrated fluid inclusion filling temperatures and δ18O measurements in secondary minerals to interpret large-scale fluid transport in clastic sedimentary basins.
The approaches outlined here and others that we do not have space to discuss can be used in quantitative modeling to set chemical, temporal, and distribution constraints on the movements of meteoric, compactional, and thermobaric fluids that precipitate cements as they move through a siliciclastic sedimentary system.
Early attempts at quantitative diagenetic modeling have used simplifications of geologic parameters to reduce the number of unknowns [e.g., Bethke (1985), Bredehoeft et al. (1988), and Harrison (1990)]. Great strides have been made toward understanding diagenetic reactions and the conditions that instigate and terminate these reactions. However, we must still develop models that combine mass balance and reaction kinetics before we can build truly quantitative diagenetic models. The activities of ionic species within both open and closed systems still need quantification. For example, the availability of some ions for precipitation in cements is unclear because of competition from prospective host crystal lattices and from shifting stabilities caused by such factors as changing PCO2, Eh, or pH conditions. These variations not only are prevalent in open systems but may also be important in closed systems as diagenetic reactions proceed.
Quantification of variables that distinguish meteoric or down-flow cements from deep burial or up-flow cements may become possible as more isotopic, trace element, and fluid inclusion data become available. Studies such as the thermochronologic analysis of the Angola basin that was carried out by Walgenwitz et al. (1990) add to our data base of fluid inclusion temperatures tied to isotopic age dates. Comparing data such as these with those collected from similar settings could be used to establish quantitative limits for both down-flow and up-flow water compositions. Similar comparisons could be made using data collected from carbonate and other cements in siliciclastic sandstones.
With the use of modern settings as partial analogues and the use of stratigraphic, sedimentologic, and petrographic data, we can reconstruct sandstone architectures and draw inferences about sediment distribution and depositional pattern, original porosity and permeability characteristics, and original fluid chemistry. Geohistory reconstructions can be used to develop chronologies of geochemical environments for each sandstone body within a time-temperature-basin setting framework. These geochemical and hydrogeologic reconstructions can then be related to each paleodepositional setting. As more insight is gained into the relationships between tectonic settings, depositional settings, and diagenetic styles, diagenetic modeling will be increasingly integrated into the more quantitative geohistory analyses and computerized backstripping methods that currently are employed in basin analysis. The volumes of data and complexities of relationships between data sets will increasingly require computer-based data-handling systems.
Diagenetic modeling is an integrative process because each variable interacts with other variables in a complex manner. As a result, single aspects of the total process are difficult to isolate. Artificial intelligence in the form of expert systems is ideally suited for this type of modeling because of its ability to integrate a large amount of data from many sources and to calculate the simultaneous influences of multiple processes on a sedimentary system during successive time increments. Once models are generated for sandstones from various paleodepositional settings and cyclic positions, they can be used to predict reservoir parameters, such as porosity and permeability. A complete basin analysis designed to produce models for prediction of reservoir distributions and qualities must include diagenetic reactions because alteration of rock properties can vary from nil to complete recrystallization, removal, or replacement during the history of a basin. These alterations affect not only the porosity and permeability of a potential reservoir but also the seismic characteristics of the strata (e.g., densities and acoustic velocities) used in lithofacies reconstructions from seismic data.
Diagenetic predictions are an important part of any basin model that is designed to evaluate petroleum accumulation potentials. However, to make quantitative predictions, we need quantitative models based on knowledge of diagenetic processes. To date, attempts at diagenetic modeling have been largely qualitative. Early attempts to model diagenesis quantitatively have been based on simplifications of geologic processes and parameters. Perhaps an intermediate phase in the evolution of truly quantitative diagenetic models involves setting constraints for the geologic parameters that control diagenetic styles. Integrated petrographic studies that yield calibration data sets can be used to help set constraints on the parameters used in quantitative forward diagenetic models.
As a quantitative model becomes more precise, its applicability becomes limited to the type of basin from which the parameter constraints were determined. However, as our understanding of diagenetic processes, rates, and controls improves, models will be constructed on two levels. First, general relationships will be combined with parameter values for particular basin types. Second, precise quantitative models will be constructed by combining these general relationships with constraints derived from particular paleotectonic and paleoclimatic settings. In this way, quantitative models will be constructed to make predictions for basins within corresponding paleotectonic and paleoclimatic settings.
Aspects of the studies reported here have been supported by the Iowa Science Foundation under grant ISF-89-09; the Iowa Department of Natural Resources, Geological Survey Bureau; and the Graduate School of the University of Iowa. We thank William M. Benzel, Wendy J. Harrison, William C. Ross, and Sharon Stonecipher for making suggestions that were critical in our revisions of this paper.
Anderson, T. F., and Arthur, M. A., 1983, Stable isotopes of oxygen and carbon and their application to sedimentologic and environmental problems; in, Stable Isotopes in Sedimentary Geology, Arthur, M. A., ed.: Society of Economic Paleontologists and Mineralogists, Short Course Notes 10, p. 1- 151
Berner, R. A., and Holdren, G. R., 1979, Mechanisms of feldspar weathering II--observations of feldspar from soils: Geochimica et Cosmochimica Acta, v. 43, p. 1, 173-1,186
Bethke, C. M., 1985, A numerical model of compaction-driven ground-water flow and heat transfer and its application to paleohydrology of intercratonic sedimentary basins: Journal of Geophysical Research, v. 90, p. 6,817-6,828
Bjørlykke, K., 1988, Sandstone diagenesis in relation to preservation, destruction, and creation of porosity; in, Diagenesis, Chilingarian, C. V., and Wolf, K. H., eds.: Developments in Sedimentology 41, Elsevier, Amsterdam, 591 p.
Bloch, S., McGowen, J. H., Duncan, J. R., and Brizzolara, D. W., 1990, Porosity prediction, prior to drilling, in sandstones of the Kekiktuk Formation (Mississippian), North Slope of Alaska: American Association of Petroleum Geologists, Bulletin, v. 74, p. 1,371-1,385
Boles, J. R., 1982, Active albitization of plagioclase, Gulf Coast Tertiary: American Journal of Science, v. 282, p. 165-180
Boles, J. R., 1984, Secondary porosity reactions in the Stevens Sandstone, San Joaquin Valley, California; in, Clastic Diagenesis, McDonald, D. A., and Surdam, R. C., eds.: American Association of Petroleum Geologists, Memoir 37, p. 217-224
Boles, J. R., and Franks, S. G., 1979, Clay diagenesis in Wilcox sandstones of southwest Texas--implications of smectite diagenesis on sandstone cementation: Journal of Sedimentary Petrology, v. 49, p. 55-70
Bredehoeft, J. D., Djevanshir, R. D., and Belitz, K. R., 1988, Lateral fluid flow in a compacting sand-shale sequence--South Caspian basin: American Association of Petroleum Geologists, Bulletin, v.72,p.416-424
Brenner, R. L., 1989, Stratigraphy, petrology, and paleogeography of the upper part of the Cherokee Group (Middle Pennsylvanian), eastern Kansas, northeastern Oklahoma: Kansas Geological Survey, Geology Series 3, 70 p. [available online]
Bunker, B. J., Witzke, B. J., Watney, W. L., and Ludvigson, G. A., 1988, Phanerozoic history of the central midcontinent, United States; in, The Geology of North America, v. D-2, Sedimentary Cover--North American Craton, Sloss, L. L., ed.: Geological Society of America, p. 243-260
Cecil, C. B., Stanton, R. W., Neuzil, S. G., Dulong, F. T., Ruppert, L. F., and Pierce, B. S., 1985, Paleoclimate controls, Late Paleozoic sedimentation, and peat formation in the central Appalachian basin (USA): International Journal of Coal Geology, v. 5, p. 195-230
Cross, T. A., ed., 1990, Quantitative dynamic stratigraphy: Prentice Hall, Englewood Cliffs, New Jersey, 625 p.
Crossey, L. J., Surdam, R. C., and Lahann, R., 1986, Application of organic/inorganic diagenesis to porosity prediction; in, Roles of Organic Matter in Sediment Diagenesis, Gautier, D. L., ed.: Society of Economic Paleontologists and Mineralogists, Special Publication 38, p. 147-155
Curtis, C. D., 1978, Possible links between sandstone diagenesis and depth-related geochemical reactions occurring in enclosing mudstones: Journal of the Geological Society of London, v. 135, p. 107-118
Curtis, C. D., 1990, Aspects of climatic influence on the clay mineralogy and geochemistry of soils, palaeosols, and clastic sedimentary rocks: Journal of the Geological Society of London, v. 147, p. 351-357
Dickinson, W. R., 1985, Interpreting provenance relations from detrital modes of sandstones; in, Provenance of Arenites, Zuffa, G. G., ed.: Reidel Publishing Co., Boston, p. 333-361
Dickinson, W. R., 1988, Provenance and sediment dispersal in relation to paleotectonics and paleogeography of sedimentary basins; in, New Perspectives in Basin Analysis, Kleinspehn, K. L., and Paola, C., eds.: Springer-Verlag, New York, p. 3-25
Dickinson, W. R., and Suczek, C. A., 1979, Plate tectonics and sandstone compositions: American Association of Petroleum Geologists, Bulletin, v. 63, p. 2,164-2,182
Dickinson, W. R., and Valloni, R., 1980, Plate settings and provenance of sands in modern ocean basins: Geology, v. 8, p. 82-86
Dogan, A. U., 1984, Stratigraphy, petrology, depositional and postdepositional histories and their effects upon reservoir properties of the Parkman Formation of the Mesaverde Group, Powder River Basin, Wyoming: Ph.D. dissertation, University of Iowa, Iowa City, 265 p.
Fournier, R. O., and Potter, R. W., 1982, An equation correlating the solubility of quartz in water from 25° to 900°C at pressures up to 10,000 bars: Geochimica et Cosmochimica Acta, v. 46, p. 1,969-1,973
Friedman, I., and O'Neil, J. R., 1977, Compilation of stable isotope fractionation factors of geochemical interest: US Geological Survey, Professional Paper 440-KK, p. KK1-KK12 [available online]
Galloway, W. E., 1984, Hydrogeologic regimes of sandstone diagenesis; in, Clastic Diagenesis, McDonald, D. A., and Surdam, R. C., eds.: American Association of Petroleum Geologists, Memoir 37, p. 3-13
Girard, J. P., Savin, S. M., and Aronson, J. L., 1989, Diagenesis of Lower Cretaceous arkoses of the Angola margin--petrology, K/ Ar dating, and 180/160 evidence: Journal of Sedimentary Petrology,v.59,p.519-538
Girard, J. P., Savin, S. M., Aronson, J. L., and Walgenwitz, F., 1988, Diagenesis of the Lower Cretaceous arkoses of Angola-petrology, K/Ar, 18O/16O, and fluid inclusions: Geological Society of America, Abstracts with Programs, v. 20, no. 5, p. 345
Graham, S. A.,McCloy, C., Hitzman, M., Ward, R., and Turner, R., 1984, Basin evolution during change from convergent to transform continental margin in central California: American Association of Petroleum Geologists, Bulletin, v. 68, p. 233-248
Guidish, T. M., Kendall, C., Lerche, I., Toth, D. J., and Yarzab, R. F., 1985, Basin evaluation using burial history calculations--an overview: American Association of Petroleum Geologists, Bulletin, v. 69, p. 92-105
Harris, N. B., 1989, Reservoir geology of Fangst Group (Middle Jurassic), Heidrun Field, offshore mid-Norway: American Association of Petroleum Geologists, Bulletin, v. 73, p. 1,4151,435.
Harrison, W. J., 1990, Modeling fluid/rock interactions in sedimentary basins; in, Quantitative Dynamic Stratigraphy, Cross, T. A., ed.: Prentice Hall, Englewood Cliffs, New Jersey, p. 195-231
Heckel, P. H., 1977, Origin of phosphatic black shale facies in Pennsylvanian cyclothems of the midcontinent North America: American Association of Petroleum Geologists, Bulletin, v. 61, p. 1,045-1,068
Heckel, P. H., 1983, Diagenetic model for carbonate rocks in midcontinent Pennsylvanian eustatic cyclothems: Journal of Sedimentary Petrology, v. 53, p. 733-759
Heckel, P. H., 1984, Changing concepts of midcontinent Pennsylvanian cyclothems, North America: Ninth International Carboniferous Congress, Compte Rendu, v. 3, pt. 3, Southern Illinois University, Carbondale, p. 535-553
Hudson, J. D., and Anderson, T. F., 1989, Ocean temperature and isotopic compositions through time: Transactions of the Royal Society of Edinburgh, Earth Sciences, v. 80, p. 183-192
Hutcheon, I., Oldershaw, A., and Ghent, E. D., 1980, Diagenesis of Cretaceous sandstones of the Kootenay Formation at Elk Valley (southeastern British Columbia) and Mt. Allen (southwestem Alberta): Geochimica et Cosmochimica Acta, v. 44, p. 1,4251,435
Ingersoll, R. V., 1983, Petrofacies and provenance of late Mesozoic forearc basin, northern and central California: American Association of Petroleum Geologists, Bulletin, v. 67, p. 1, 125-1,142
Irwin, H., Curtis, C. D., and Coleman, M. L., 1977, Isotopic evidence for source of diagenetic carbonates formed during burial of organic-rich sediments: Nature, v. 269, p. 209-213
Isbell, J. L., 1985, Palynological and sedimentological basin analysis of Lower to Middle Pennsylvanian deposits in rock Island County, Illinois, and Muscatine County, Iowa: Master's thesis, Northern Illinois University, DeKalb, 331 p.
Johnsson, M. J., 1990, Tectonic versus chemical-weathering controls on the composition of fluvial sands in tropical environments: Sedimentology, v. 37, p. 713-726
Land, L. S., 1984, Frio sandstone diagenesis, Texas Gulf Coast--a regional study; in, Clastic Diagenesis, McDonald, D. A., and Surdam, R. C., eds.: American Association of Petroleum Geologists, Memoir 37, p. 47-62
Leder, F., and Park, W. C., 1986, Porosity reduction in sandstone by quartz overgrowth: American Association of Petroleum Geologists, Bulletin, v. 70, p. 1,713-1,728
Lee, M., Aronson, J. L., and Savin, S. M., 1989, Timing and conditions of Permian Rotliegend Sandstone diagenesis, southern North Sea: American Association of Petroleum Geologists, Bulletin, v. 73, p. 195-215
Ludvigson, G. A., and Spry, P. G., 1990, Tectonic and paleohydrologic significance of carbonate veinlets in the Keweenawan sedimentary rocks of the Amoco M. G. Eischeid #1 drill hole; in, The Amoco M. G. Eischeid #1 Deep Petroleum Test, Carroll County, Iowa, Anderson, R. R., ed.: Iowa Department of Natural Resources, Geological Survey Bureau, Preliminary Investigations, Special Report Series 2, p. 153-167
Mack, G. H., James, W. C., and Thomas, W. A., 1981, Orogenic provenance of Mississippian sandstones associated with southern Appalachian-Ouachita orogen: American Association of Petroleum Geologists, Bulletin, v. 65, p. 1,444-1,456
McBride, E. F., 1985, Diagenetic processes that affect provenance determination in sandstone; in, Provenance of Arenites, Zuffa, G. G., ed.: Reidel Publishing Co., Boston, p. 95-113
McBride, E. F., 1987, Diagenesis of the Maxon Sandstone (Early Cretaceous), Marathon region, Texas--a diagenetic quartzarenite: Journal of Sedimentary Petrology, v. 57, p. 98-107
Milliken, K. L., 1988, Loss of provenance information through subsurface diagenesis in Plio-Pleistocene sandstones, northern Gulf of Mexico: Journal of Sedimentary Petrology, v. 58, p. 9921,002
Milliken, K. L., and Land, L. S., 1991, Reverse weathering, the carbonate-feldspar system, and porosity evolution during burial of sandstones (abs.). American Association of Petroleum Geologists, Bulletin, v. 75, p. 636
Molenaar, N., 1989, Eogenetic and telogenetic cementation of sandstones: Geologica Ultraiectina, no. 58, 126 p.
Moncure, G. K., Lahann, R. W., and Siebert, R. M., 1984, Origin of secondary porosity and cement distribution in a sandstone/shale sequence from the Frio Formation (Oligocene); in, Clastic Diagenesis, McDonald, D. A., and Surdam, R. C., eds.: American Association of Petroleum Geologists, Memoir 37, p. 15 I175
Morad, S., Bergan, M., Knarud, R., and Nystuen, J. P., 1990, Albitization of detrital plagioclase in Triassic reservoir sandstones from the Snorre Field, Norwegian North Sea: Journal of Sedimentary Petrology, v. 60, p. 411-425
Pettijohn, F. G., Potter, P. E., and Siever, R., 1987, Sand and sandstones, 2d ed.: Springer-Verlag, New York, 553 p.
Scherer, M., 1987, Parameters influencing porosity in sandstone--a model for sandstone porosity prediction: American Association of Petroleum Geologists, Bulletin, v. 71, p. 485-491
Sclater, J. G., and Christie, P. A. F., 1980, Continental stretching--an explanation of the post-mid-Cretaceous subsidence of the central North Sea basin: Journal of Geophysical Research, v. 85, p. 3,711-3,739
Shanmugam, G., 1985, Types of porosity in sandstones and their significance in interpreting provenance; in, Provenance of Arenites, Zuffa, G. G., ed.: Reidel Publishing Co., Dordrecht, Holland, p. 115-137
Siever, R., 1962, Silica solubility, 0°-200°C, and the diagenesis of siliceous sediments: Journal of Geology, v. 70, p. 127-150
Siever, R., 1983, Burial history and diagenetic reaction kinetics: American Association of Petroleum Geologists, Bulletin, v. 67, p. 684-689
Siever, R., 1986, Burial diagenesis of sandstones; in, Studies in Diagenesis, Mumpton, F. A., ed.: US Geological Survey, Bulletin 1,578, p. 237-248
Sullivan, M. D., Haszeldine, R. S., and Fallick, A. E., 1990, Linear coupling of carbon and strontium isotopes in Rotliegend Sandstone, North Sea--evidence for cross-formation fluid flow: Geology, v. 18, p. 1,215-1,218
Surdam, R. C., Boese, S. W., and Crossey, L. J., 1984, The chemistry of secondary porosity; in, Clastic Diagenesis, McDonald, D. A., and Surdam, R. C., eds.: American Association of Petroleum Geologists, Memoir 37, p. 127-150
Surdam, R. C., Crossey, L. J., Hagan, E. S., and Heasler, H. P., 1989, Organic-inorganic interactions and sandstone diagenesis: American Association of Petroleum Geologists, Bulletin, v. 73, p. 1-23
Taylor, T. R., 1990, The influence of calcite dissolution on reservoir porosity in Miocene sandstones, Picaroon Field, offshore Texas Gulf Coast: Journal of Sedimentary Petrology, v. 60, p. 322-334
Thornburg, T. M., and Kulm, L. D., 1987, Sedimentation in the Chile trench--petrofacies and provenance: Journal of Sedimentary Petrology, v. 57, p. 55-74
Thorne, J. A., and Watts, A. B., 1989, Quantitative analysis of North Sea subsidence: American Association of Petroleum Geologists, Bulletin, v. 73, p. 88-116
Van de Graaff, W. J. E., and Ealey, P. J., 1989, Geological modeling for simulation studies: American Association of Petroleum Geologists, Bulletin, v. 73, p. 1,436-1,444
Van Hinte, J. E., 1978, Geohistory analysis--application of micropaleontology in exploration geology: American Association of Petroleum Geologists, Bulletin, v. 62, p. 201-222
Walgenwitz, F., Paget, M., Meyer, A., Muluski, H., and Moine, P., 1990, Thermo-chronological approach to reservoir diagenesis in the offshore Angola Basin, a fluid inclusion 40Ar-39Ar and K-Ar investigation: American Association of Petroleum Geologists, Bulletin, v. 74, p. 547-563
Walter, L. M., 1986, Relative efficiency of carbonate dissolution and precipitation during diagenesis-a progress report on the role of solution chemistry; in, Roles of Organic Matter in Sediment Diagenesis, Gautier, D. L., ed.: Society of Economic Paleontologists and Mineralogists, Special Publication 3 8, p. 1-11
Weyl, P. K., 1959, Pressure solution and the force of crystallization-a phenomenological theory: Journal of Geophysical Research, v. 64, p. 2,001-2,025
Witzke, B. J., 1990, Paleoclimatic constraints for Palaeozoic palaeolatitudes of Laurentia and Euramerica; in, Palaeozoic Palaeogeography and Biogeography, McKerrow, W. S., and Scotese, C. R., eds.: Geological Society of America, Memoir 12, p. 57-73
Wood, J. R., 1986, Thermal mass transfer in systems containing quartz and calcite; in, Roles of Organic Matter in Sediment Diagenesis, Gautier, D. L., ed.: Society of Economic Paleontologists and Mineralogists, Special Publication 38, p. 169-180
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version March 16, 2010. Original publication date 1991.
URL=http://www.kgs.ku.edu/Publications/Bulletins/233/Brenner/index.html