Kansas Geological Survey, Bulletin 223, part 2, originally published in 1981
Originally published in 1981 as Kansas Geological Survey Bulletin 223, part 2. This is, in general, the original text as published. The information has not been updated.
Exploration for mineral resources is important to the State of Kansas as sell as to the United States as a whole. The Midcontinent region, because of its relatively thick sedimentary cover, is one of the least explored areas, with the exception of exploration for oil, gas, and coal.
This part of the study dealing with the stratiform or bedded copper occurrences in south-central Kansas focuses on the kinds of ore minerals present in the rocks. Electron-microprobe and ore-microscopy studies clearly show that they occur in a definite and predictable sequence, with the more copper-rich minerals always occurring nearer to the present-day surface.
This type of information is invaluable because it allows us to formulate ideas and present possible models for mineralization. In turn, models are very important in making decisions regarding future or additional exploration programs. Based upon our evaluation of the materials examined, we think it unlikely that economic concentrations of copper will be found in the Permian strata in Kansas. However, the possibility exists that large quantities of copper may be present in older rocks.
This study is the first report documenting the zonation of the copper mineralization and the occurrence of the mineral anilite in stratiform copper deposits in the Midcontinent.
Subeconomic concentrations of copper sulfide minerals are irregularly distributed throughout the Lower Permian Wellington Formation and the Ninnescah Shale of south-central Kansas. The host rocks consist principally of gray shales and siltstones with lesser argillaceous dolomites and limestones in which the sulfides occur principally as replacements of earlier diagenetic pyrite or as irregular stringers.
Although two spatially distinct and separate sulfide assemblages are identified on the basis of drill hole information, the sequence of ore minerals indicates that a descending copper-rich solution percolating downward was slowly depleted of its copper content
In the northern portion of the area, pyrite is replaced by chalcopyrite and bornite at shallower depths, while in the southern part of the area pyrite is replaced by "chalcocite-like" minerals. It is suggested that pH and oxygen fugacity are controlling factors responsible for the different assemblages.
Electron microprobe studies indicate that no true chalcocite (Cu2S) is present; instead two phases having the compositions Cu1.78±0.04S (similar to anilite) and Cu1.91±0.03S (similar to djurleite) were identified. The mineral anilite has not been reported before associated with the Midcontinent copper occurrences.
We propose that the mafic rocks associated with the Central North American Rift System were the source rocks of the copper that was subsequently introduced into the Permian basin and adsorbed onto the sediments. Oxidizing ground waters were responsible for redissolving the copper and secondarily concentrating it in a reduced environment.
Redbed-associated copper deposits are stratibound copper sulfide occurrences that exist as layers or disseminations in organic-rich shales and carbonate beds and as replacements of organic material in continental sandstones. Although the ore occurs in dark-colored, reduced sediments, the host sequence is generally characterized by substantial amounts of red, oxidized clastics. In Kansas, Oklahoma, and Texas, copper sulfide mineralization associated with shale rather than sandstone is of greater economic significance (Johnson, 1976).
Ore-grade material was mined from the Creta copper-bearing shale in southwestern Oklahoma from October 1965 to February 1975, when a drop in the price of copper forced the mine to close. The Creta copper shale has an average copper content of 2.3 volume percent, and chalcocite (Cu2S) is the mineral that was mined. At the nearby Magnum copper prospect, malachite [CuCO3Cu(OH)2] is the most abundant copper mineral that has been identified, although chalcocite may be the dominant ore mineral in the subsurface. The Magnum site averages one percent copper by volume.
In Texas, three prospects have been identified in the San Angelo Formation. In each case the ore mineral is chalcocite, which weathers to malachite and azurite [Cu3(CO3)2(OH)2] near the surface (Johnson, 1974). The mineralization here occurs in shales and mudstones associated with gypsum and dolomite much as in the Creta and Magnum deposits of Oklahoma. Copper content of the Texas prospects averages between one and two volume percent.
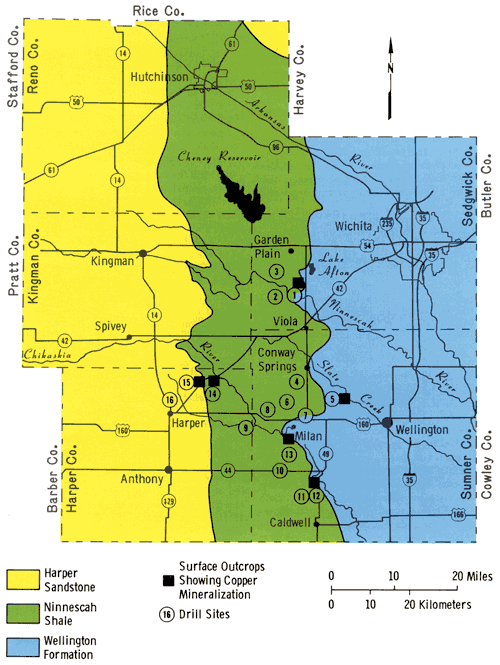
To better understand the nature and distribution of the copper mineralization in the Midcontinent region, 16 holes were drilled in Harper, Sedgwick, and Sumner counties in south-central Kansas during the summer of 1975. The location of the sites was based upon the known distribution of copper mineralization in outcrop in this study area. The drill site locations are shown in Figure 1.
Figure 1--Generalized map showing the distribution of the major Permian rocks units, the location of surface outcrops containing copper mineralization, and the location of the drill holes.

Local stratigraphy and host rock lithology of the copper sulfide occurrences in Kansas are discussed in Part 1 of this bulletin. Part 2 describes the copper sulfide minerals and places them in paragenetic sequence. It also examines the relationship of the host rock and its depositional history to the copper sulfide mineralization.
Norton (1939) noted the presence of copper sulfides in the Permian redbeds of Kansas. He stated that chalcopyrite (CuFeS2) and malachite are the ore minerals, but did not provide further details concerning the mineralization.
Hill (1967) examined copper mineralization in the greenish-gray shales of the Ninnescah Shale, in the Runnymede Sandstone Member of the Ninnescah Shale, and in the Milan Limestone Member that marks the top of the underlying Wellington Formation. Traverses were made from Kingman County eastward into Sedgwick County and from Harper County eastward into Sumner County. Traverse samples were analyzed by x-ray spectroscopy for copper; the copper-bearing mineral in each sample was found to be malachite. Among 411 samples collected along the traverses, the highest concentrations of copper were detected in the Runnymede Sandstone Member.
Waugh and Brady (1976) examined the Ninnescah Shale and the Milan Limestone Member of the Wellington Formation. More than 400 samples were obtained from 120 localities in Harper, Kingman, Sedgwick, and Sumner counties. The highest concentrations of copper they found were in the Runnymede Sandstone Member of the Ninnescah Shale in Harper County. Waugh and Brady (1976) identified the copper-bearing minerals as malachite in association with a small amount of azurite.
Long and Angino (1976) investigated copper mineralization in the Milan Limestone Member and reported chalcocite, covellite (CuS), and malachite. The highest copper enrichment found by atomic absorption spectrophotometry was about 1.5 percent, and no zinc enrichment was detected in any of the outcrops sampled. Long and Angino considered the copper sulfide mineralization to be late diagenetic, low temperature, and sabkha-related.
The present study commenced with the selection of samples from the cores that had previously been split in half and examined with a binocular microscope. Fifty-two paired thin sections and polished sections and 11 additional thin sections were prepared.
Following detailed examination of the polished and thin sections, seven samples were prepared for analysis with the Indiana University ETEC automated electron microprobe. The Magic IV correction program, prepared by J. W. Colby of Bell Telephone Laboratories (Allentown, Pennsylvania) and modified for use on the PDP-11 computer by L. Finger of the Carnegie Institution (Geophysical Laboratory), was used for making ZAF (atomic number, absorbance, and fluorescence) corrections of data from the microprobe.
Various dolomite and shale units had previously been analyzed by atomic absorption spectrophotometer for copper, lead, and zinc at the Kansas Geological Survey.
The host rocks for the copper sulfide mineralization in Kansas are the shale, siltstone, and carbonate of the Wellington Formation and the Ninnescah Shale, which are discussed in some detail in Part 1 of this bulletin. The copper-bearing minerals occur principally as the result of solution (in vugs) and replacement processes. The most common replacement process is that of the megaspore Triletes by sulfides. Mineralization also occurs in vertical stringers that may be related to the activity of burrowing organisms, or dessication features.
Veinlets are commonly associated with copper sulfide mineralization in the thin carbonate units. Large euhedral carbonate grains rim the veinlets and these in turn are surrounded and partially replaced by younger, large euhedral quartz crystals. A second generation of very fine grained quartz is the most abundant mineral phase in the veinlets, and this phase corrodes the surface of the older, larger grains of euhedral quartz. Figure 2 illustrates copper sulfide grains in micritic dolomite, and Figure 3 shows large euhedral quartz grains and younger, finer grained quartz in a veinlet.
Figure 2--Copper sulfide grains (black) in micritic dolomite. Drill hole 10, 44.0 m deep, polarized transmitted light. The field width is 1.8 mm.
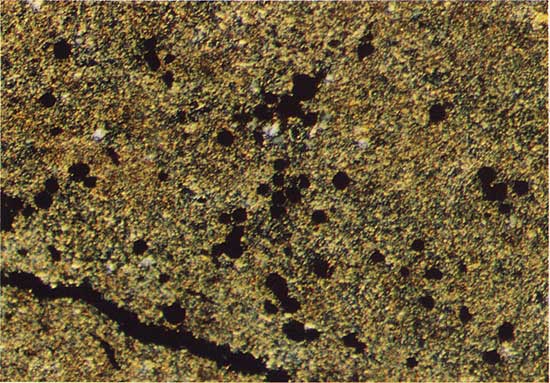
Figure 3--Copper sulfide grains (black), large euhedral quartz grains (light gray), and younger fine-grained quartz in a veinlet. Drill hole 7, 16.3 m deep, plain transmitted light. The field width is 1.8 mm.
Because redbeds in the upper part of the Wellington Formation are discontinuous, the entire formation can be considered as one thick greenish-gray shale as far as the migration of any ore-forming fluid is concerned. According to Swineford (1955), fully 80 percent of the Wellington is shale and silty shale, but only 15 percent is red shale.
Ripple marks and mud cracks visible in surface outcrop and core in the study area suggest a shallow marine (lagoonal) environment of deposition. Both mechanical and biological agents have extensively reworked many of the beds that contain copper sulfides and are responsible for creating the veinlets that have since been filled with the carbonate so often associated with copper sulfide grains.
Covellite (CuS) occurs as a near-surface alteration product of primary copper sulfides in drill hole 7. Within 7 m of the surface, azurite [Cu3(CO3)2(OH)2] surrounds and has replaced grains of "chalcocite" (a chalcocite-like phase described below), which have partially altered to covellite (Fig. 4). The azurite is in turn rimmed by malachite [CuCO3CU(OH)2], which penetrates the azurite along cracks (Fig. 5).
Figure 4--Chalcocite-like phase (light blue) partially replaced by covellite (dark blue) and surrounded by azurite (light gray). Drill hole 7, 6.0 m deep, plain reflected light. The field width is 3.6 mm.

Figure 5--Malachite (green) penetrating and replacing azurite (brown). Covellite (orange) is partially replaced by the azurite. Drill hole 7, 6.0 m deep, polarized reflected light. The field width is 1.8 mm.
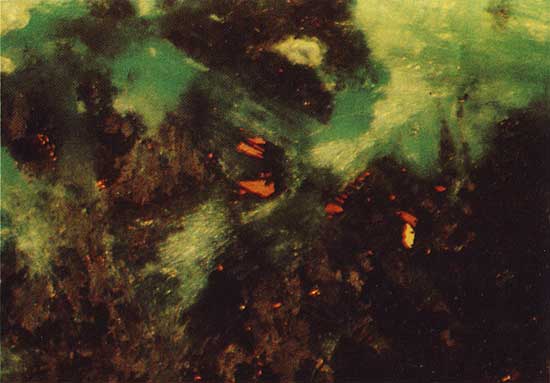
The chalcocite-like phase is present in the southern part of the study area where it replaces pyritized spores (Fig. 6). A mineral identified as chalcocite is reported to replace pyritized spores in the redbed copper ore deposit at Creta in southwestern Oklahoma (Hagni and Gann, 1976). In Kansas the chalcocite-like phase also occurs as vertical stringers in greenish-gray shale, in structures that may have been formed by burrowing organisms (Fig. 7).
Figure 6--Chalcocite-like phase (blue-green) replacing pyritized spores. Drill hole 3, 18.5 m deep, plain reflected light. The field width is 1.8 mm.
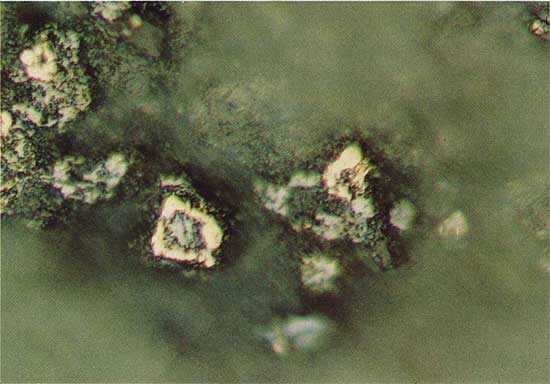
Figure 7--Chalcocite-like phase (light blue-green) and covellite (darker blue-green) in a vertical stringer (burrow?). Drill hole 13, 26.5 m deep, plain reflected light. The field width is 1.8 mm.
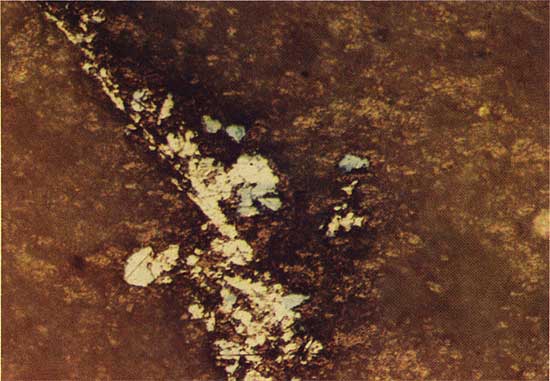
At drill location 15 the chalcocite-like phase is clearly related to the replacement of spores. Core from drill holes 10 and 11 contain the chalcocite-like phase in vertical stringers, and in drill hole 13 the sulfide grains have characteristics of both spore-related and vertical stringer mineralization. The depth at which the chalcocite-like phase first appears increased from 26.52 m at drill location 13 to 32.67 m at drill location 15.
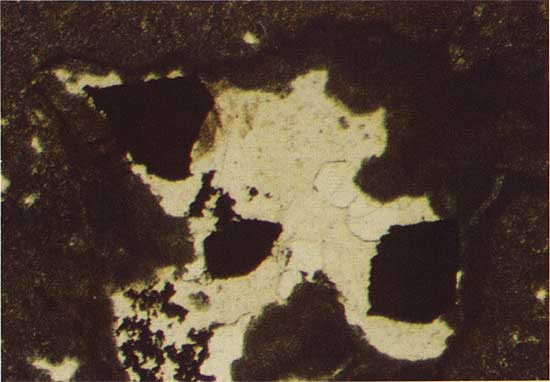
A transition between ore mineralogies at the southern and northern parts of the study area occurs at drill location 7. Grains of the chalcocite-like phase are found 6 m below the surface; and grains of chalcopyrite (CuFeS2), partially replaced by bornite (Cu5FeS4), occur 16.95 m beneath the surface in the same core.
The chalcocite-like phase replaces bornite at a depth of 22.86 m in drill hole 2, and bornite is clearly a replacement of chalcopyrite in drill holes 2 and 3 (Fig. 8). Root-like patches of bornite intrude into the chalcopyrite and progressively replace more and more chalcopyrite in grains nearer the surface. Bornite is first identified at a depth of 15.91 m in drill hole 3, and at 22.81 m in drill hole 2 farther to the south. The chalcopyrite occurs as an irregular growth without clear replacement texture around pyrite framboids (Fig. 9), and as replacement of pyrite. The depth at which chalcopyrite is first identified increases from 18.41 m in drill hole 3 to 25.45 m in drill hole 2.
Figure 8--Bornite (light brown) replacing chalcopyrite (yellow). Drill hole 3, 15.9 m deep, plain reflected light. The field width is 1.8 mm.
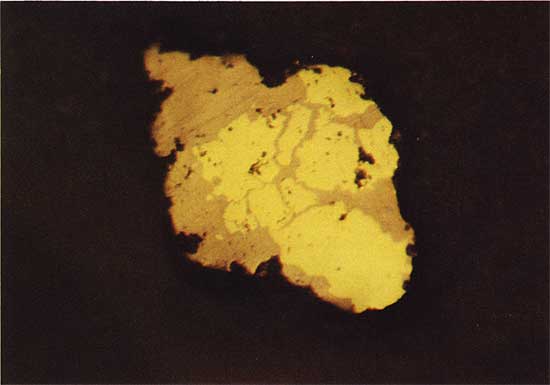
Figure 9--Chalcopyrite (yellow) as an irregular growth around pyrite (white). Drill hole 3, 18.5 m deep, plain reflected light. The field width is 1.8 mm.
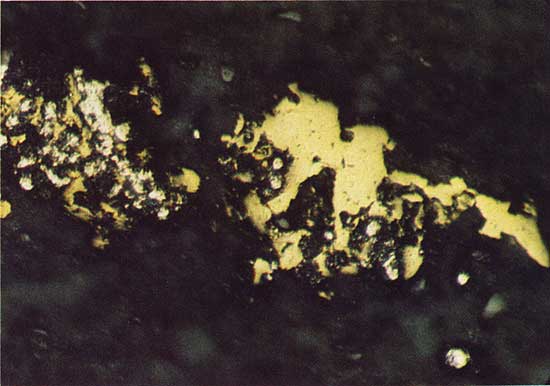
Under reflected light, a dark-blue isotropic material is observed as a replacement of bornite grains in the cored material (Fig. 10). Digenite (Cu1.8S), a copper sulfide that has these optical properties, is reported from the Prewitt copper shale of Oklahoma as an intergrowth with chalcocite (Cu2S) (Hagni and Gann, 1976). Morimoto and Gyobu (1971) have determined that digenite is stable in the copper-iron-sulfur field and contains about one percent iron. Electron microprobe analysis showed the mineral to be digenite (Table 1).
Figure 10--Digenite (blue-green) replacing bornite (light brown). Drill hole 2, 22.9 m deep, plain reflected light. The field width is 0.68 mm.
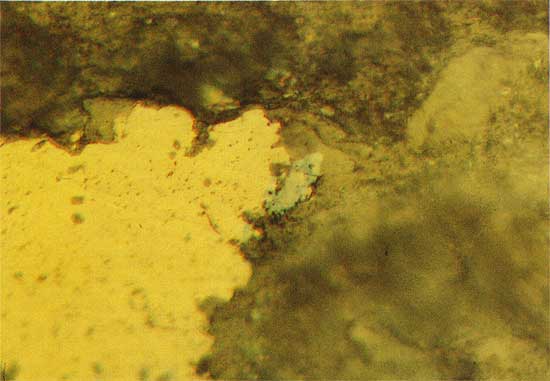
Table 1--Results of electron microprobe analysis for chalcopyrite, bornite, and digenite from drill hole 2 (weight percent).
| Specimen | %Cu | %Fe | %S | Total % | Formula | |
|---|---|---|---|---|---|---|
| Chalcopyrite in chalcopyrite grains, 22.86 m depth |
1a | 34.93 | 30.85 | 35.31 | 101.09 | Cu1.01Fe1.01S2 |
| 1b | 33.45 | 30.46 | 34.58 | 98.48 | Cu1.01Fe1.05S2 | |
| 1c | 34.64 | 31.08 | 34.92 | 100.64 | Cu1.00Fe1.02S2 | |
| 1d | 33.86 | 30.18 | 35.17 | 99.21 | Cu.97Fe.98S2 | |
| 1e | 35.11 | 30.06 | 34.95 | 100.12 | Cu1.01Fe.99S2 | |
| 1f | 35.79 | 30.92 | 35.19 | 101.90 | Cu1.02Fe1.01S2 | |
| 1g | 35.06 | 30.64 | 35.65 | 101.35 | Cu1.00Fe1.00S2 | |
| 1h | 33.76 | 30.49 | 33.79 | 98.04 | Cu1.00Fe1.04S2 | |
| 1i | 34.73 | 31.15 | 34.52 | 100.40 | Cu1.01Fe1.03S2 | |
| 1j | 34.09 | 30.30 | 34.95 | 99.34 | Cu.98Fe.99S2 | |
| Average | 34.54 | 30.61 | 34.90 | 100.06 | Cu1.00Fe1.01S2 | |
| Chalcopyrite in chalcopyrite grains, 22.77 m depth |
2a | 35.13 | 29.69 | 34.87 | 99.69 | Cu1.02Fe98S2 |
| 2b | 34.92 | 29.09 | 35.57 | 99.58 | Cu.99Fe.94S2 | |
| 2c | 35.15 | 29.79 | 35.43 | 100.37 | Cu1.00Fe.96S2 | |
| 2d | 35.04 | 30.24 | 34.54 | 99.82 | Cu1.02Fe1.00S2 | |
| 2c | 34.38 | 28.96 | 35.05 | 98.39 | Cu.99Fe.95S2 | |
| 2f | 35.03 | 29.30 | 34.59 | 98.92 | Cu1.02Fe.97S2 | |
| Average | 34.94 | 29.51 | 35.01 | 99.46 | Cu1.01Fe.97S2 | |
| Chalcopyrite in chalcopyrite grains, 22.77 m depth |
3a | 35.04 | 30.24 | 34.54 | 99.32 | Cu1.02Fe1.00S2 |
| 3b | 34.38 | 28.96 | 35.05 | 98.39 | Cu.99Fe.95S2 | |
| 3c | 35.03 | 29.30 | 34.59 | 98.92 | Cu1.02Fe.97S2 | |
| 3d | 35.27 | 29.35 | 35.38 | 97.08 | Cu1.01Fe.95S2 | |
| Average | 34.93 | 29.46 | 34.89 | 98.55 | Cu1.01Fe.97S2 | |
| Bornite in chalcopyrite-bornite grains, 22.77 m depth |
4a | 61.03 | 10.26 | 25.02 | 96.31 | Cu4.92Fe.94S4 |
| 4b | 61.17 | 10.73 | 25.73 | 97.63 | Cu4.80Fe.96S4 | |
| 4c | 61.01 | 10.46 | 25.54 | 97.01 | Cu4.82Fe.94S4 | |
| 4d | 62.20 | 10.32 | 25.51 | 98.03 | Cu4.92Fe.93S4 | |
| 4e | 61.32 | 10.54 | 25.38 | 97.24 | Cu4.87Fe.95S4 | |
| 4f | 60.86 | 10.71 | 25.47 | 97.04 | Cu4.82Fe.96S4 | |
| 4g | 61.24 | 10.47 | 25.34 | 97.05 | Cu4.87Fe.95S4 | |
| Average | 61.26 | 10.50 | 25.43 | 97.19 | Cu4.86Fe.95S4 | |
| Digenite in a bornite grain, 22.77 m depth |
5a | 72.68 | 1.70 | 21.76 | 96.14 | Cu1.68Fe.048S |
| 5b | 70.92 | 2.65 | 23.29 | 96.86 | Cu1.53Fe.065S | |
| 5c | 71.44 | 1.88 | 22.36 | 95.68 | Cu1.62Fe.048S | |
| 5d | 70.65 | 2.91 | 22.70 | 96.26 | Cu1.57Fe.074S | |
| Average | 71.42 | 2.28 | 22.53 | 96.23 | Cu1.60Fe.059S |
Pyrite (FeS2) is the ore mineral found at the greatest depth in shale and siltstone in most of the cores. Pyrite is the first sulfide to form and occurs mostly as a diagenetic replacement of the cases of the megaspore Triletes (Fig. 11). Most authors agree that in this type of deposit pyrite is syngenetic. Wherever spores are in contact with quartz-filled veinlets of carbonate (dolomite?), pyritization is complete. These veinlets occur in greenish-gray shales and in dolomite beds. Mineralized spores also occur in fine-grained carbonate units that lack veinlets, while dense shales that lack veinlets contain unreplaced spores (Fig. 12). No pyrite or chalcopyrite has been observed in any of the carbonate units.
Figure 11--Megaspores replaced by pyrite, from an outcrop in the SE sec. 20, T. 31 S., R. 2 W., plain reflected light. The field width is 1.8 mm.
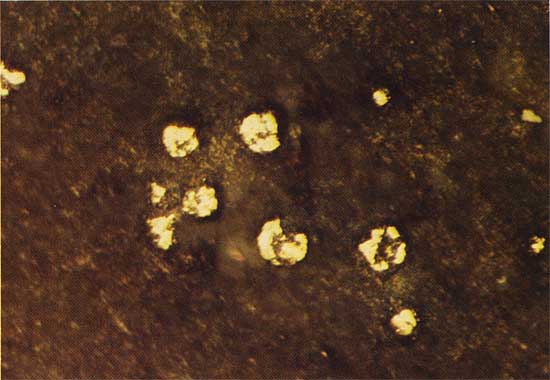
Figure 12--Unreplaced megaspores of the gentis Triletes, from an outcrop in the SE sec. 27, T. 31 S., R. 2 W., plain transmitted light. The field width is 6.0 mm.

Sulfide mineral chemistry was investigated with an ETEC electron microprobe. This was done to determine the degree to which mineral formulas differ from the ideal in sulfides that have been partially replaced by other ore minerals. The following minerals were analyzed: chalcopyrite, bornite, and digenite from drill hole 2; "chalcocite" from drill hole 13; "chalcocite," covellite, and azurite from drill hole 7.
Chalcopyrite from drill location 2 was analyzed in grains composed wholly of chalcopyrite; the results of these analyses are presented in Table 1. The average formulas for three sets of chalcopyrite analyses were Cu1.00Fe1.01S2, Cu1.01Fe.97S2, and Cu1.01Fe.97S2. Bornite was analyzed in a bornite-chalcopyrite grain and the average bornite formula was Cu4.86Fe.95S4. Sulfur-rich bornites are said to be common in redbed copper deposits (Brett and Yund, 1964), and are thought to have formed at temperatures below 75°C.
On the basis of electron microprobe analysis, the digenite can be definitely identified as digenite. The average of all analyses is Cu1.60Fe.059S. Morimoto and Gyobu (1971) report that Cu6.9Fe0.1S4 (Cu1.72Fe.025S) is nearly in the center of the digenite stability field. The grain that was probed contained digenite as a partial replacement of bornite.
Judging from its optical properties, the chalcocite-like phase that was probed in drill hole 13 was a grain of chalcocite. Table 2 lists the electron microprobe results and reveals that the average composition of the probed grain was Cu1.90S. This is close to the composition of djurleite (Cu1.96S), a copper sulfide optically identical to chalcocite. Detailed examination of the probed section from drill hole 13 revealed that a light sulfide phase and a dark sulfide phase existed in this possible djurleite phase. Both phases were analyzed and the dark phase was found to be relatively copper-rich with an average composition of Cu1.95S. The lighter phase was slightly poorer in copper with an average composition of Cu1.86S (Table 2).
Table 2--Results of electron microprobe analysis for chalcocite-like phase from drill hole 13 (weight percent)
| Specimen | %Cu | %S | Total % | Formula | |
|---|---|---|---|---|---|
| Chalcocite-like phase (Cu-rich), 26.12 m depth |
6a | 78.78 | 21.46 | 100.24 | Cu1.86S |
| 6b | 80.45 | 21.73 | 102.18 | Cu1.86S | |
| 6c | 78.79 | 21.88 | 100.67 | Cu1.81S | |
| 6d | 79.14 | 21.53 | 100.67 | Cu1.85S | |
| 6e | 79.08 | 21.78 | 100.86 | Cu1.83S | |
| 6f | 78.33 | 21.98 | 100.31 | Cu1.80S | |
| 6g | 78.61 | 21.76 | 100.37 | Cu1.82S | |
| 6h | 78.49 | 20.88 | 99.37 | Cu1.88S | |
| 6i | 78.49 | 21.21 | 99.70 | Cu1.87S | |
| 6j | 78.06 | 21.25 | 99.31 | Cu1.86S | |
| 6k | 78.22 | 20.79 | 99.01 | Cu1.90S | |
| 6l | 79.13 | 21.23 | 100.36 | Cu1.88S | |
| 6m | 78.70 | 20.94 | 99.64 | Cu1.89S | |
| 6n | 78.46 | 20.62 | 99.08 | Cu1.92S | |
| Average | 78.77 | 21.36 | 100.13 | Cu1.86S | |
| Chalcocite-like phase (Cu-poor), 26.12 m depth |
7a | 79.51 | 20.86 | 100.37 | Cu1.93S |
| 7b | 80.38 | 20.79 | 101.17 | Cu1.95S | |
| 7c | 79.15 | 20.63 | 99.78 | Cu1.94S | |
| 7d | 79.43 | 20.87 | 100.30 | Cu1.93S | |
| 7e | 78.73 | 20.76 | 99.49 | Cu1.91S | |
| 7f | 79.31 | 19.98 | 99.29 | Cu2.01S | |
| 7g | 80.56 | 20.67 | 101.23 | Cu1.96S | |
| 7h | 79.31 | 20.56 | 99.87 | Cu1.95S | |
| 7i | 79.48 | 20.61 | 100.09 | Cu1.95S | |
| 7j | 80.15 | 20.76 | 100.90 | Cu1.95S | |
| 7k | 79.75 | 20.87 | 100.62 | Cu1.94S | |
| 7l | 80.46 | 20.88 | 101.34 | Cu1.95S | |
| Average | 79.68 | 20.68 | 100.37 | Cu1.95S |
Results of the electron microprobe analysis of chalcocite-like grains in drill hole 7 are listed in Table 3. Not clearly in a grain that was originally a spore, the mineral in this sample has a composition unlike that of djurleite in drill hole 13. The average composition is Cu1.73S, similar to the composition of anilite (Cu1.75S). Anilite is said to be much like chalcocite in appearance (Morimoto and Koto, 1969).
Table 3--Results of electron microprobe analysis for chalcocite-like phase and covellite from drill hole 7 (weight percent).
| Specimen | %Cu | %S | Total % | Formula | |
|---|---|---|---|---|---|
| Chalcocite-like phase in chalcocite-covellite grain, 6 m depth |
8a | 76.22 | 21.66 | 97.88 | Cu1.78S |
| 8b | 76.15 | 22.50 | 93.65 | Cu1.70S | |
| Sc | 77.43 | 22.63 | 100.06 | Cu1.73S | |
| 8d | 76.45 | 22.71 | 99.16 | Cu1.69S | |
| 8e | 77.05 | 22.11 | 99.16 | Cu1.75S | |
| 8f | 76.89 | 22.11 | 99.00 | Cu1.74S | |
| 8g | 76.70 | 22.50 | 99.20 | Cu1.71S | |
| 8h | 75.55 | 21.99 | 97.54 | Cu1.73S | |
| 8i | 76.32 | 22.30 | 98.62 | Cu1.72S | |
| 8j | 76.73 | 22.00 | 98.73 | Cu1.76S | |
| Average | 76.55 | 22.25 | 98.80 | Cu1.73S | |
| Covellite in chalcocite-covellite grain, 6 m depth |
9a | 67.14 | 32.70 | 99.84 | Cu1.04S |
| 9b | 65.29 | 32.90 | 98.19 | Cu1.00S | |
| 9c | 68.80 | 30.26 | 99.06 | Cu1.14S | |
| 9d | 67.07 | 31.33 | 98.40 | Cu1.00S | |
| 9e | 73.33 | 25.63 | 98.96 | Cu1.44S | |
| 9f | 66.81 | 33.89 | 100.70 | Cu0.99S | |
| 9g | 65.85 | 32.31 | 98.16 | Cu1.03S | |
| 9h | 66.17 | 32.42 | 98.59 | Cu1.02S | |
| 9i | 64.51 | 32.41 | 96.92 | Cu1.00S | |
| Average | 67.22 | 31.54 | 98.76 | Cu1.08S |
Recent electrochemical studies of the copper-sulfur system by Potter (1977) have highlighted the phase complexities between Cu1.7S and Cu2.0S. The mineral assemblages found in south-central Kansas confirm these complexities in natural samples. The varied stoichiometries of the copper sulfide minerals in Kansas probably resulted from phase transformations after precipitation from fluids with slightly varying copper-sulfur ratios.
The analyses of samples from drill hole 13 (Table 2) fall within a compositional range that, according to Potter's study, should contain a djurleite-anilite mixture. In particular, the Cu1.87S phase probably represents a metastable crystallization product. The analyses of samples from drill hole 7 indicate a copper to sulfur ratio that, according to Potter (1977), should allow both anilite and covellite to form at the low (less than 75°C) temperatures postulated for redbed copper deposits.
The section from drill hole 7 contains covellite as well as anilite. Electron microprobe results indicate that the average composition of the covellite is Cu1.08S, quite close to the ideal formula (CuS).
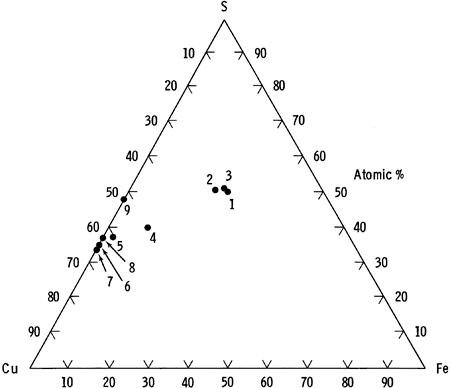
The identification of azurite in the cores was not definitely resolved by optical methods. Comparison of copper content in suspected azurite with that predicted by its ideal formula (shown in Table 4) strongly suggests that the mineral is azurite. A ternary diagram of the copper-iron-sulfur system, showing the average microprobe analysis for each mineral type, is depicted in Figure 13.
Table 4--Results of electron microprobe analysis for azurite from drill hole 7 (weight percent )
| Specimen | Experimental %Cu |
Formula %Cu |
Difference (Formula %Cu minus Exp. %Cu) |
|
|---|---|---|---|---|
| Azurite surrounding a chalcocite grain, 6 m depth |
10a | 50.22 | 55.31 | 5.09 |
| 10b | 50.65 | 55.31 | 4.66 | |
| 10c | 49.61 | 55.31 | 5.70 | |
| 10d | 51.64 | 55.31 | 3.67 | |
| 10e | 50.82 | 55.31 | 4.49 | |
| 10f | 50.67 | 55.31 | 4.64 | |
| 10g | 49.75 | 55.31 | 5.56 | |
| 10h | 51.49 | 55.31 | 3.82 | |
| 10i | 53.22 | 55.31 | 2.09 | |
| 10j | 51.79 | 55.31 | 3.52 | |
| 10k | 56.46 | 55.31 | -1.15 | |
| 10l | 54.98 | 55.31 | 0.33 | |
| 10m | 52.10 | 55.31 | 3.21 | |
| Average | 51.80 | 55.31 | 3.51 |
Figure 13--Electron microprobe data for the copper-iron-sulfur system.
| Analyzed Phase | Average Formula |
|---|---|
| 1. Chalcopyrite in pure chalcopyrite grains | Cu1.00Fe1.01S2 |
| 2. Chalcopyrite in pure chalcopyrite grains | Cu1.01Fe.97S2 |
| 3. Chalcopyrite in chalcopyrite-bornite grains | Cu1.01Fe.97S2 |
| 4. Bornite in chalcopyrite-bornite grains | Cu4.86Fe.95S4 |
| 5. Digenite phase | Cu1.60Fe.059S |
| 6. Anilite-djurleite intermediate phase | Cu1.86S |
| 7. Djurleite | Cu1.95S |
| 8. Anilite | Cu1.73S |
| 9. Covellite | Cu1.08S |
Atomic absorption analyses of non-oxidized carbonates and shales from drill holes 10 and 13 are. shown in Tables 5 and 6. Copper, lead, and zinc values were determined in parts per million, and the only metal that showed a pronounced anomaly was copper. In those intervals of the core in which copper concentrations reach their highest level (several thousand parts per million), mineralization can be seen with the unaided eye.
Samples for polished sections were collected wherever mineralization was visible in the core. Atomic absorption data are included to demonstrate that mineralization detectable with the unaided eye and binocular microscope is indeed a reliable method of assessing the number and approximate thickness of the mineralized beds. The data also show the absence of associated lead or zinc anomalies.
Table 5--Results of atomic absorption spectrophotometry of samples from drill hole 10. *Sulfide mineralization detected by microscopy. Analysis carried out by O. Karmie Galle, Kansas Geological Survey.
| Depth (Meters) |
Cu (ppm) | Pb (ppm) | Zn (ppm) | Rock Type |
|---|---|---|---|---|
| 27.89-27.94 | 5.2±0.1 | 0.1±0.1 | 35.7±1.5 | gray shale |
| 32.16-32.26* | 5260.0±64.7 | 5.4±0.3 | 19.0±1.4 | dolomite |
| 32.26-32.31* | 3280.0±65.1 | 18.8±1.5 | 31.5±1.4 | gray dol. shale |
| 32.31-32.38 | 810.0±66.1 | 0.1±0.1 | 49.7±1.4 | gray shale |
| 33.68-33.86 | 2.3±0.1 | 0.1±0.1 | 18.2±1.5 | gray shale |
| 34.11-34.34 | 8.6±0.3 | 0.9±0.1 | 29.9±1.5 | gray shale |
| 43.16-43.33 | 3.5±0.3 | 0.1±0.1 | 42.3±1.6 | gray shale |
| 43.99-44.04* | 3980.0±66.1 | 0.1±0.1 | 54.3±1.4 | gray shale |
| 44.04-44.07* | 2690.0±132.0 | 2.4±0.3 | 31.4±0.3 | gray shale |
| 44.07-44.13 | 12600.0±134.0 | 3.9±0.3 | 22.1±0.3 | dolomite |
| 44.13-44.16 | 85.1±2.7 | 11.1±1.5 | 23.3±0.3 | dolomite |
| 44.16-44.20* | 240.0±2.7 | 13.4±1.5 | 30.1±0.3 | dolomite |
| 44.20-44.29 | 25.2±0.4 | 0.2±0.1 | 32.3±0.3 | dolomite |
| 44.29-44.35 | 273.0±2.9 | 0.1±0.1 | 26.1±1.5 | dol. & gray shale |
| 44.35-44.42 | 3.9±0.1 | 0.1±0.1 | 50.0±1.5 | red shale |
| 44.42-44.60 | 3.3±0.1 | 0.1±0.1 | 42.1±1.4 | gray & red shale |
| 44.93-44.98 | 2.7±0.1 | 0.1±0.1 | 43.0±1.4 | gray shale |
| 45.41-45.54 | 3.2±0.1 | 0.1±0.1 | 45.4±1.4 | gray shale |
| 45.54-45.57 | 1.3±0.1 | 0.1±0.1 | 11.3±0.3 | red shale |
| 45.57-45.82 | 3.1±0.1 | 0.1±0.1 | 42.6±1.5 | gray shale |
| 45.82-46.02 | 2.1±0.1 | 0.1±0.1 | 25.4±1.5 | red shale & ls. |
| 46.02-46.18 | 3.7±0.1 | 0.1±0.1 | 27.5±1.5 | gray shale |
| 46.18-46.33 | 55.9±0.4 | 0.3±0.1 | 24.2±0.3 | dol. & gray shale |
| 46.33-46.41 | 11.4±0.3 | 0.3±0.1 | 32.0±0.3 | ls. & gray shale |
| 46.41-46.50 | 12.2±0.6 | 0.8±0.1 | 27.2±1.3 | dol. & gray shale |
| 46.50-46.53 | 6.7±0.6 | 0.5±0.1 | 46.0±1.3 | gray shale |
| Cu Max = 12600.0 | Pb Max = 18.8 | Zn Max = 54.3 |
| Cu Ave = 1129.7 | Pb Ave = 2.3 | Zn Ave = 33.2 |
| Cu Min = 1.3 | Pb Min = 0.1 | Zn Min = 11.3 |
Table 6--Results of atomic absorption spectrophotometry of samples from drill hole 13. *Sulfide mineralization detected by microscopy. Analysis carried out by O. Karmie Galle, Kansas Geological Survey.
| Depth (Meters) |
Cu (ppm) | Pb (ppm) | Zn (ppm) | Rock Type |
|---|---|---|---|---|
| 14.17-14.86 | 7.9±0.9 | 1.5±0.1 | 35.3±2.1 | gray & red shale |
| 14.86-14.99 | 202.0±1.7 | 1.2±0.1 | 42.7±2.1 | gray shale |
| 14.99-15.06* | 4490.0±101.0 | 4.2±0.1 | 32.6±0.6 | limestone |
| 15.06-15.12 | 427.0±1.7 | 2.8±0.1 | 32.8±2.1 | limestone |
| 15.12-15.19 | 266.0±1.7 | 1.2±0.1 | 33.9±2.1 | gray shale |
| 15.54-15.67 | 5.8±0.1 | 2.7±0.1 | 34.8±2.2 | gray & red shale |
| 15.67-15.77 | 18.2±0.1 | 5.1±0.1 | 30.0±0.4 | limestone |
| 15.77-15.85 | 16.1±0.1 | 5.1±0.1 | 29.0±2.2 | gray shale |
| 15.85-16.15 | 6.6±0.1 | 2.2±0.1 | 44.2±2.1 | red shale |
| 25.98-26.00 | 4.0±0.1 | 0.1±0.1 | 4.9±0.4 | gypsum |
| 26.00-26.11* | 1770.0±10.4 | 2.1±0.1 | 51.0±2.1 | gray shale |
| 26.11-26.14* | 6420.0±98.9 | 4.7±0.3 | 35.6±0.5 | dolomite |
| 26.14-26.18* | 119.0±7.9 | 3.2±0.1 | 28.8±0.5 | dolomite |
| 26.18-26.24* | 1080.0±10.5 | 2.8±0.1 | 24.2±0.4 | dolomite |
| 26.24-26.29* | 1400.0±8.5 | 3.0±0.1 | 30.4±0.4 | dolomite |
| 26.29-26.31* | 99.0±3.5 | 2.2±0.1 | 34.3±1.3 | dolomite |
| 26.31-26.39 | 113.0±3.4 | 4.1±0.1 | 28.7±1.3 | dolomite |
| 26.42-26.49 | 7.1±0.2 | 1.0±0.1 | 67.0±1.3 | gray shale |
| 26.54-26.64* | 940.0±40.1 | 2.4±0.1 | 51.8±0.5 | ls. & gray shale |
| 27.74-27.87 | 3.2±0.1 | 0.6±0.1 | 60.2±1.3 | gray shale |
| 27.87-27.91 | 2.5±0.1 | 0.2±0.1 | 41.3±1.2 | gray & red shale |
| 27.94-28.02 | 3.4±0.1 | 0.7±0.1 | 51.1±1.2 | gray shale |
| 28.02-28.17 | 3.5±0.1 | 0.5±0.1 | 71.0±1.3 | gray shale |
| 28.17-28.27 | 4.5±0.1 | 0.7±0.1 | 56.2±1.2 | ls. & gray shale |
| 28.65-28.67 | 3.4±0.1 | 0.4±0.1 | 70.4±1.2 | ls. & gray shale |
| 28.67-28.80 | 2.6±0.1 | 1.2±0.1 | 41.5±1.2 | gray shale |
| 28.89-28.92* | 45.8±0.4 | 1.5±0.1 | 38.7±1.3 | limestone |
| 28.92-28.99* | 37.6±0.4 | 1.7±0.1 | 34.5±1.3 | limestone |
| Cu Max = 6420.0 | Pb Max = 5.1 | Zn Max = 71.0 |
| Cu Ave = 625.0 | Pb Ave = 2.1 | Zn Ave = 40.6 |
| Cu Min = 2.5 | Pb Min = 0.1 | Zn Min = 4.9 |
Pyrite is the first sulfide to form in the stratiform redbed copper deposits of south-central Kansas. In the northern part of the study area, chalcopyrite may form at the same time as the pyrite or as a replacement of it. Bornite occurs as a later replacement of chalcopyrite; and digenite, in turn, replaces the bornite.
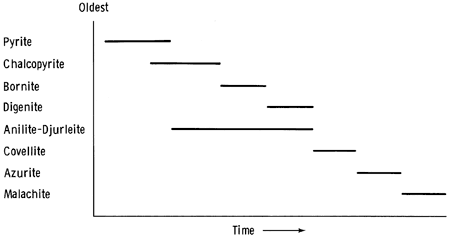
Anilite and djurleite are found in the southern part of the study area. Potter (1977) wrote that djurleite, anilite, and covellite, in turn, become the stable copper-sulfur phase at low temperatures with decreasing copper-sulfur ratios. The paragenetic sequence is thus djurleite, djurleite plus anilite, anilite, anilite plus covellite, and covellite. Such a sequence might be represented by the copper-rich phase (djurleite) and copper-poor phase (an anilite-djurleite intermediate phase), which occur at a depth of 26.12 m in drill hole 13; the anilite, which is partially replaced by covellite 6 m beneath the surface in drill hole 7; and the covellite, which persists in areas where azurite has replaced anilite at the same depth in drill hole 7. Figure 14 is a paragenetic diagram for the sulfides found in the study area.
Figure 14--Paragenetic sequence for copper carbonates and copper sulfides in the study area.
The two observed sulfide assemblages, bornite-chalcopyrite-pyrite and chalcocite-pyrite, which are spatially separated and suggested to be produced by a descending copper-bearing ore fluid reacting with diagenetic pyrite, support theories stressing the importance of groundwater in such processes (Rose, 1976).
Extrapolation of solubility data of Crerar and Barnes (1976) for the system copper-iron-sulfur indicates that CuCl0 is the dominant cuprous complex at mCl- in excess of 10-3. However, at mCl- in excess of about 10-1.5 the CuCl3-2 complex may be the dominant species (Rose, 1976).
Ripley and others (1980) use the molalities Fe2+ = 10-4, H2S = 10-4, Cl- = 10-1 to show a log aCuCl0 - pH - log fO2 diagram which aids them in explaining the possible reactions taking place.
For the transformation of pyrite into chalcocite-like minerals, which is the important reaction in the southern part of the area, the following simple reaction can be written:
FeS2 + H2O + 2CuCl0 = Cu2S + Fe 2+ + 2Cl- + 1/2 O2 + H2S
The main controlling factor for the production of chalcocite rather than chalcopyrite seems to be pH. A drop in aCuCl0 would favor the above reaction at a PH of about 5.5 and a log fO2 of -54. At a higher pH and the same fO2 of -54, the sequence pyrite-chalcopyrite-bornite may be produced by continuous depletion of copper from an ore fluid (drop in aCuCl0) according to the following set of general reactions:
Of course, changes in temperature and the concentration of iron also effect a shift in the pyrite-chalcocite-chalcopyrite triple point.
The presence of mineralized carbonate beds permits an evaluation of the lowest pH conditions to be expected, Ripley and others (1980) calculated that the pH conditions suggested for the production of the ore minerals are quite consistent with the presence of carbonates within the sequence.
Covellite, azurite, and malachite are recent and are directly related to the oxidation of sulfides by groundwater. The depths at which pyrite, chalcopyrite, bornite, digenite, and anilite-djurleite first appear in the cores increase to the south in the study area.
The apparent depth zonation is not related to depth beneath the present land surface, but probably is the result of a basin-ward decrease in the amount of primary copper contained in the sedimentary sequence during Permian time. The upper part of the Wellington Formation in Kansas is a regressive marine sequence. Cheney and Jensen (1962) wrote that in the basin of sedimentation original pyrite would be replaced by copper sulfides formed from progressively less copper-rich solutions in the direction of sediment transport. This would create a basin-ward zonation of chalcocite → bornite → chalcopyrite → pyrite, which is the zonation found with increasing depth (i.e., further basin ward) in the cores.
The Wichita Mountains in southern Oklahoma have been proposed as a source of copper for sulfides in Oklahoma redbeds. Cox (1978) reported that gabbroic and anorthositic rocks contain an average of 140 ppm and 20 ppm copper, respectively. Fay (1975) suggested that chalcopyrite in Ouachita sediment could have released copper to Permian rivers and thereby could have supplied this metal to the Wellington Formation of north-central Oklahoma.
Brown (1971) wrote that the Nonesuch Shale of Michigan has a copper sulfide zonation that is, in descending order, pyrite-chalcopyrite-bornite-digenite-djurleite-chalcocite. In this formation the source of copper is the Copper Harbor Conglomerate, which lies directly beneath the Nonesuch. Therefore, with increased distance from the source of metal, the same zonation is found as in the upper Wellington.
Rose (1976) believed that the mineralizing fluid in redbed copper deposits is a cuprous chloride-rich brine in equilibrium with hematite, quartz, feldspar, and mica at temperatures less than 75° C. He wrote that evaporates associated with the redbeds could have supplied the chloride anions, and suggested that this mechanism could also have been important in the origin of the European Kupferschiefer and African Zambian ore deposits.
Although cuprous chloride complexes are generally accepted as the mineralizing fluid in this type of ore deposit, the origin of the fluid is a matter of debate. Davidson (1965) believed that the chloride brines are generated by dissolution of evaporites in the host rock, but Renfro (1974) suggested a sabkba-type origin for stratiform metalliferous deposits. Sabkhas (evaporite flats) form along the margins of a regressive sea such as that known to have existed in the study area during Permian time. According to Renfro's model, ore deposition occurs when metal-bearing non-saline meteoric water encounters reduced areas of coastal algal mats. The model favored for the deposition of the copper deposits along the eastern margin of the Permian epicontinental sea is discussed in detail in Part 1 of this bulletin. Basically, the model recognizes a source for the copper either within or along the margins of the sea with subsequent adsorption of the dissolved copper on the reduced sediments. We believe that the majority of the sediments were deposited in a reduced environment. At a later time, the sea retreated and the sediments became exposed to the air. Downdip-migrating oxygenated meteoric water oxidized the sediments and dissolved the evaporites and copper. Copper was reprecipitated and concentrated in a reduced environment downdip from the surface. Oxygen content of the water was a major factor controlling the precipitation of the copper. The dissolved evaporitic minerals were probably rich enough in magnesium to dolomitize many of the carbonate units to varying degrees. Mineralization associated with the carbonates is almost always restricted to the dolomitized beds.
The major conclusions reached from this study are:
Brett, R., and Yund, R. A., 1964, Sulfur-rich bornites: The American Mineralogist, v. 49, no. 7-8, p. 1084-1098.
Brown, R., 1971, Zonation in the White Pine Copper, Ontonagan County, Michigan: Economic Geology, v. 66, no. 4, p. 543-573.
Cheney, E. S., and Jensen, M. L., 1962, Comment on The origin of some strata-bound sulfide ore deposits, by C. F. Davidson, 1962, (in Economic Geology, v. 57, no. 2, p. 265-2-14): Economic Geology, v. 57, no. 4, p. 624-627.
Cox, R. E., 1978, Subsurface geochemical exploration of strata-bound copper in Lower Permian redbeds of north-central Oklahoma: M.S. thesis, Oklahoma State University, 117 p.
Crerar, D. A., and Barnes, H. L., 1976, Ore solution chemistry, V. Solubilities of chalcopyrite and chalcocite assemblages in hydrothermal solution at 200° to 350° C: Economic Geology, v. 71, no. 4, p. 772-794.
Davidson, C. F., 1965, A possible mode of origin of stratibound copper ores: Economic Geology, v. 60, no. 5, p. 942-954.
Fay, R. O., 1975, A possible origin for copper in Oklahoma: Oklahoma Geology Notes, v. 35, no. 4, p. 151-153.
Hagni, R. D., and Gann, D. E., 1976, Microscopy of copper ore at the Creta Mine, Southwestern Oklahoma, in Johnson, K. S., and Croy, R. L., eds., Stratiform copper deposits of the Midcontinent region, a symposium: Oklahoma Geological Survey Circular 77, p. 40-50.
Hill, W. E. Jr., 1967, Copper in redbeds of south-central Kansas: Kansas Geological Survey, Bulletin 187, pt. 1, p. 13-14.
Johnson, K. S., 1976, Permian copper shales of southwestern Oklahoma, in Johnson, K. S., and Croy, R. L., eds., Stratiform copper deposits of the Midcontinent region, a symposium: Oklahoma Geological Survey Circular 77, p. 3-14.
Johnson, K. S., 1974, Permian copper shales of Southwestern United States, in Bartholomé, Paul, ed., Gisements stratiforme et provinces cuprifères: Société Geologique de Belgique, p. 383-393.
Long, D. T., and Angino, E. E., 1976, Occurrence of copper sulfide in the (Permian age) Milan Dolomite, south-central Kansas: Economic Geology, v. 71, no. 3, p. 656-661.
Morimoto, N., and Gyobu, A., 1971, The composition and stability of digenite: The American Mineralogist, v. 56, no. 11-12, p. 1889-1909.
Morimoto, N., and Koto, K., 1969, Anilite, Cu7S4, a new mineral: The American Mineralogist, v. 54, no. 9-10, P. 1256-1268.
Norton, G. H., 1939, Permian redbeds of Kansas: American Association of Petroleum Geologists Bulletin, v. 23, no. 12, p. 1751-1819.
Potter, R. W., II, 1977, An electrochemical investigation of the system copper-sulfur: Economic Geology, v. 72, no. 8, p. 1524-1542.
Renfro, A. R., 1974, Genesis of evaporite-associated stratiform metalliferous deposits--a sabkha process: Economic Geology, v. 69, no. 1, p. 33-45.
Ripley, E. M., Lambert, M. W., and Berendsen, P., 1980, Mineralogy and paragenesis of redbed copper mineralization in the Lower Permian of south-central Kansas: Economic Geology, v. 75, no. 5, p. 722-729.
Rose,, A. W., 1976, The effect of cuprous chloride complexes in the origin of redbed copper and related deposits: Economic Geology, v. 71, no. 6, p. 1036-1048.
Swineford, A., 1955, Petrography of Upper Permian rocks in south-central Kansas: Kansas Geological Survey, Bulletin 111, 179 p. [available online]
Waugh, T. C., and Brady, L. L., 1976, Copper occurrences associated with Permian rocks in south-central Kansas, in Johnson, K. S., and Croy, R. L., eds., Stratiform copper deposits of the Midcontinent region, a symposium: Oklahoma Geological Survey, Circular 77, p. 76-79.
Kansas Geological Survey, Mineralogy of Copper Sulfides, Lower Permian Redbeds, South-Central Kansas
Placed on web June 30, 2009; originally published in Oct. 1981.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/223_2/index.html