Kansas Geological Survey, Bulletin 211, Part 4, p. 23-27
Stoneware potters often complain about two problems occurring with their ware; it is cracked when removed from the kiln or the glaze pops off the surface after the glaze is fired. A body used for years may suddenly develop these problems. The potter assumes that nothing has changed in the method of handling or firing the body. This is probably true, but some small change in final fired-body composition has occurred. This problem is not new; it has been going on as long as potters have been firing stoneware. It's just that often one forgets how the old problems were solved. Most pottery books mention the cracking problem and imply that if you are careful and don't anger the "pottery gods" too often, the problem can be lived with.
It is the purpose of this note to give some insight into the source of the trouble and some steps that may be taken to correct it. No specific body compositions are referred to, nor is a typical composition listed, because there are so many variations of stoneware compositions in use. Only ideas for problem solutions are presented. How the ideas presented are used to solve your problem will depend upon how much control you have over the stoneware body formulation. Sometimes the simplest solution is to change to another clay body.
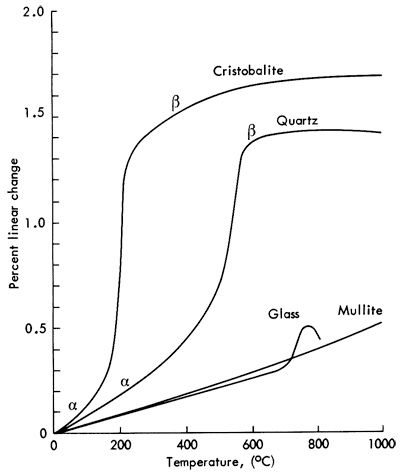
X-ray diffraction analysis and thermal expansion measurements of the broken ware always show an excessive amount of the high temperature phase of quartz known as cristobalite to be present. Vitrified stoneware expands in size when heated and shrinks when cooled. This change in length is called thermal expansion.
The troublesome thermal expansion curve for the cristobalite form of quartz is shown in Figure 1 along with the thermal expansion of other phases normally present in vitrified stoneware. Note the very large percent linear change for cristobalite at about 200°C (392°F). This is the cause of the trouble as it changes from the β to α form during cooling. The α and β refer to different arrangements of the atoms within the crystal structure, β always referring to the high-temperature structure in American literature. (It is common in European literature to reverse the meaning of the symbols.) This reaction happens upon heating and cooling each time the ware is thermally cycled. If the ware comes out of the kiln broken, the trouble is caused by the β form changing to the α form causing it to break in the first cooling period. The quartz expansion curve shows a similar behavior, but the transformation occurs at a higher temperature and is of a lesser magnitude than that of the cristobalite so it is less troublesome in most bodies. The glass expansion is characteristic of a glass in that the expansion is linear up to a region just below its softening temperature. Mullite has the ideal thermal expansion curve for a stoneware body that is to be used as ovenware. Note the lack of any irregularities in the curve. It is smooth and continually increasing with increasing temperature.
Figure 1--Thermal expansion curves for the phases present in fired stoneware.

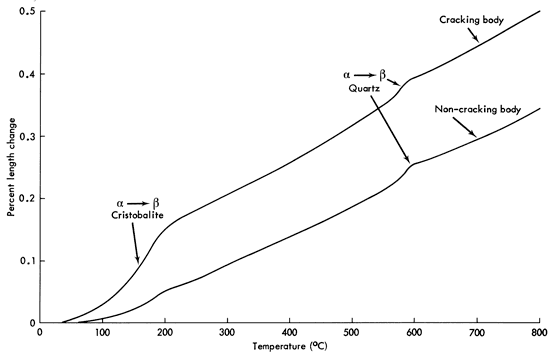
Cristobalite is the high-temperature form of quartz with its region of equilibrium stability above 1470°C (2678°F). How can this cause trouble if the body has never been fired higher than cone 9 or 10, a maximum of 1285°C (2345°F)? The 1470°C temperature applies only if you are starting with pure quartz and converting it to cristobalite. A stoneware body is not pure quartz but is mostly made up of clays which contain some free quartz. Note that in the expansion curves for stoneware bodies in Figure 2 the α and β transformation for quartz is still visible, showing that much of the original quartz was not transformed to cristobalite or dissolved in the glass phase.
Figure 2--Thermal expansion curves for a cracking and non-cracking fired stoneware body.
Most of the stoneware bodies are formulated around clays containing the clay mineral kaolinite, which when heated undergoes the following set of reactions:
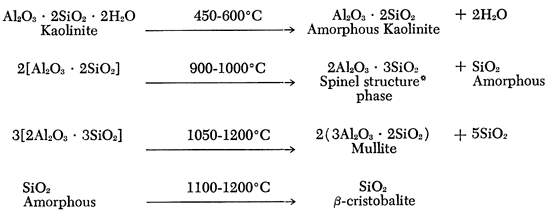
[Note: In above reactions, this (*) refers to a spinel crystal structure. it does not mean the compound MgO • Al2O3, spinel, is formed.]
It is the last reaction that causes the problem. If it can be prevented, or retarded, the amount of cristobalite will be held to a minimum. Why the cristobalite forms at temperatures below its temperature of stability is one of the interesting quirks of the crystallization phenomena. Amorphous silica has a random arrangement of silicon and oxygen ions similar to that of silica glass. However, this is a highly unstable state for the 1285°C temperature and the ions will arrange in the most loosely packed crystalline arrangement possible, which is β-cristobalite. Once in this form, the change to another crystalline arrangement is normally slow. Thus, there is a good chance of having some cristobalite present in the fired ware.
At the same time the transformation of the amorphous silica into cristobalite is occurring, another reaction is going on that is removing some of the amorphous quartz which is reacting with the melting fluxes to form the liquid phase that is vitrifying the body. Since the atomic arrangement of amorphous silica is closer to that of the liquid phase than is that of the crystalline quartz, the amorphous silica will be dissolved at a faster rate than the other crystalline forms of quartz. The liquid phase can only dissolve a certain amount of silica, depending upon its overall chemistry. The remaining silica is available for crystalline phase formation. If the liquid phase becomes supersaturated with silica as it cools, there is a high probability that the excess will precipitate out as crystalline cristobalite. A small amount of cristobalite is beneficial towards developing the proper compressive stress in the glaze to prevent crazing and increase the strength of the fired ware; only an excess causes trouble.
Before suggesting remedies for the problem, let's look at how the thermal expansion behavior of the body can cause both the body cracking and the glaze popping problem. Thermal expansion is the change of the length or volume of the solid as the temperature changes. Normally a solid expands when heated and shrinks when cooled. A small thermal expansion change means good thermal shock resistance while a large thermal expansion change means poor thermal shock resistance of the body. Why?
A thermal gradient is established in the ware when it is cooled. The slower the cooling rate the smaller is this gradient between the hotter inside and the cooler outside of the wall. The cooler outside wall has contracted more than the hotter interior, creating a tension or pulling apart in the outer wall because the interior is trying to keep it from contracting. Tension forces are building in the outer surface and when this force exceeds the tensile strength of the body, which is quite low, a crack begins and the wall breaks. The problem is dealt with by reducing the rate of cooling or by reducing the thermal expansion value so that it is difficult to establish a large differential of length change during cooling. What makes the α to β transformation so troublesome is that no ions have to move and rearrange to produce the change in volume. The change occurs almost instantaneously by changing a bond angle between the silicon and oxygen ion when the transformation temperature is reached. It occurs over a small temperature range in the expansion curve because it takes time for the interior of the ware wall to reach the transformation temperature. The transformations occur upon both heating and cooling as long as the crystalline phase is present. If you want cristobalite present in fairly large amounts, the cooling rate must be quite slow between 250°C (482°F) and room temperature to prevent the buildup of excessive tensile forces. Close the kiln door during this range, or at least don't let cooling air blow directly onto the ware. How fast the stoneware body can be cooled depends largely upon the thermal expansion behavior below 250°C (Figure 2 shows a fairly linear rate of expansion above this temperature). This does not mean that the body can be cooled from maturing temperature to 250°C in one hour. Find out the limitations of your ware and also the thermal shock limitations of the refractories in your kiln.
According to Lawrence (page 126) cracks originating because of the quartz inversion can be distinguished from cracks caused by the cristobalite inversion. Both cracks will be sharp, but because the cristobalite crack occurred at a much lower temperature, its surface will be dull while the surface of the higher temperature crack will be smooth and glossy.
Porous bodies may have a considerable amount of cristobalite present and still have reasonably good thermal shock resistance because the pore structure retards the spread of the crack. The cracks are still there, but they don't grow and cause immediate failure. After many thermal cycles, pieces of the ware will develop cracks of major size. Vitreous ovenware should have a minimum of cristobalite material present as the ware has no porous structure to block crack development and growth.
Glaze popping (dunting) also occurs below 255°C because the body is shrinking much faster than the glaze is shrinking. This difference in the two rates of length change causes a large amount of compressive stress to develop in the thin layer of glaze. The glaze buckles and pops off the surface. The cure is to lower the thermal expansion of the body in the last few hundred degrees of cooling temperature. Raising the thermal expansion of the glaze is another solution but it may create crazing problems that the method of lowering body expansion avoids. A good glaze fit occurs when the thermal expansion of the glaze is just slightly less than the thermal expansion of the body in all temperature regions where the glaze is solidly attached to the body. If given a choice, most potters would rather have the glaze craze than pop off the surface.
Now that we know that cristobalite is causing the problem, here are some steps that can be taken to minimize or control the amount formed.
The maximum amount of cristobalite formed from the decomposition of the kaolinite appears to be in the temperature range of 1250-1300°C (cone 7-10). Lowering the firing temperature without changing the flux content of the body may increase the porosity and may not properly mature the currently used glaze.
Most stoneware bodies contain brick grog which often has well-developed particles of cristobalite present. Such particles are difficult to dissolve in the liquid phase and will act as growth sites for the transformation of the amorphous quartz into cristobalite and for the precipitation of excess silica in the glass phase as cristobalite during cooling. It is best to use grogs low in cristobalite content, such as calcined kyanite or synthetic mullite (3Al2O3 • 2SiO2). Some commercial suppliers are using such grogs in their stoneware. Brick grog is often made by crushing scrap bricks; hence, the quality may vary widely from source to source.
Another method of reducing the cristobalite content is to dissolve the amorphous silica material in the high temperature liquid phase. If the body has five percent potash feldspar present, increase it to ten percent. This will increase the glass content and probably allow a lowering of the firing temperature. It may remove some of the iron color. If iron color is important, try adding three to five percent calcium fluoride (minus 200 mesh) in addition to the flux already present. This flux tends to retain the iron spots and there is a small amount of iron in natural calcium fluoride.
When the flux content is increased, the amount of liquid phase also increases at the maturing temperature, which may cause slumping. Always test fire small quantities of the body before preparing large amounts.
Beware of using fluxes high in sodium oxide content. Sodium oxide may accelerate the rate of conversion of amorphous quartz into cristobalite. Some claim that there is no difference between the use of soda spar and potash spar in stoneware bodies. It is best to use only the potash spar.
Why does reduction firing cause more cristobalite to be formed in some bodies than others? The amount of iron oxide (Fe2O3) is often the reason. Ferric oxide (Fe2O3) is reduced to ferrous oxide (FeO) in the reduction firing. The ferrous oxide melts and penetrates into the amorphous silica structure and promotes the formation of cristobalite. Such a compound is called a mineralizer. Thermal expansion measurements of all iron-containing stoneware bodies showed that reduction firing increased the amount of cristobalite present.
Keep the iron content as low as possible if you fully reduce the ware. If iron spots are important in the glaze, try to formulate the glaze so that the spot material is in the glaze and not derived from the contact of the glaze and the body.
Fire under mild oxidation atmosphere and reduce only as necessary to produce the glaze effect needed. It is not necessary to have the body saturated with black carbon into the center of the wall. This does nothing beneficial for the body. A reduction firing should not belch black smoke, as only the carbon monoxide present is affecting the reduction process.
The longer the body is held at peak temperature the more time is available for rearrangement of the silicon and oxygen atoms into the cristobalite structure. This is somewhat balanced by the glass-forming reaction which dissolves more quartz over a longer time period. However, the latter may saturate the glass composition and cause cristobalite to precipitate during cooling. Experiment and find out the best soaking time for the body.
This is a difficult question to answer as the average potter has no way to measure the quantity present. The best suggestion is to give the fired stoneware a mild thermal shock by removing it from the kiln at 250°C (500°F) and allowing it to rapidly cool to room temperature in a static air condition (no wind blowing across the piece). If it doesn't break, the amount of cristobalite present is not excessive. Figure 2 shows the thermal expansion for two stoneware bodies. The cracking stoneware body shows a rapid increase in thermal expansion between 100 and 200°C. It is this rapid expansion that is cracking the body. The non-cracking body is a similar composition that has been modified by replacing the brick grog with calcined kyanite grog and increasing the potash spar from 5 to 10 parts by weight. Its thermal expansion curve increases at a much slower rate. Both curves shows a small inversion just below 600°C due to the α-β quartz transformation showing that some of the quartz has not been dissolved in the liquid phase nor has it been transformed to cristobalite.
A stoneware body that can tolerate moderate thermal shock will have an expansion curve similar to the non-cracking curve. For non-ovenware items the amount of cristobalite can be increased. Remember that small amounts of cristobalite are not bad as they provide some compression in the glaze and reduce the tendency for glazes to craze.
For a good summary of applied science and the potter I suggest reading CERAMIC SCIENCE FOR THE POTTER by W. G. Lawrence, published in 1972 by the Chilton Book Company, Philadelphia, Pa.
Kansas Geological Survey
Comments to webadmin@kgs.ku.edu
Web version updated June 18, 2010. Original publication date April 1978.
URL=http://www.kgs.ku.edu/Publications/Bulletins/211_4/bauleke.html