Kansas Geological Survey, Bulletin 202, pt. 1, originally published in 1971
Originally published in 1971 as part of Kansas Geological Survey Bulletin 201, pt. 1, p. 11-13. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version of the complete bulletin (7 MB) is also available.
Differential thermal analysis (DTA) curves of Kansas montmorillonites will most likely be those of an "abnormal" montmorillonite. No sample showed the single dehydroxylation reaction peak at 725°C of a normal montmorillonite. The predominant dehydroxylation reaction occurred at 525°C, although, especially in samples from Phillips County, two dehydroxylation peaks at 525° and 675°C were often present in a sample. Most curves had the typical two-peak endothermic water loss reactions in the 100-200°C range associated with calcium montmorillonite. Caliche (calcium carbonate) was a common impurity in Wallace County materials. All were very low in organic matter. Based on fired color, Wallace County material has a higher iron content than material from Phillips County.
The geology of the Kansas montmorillonite clay deposits in Phillips and Wallace counties has been adequately described by Ives (1960). Large, noncontaminated samples were obtained by drilling 24-inch holes at the sample locations listed in Table 1. The drill bucket was emptied every 12-14 inches of hole depth up to a maximum depth of 42 feet. Only the montmorillonitic clay zones were sampled.
Table 1--Sample locations.
| Phillips County | |
|---|---|
| Ranch | Location |
| Adee | Sec. 11, T 1 S, R 18 W |
| O'Neill | Sec. 10, T 1 S, R 18 W |
| Smith | Sec. 25, T 1 S, R 20 W |
| Wallace County | |
| Ranch | Location |
| Abrahamson | Sec. 19, T 12 S, R 41 W |
| Swartz | Sec. 30, T 12 S, R 41 W |
| Craft | Sec. 29, T 12 S, R 41 W |
Many of the Wallace County deposits were of the pocket and channel type. A good deposit would be drilled, yet 50 feet away no montmorillonite would be found. Phillips County deposits appeared to be more continuous across the region.
Earlier X-ray studies by the clay mineralogist of the State Geological Survey identified the clay mineral present as of the montmorillonite type (Paul Franks, personal communication, 1960). The purpose of this study was to compare the thermal decomposition behavior of the two deposits. A DTA was run on each sample collected. The 10-mg pellet sample method (Tien and Bauleke, 1970) was used in conjunction with the micros ample holder in a STONETRACOR Model 220 differential thermal analyzer. [Note: Mention of companies or products is not to be considered an endorsement.] Care was exercised to eliminate impurities in these very small samples.
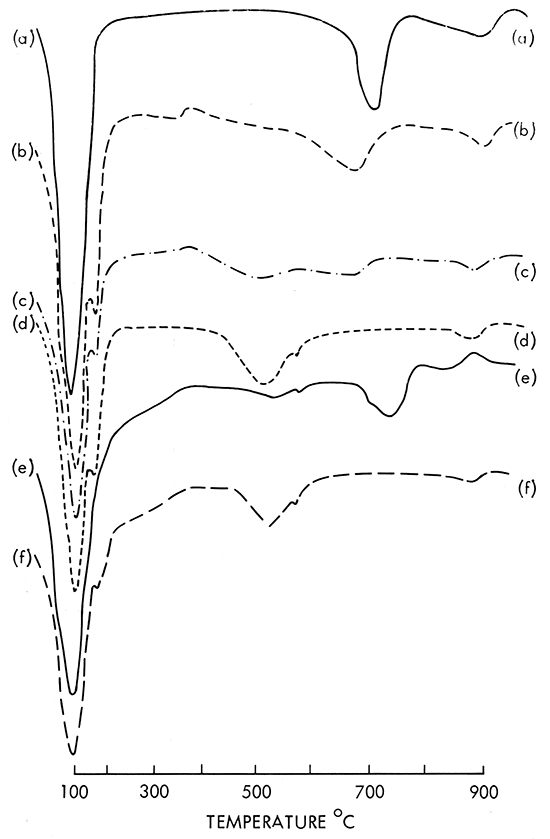
Representative data curves of the 24 samples from Phillips County and the 32 samples from Wallace County along with a reference curve from a South Dakota montmorillonite are shown in Figure 1.
Figure 1--DTA curves (10 mg, heating rate 20°C/min.; recording speech 0.2 in/min.; amplification 150 microvolts). (a) South Dakota montmorillonite, A.P.I. sample H-27. (b) Phillips County, Smith ranch. (c) Phillips County, Smith ranch. (d) Phillips County, O'Neill ranch. (e) Wallace County, Abrahamson ranch, caliche decomposition at 750°C. (f) Wallace County, Craft ranch.

A curve can be divided into three distinct reaction regions: (1) below 200°C, release of adsorbed molecular water; (2) between 400-800°C, release of water from the loss of OH ions (called dehydroxylation) in the crystal structure; (3) above 800°C, an endoexothermic reaction representing the decomposition of the crystal lattice. No curve from the Kansas montmorillonite samples exactly matches the normal DTA curve for the South Dakota montmorillonite. The South Dakota material is the sodium type with an expanding lattice that forms a stable gel with water. Kansas montmorillonites are of the calcium type with an expanding lattice, but reaction with water does not form the same type of stable gel as does the sodium montmorillonite.
The DTA curve geometry of the Kansas montmorillonites differs from the South Dakota montmorillonite in the first and second regions. The last hightemperature reaction, controlled by the chemistry of the minerals, is quite similar in all curves. Endothermic reactions in regions one and two are influenced by the crystal structure. The Kansas calcium montmorillonite has two endothermic peaks for the release of molecular water but the sodium montmorillonite shows only one. The double peak indicates that the molecular water is held by two distinctly different bonding energies.
Most of the Kansas montmorillonite samples showed that dehydroxylation occured in the temperature range between 525° and 675°C. Such low-temperature behavior is reported in the literature (Mackenzie, 1964, p. 230) and given the name "abnormal" montmorillonite.
X-ray diffraction does not appear to distinguish between the two different types of structure, only DTA detects the difference. But because DTA detects this difference, the DTA curve for an "abnormal" montmorillonite can very often be misinterpreted as being that of an illite structure. Therefore, DTA curves should not be used as the sole identifier of montmorillonite or illite clay minerals. A summary of the frequency of normal-"abnormal" patterns for Kansas montmorillonite samples is given in Table 2.
Table 2--Type of DTA pattern.
| County | Normal % |
"Abnormal" % |
Mixed % |
|---|---|---|---|
| Phillips | 17 | 25 | 58 |
| Wallace | 0 | 92 | 8 |
The term "normal" (above) means that there was only one distinct peak at 675°C. Thermal decomposition may have begun as low as 400°C, but only the peak or minimum on the curve was measured.
No sample from Wallace County showed evidence of the presence of only normal montmorillonite. Of the Phillips County samples, 17 percent showed normal behavior, and 58 percent showed a behavior indicative of a mixture of both types of minerals. By contrast, the Wallace County material showed normal reaction in only 8 percent of the samples and it was always associated with the "abnormal" material.
It is postulated (Hill, 1953) that the "abnormal" thermal behavior is caused by some random-type disorder within the crystal lattice. If so, one might ask these questions: Is this disorder a step in the decomposition process of volcanic ash? Is the crystal structure of the Kansas montmorillonite approaching that of the Wyoming-South Dakota montmorillonites? Has the Kansas montmorillonite been altered due to the weathering sequence? At present, the answers are not known.
Deposits from both Phillips and Wallace counties are relatively free from organic matter as shown by the small amount of exothermic activity between 300° and 500°C. No Phillips County sample contained calcium carbonate disseminated throughout the montmorillonite sample, yet 40 percent of the Wallace County sample had a recognizable calcium carbonate decomposition peak at 750°C. All samples having this characteristic peak also reacted with dilute hydrochloric acid. Small lumps of white caliche were found in Wallace County material. Some areas of Phillips County are also reported to contain caliche (A. Hornbaker, personal communication, 1969).
Interestingly, when the calcium carbonate decomposition peak was present (which must mean that an abundance of calcium ions was available to any groundwater), the distinct second dehydration peak occurring at 180°C had been transformed into a broad change-of-slope area. This occurred with all samples in Wallace County having the calcium carbonate peak; when the calcium carbonate decomposition peak was absent the second dehydration peak was present.
Quartz as an impurity was often present as shown by the small endothermic reaction at 575°C in curves d, e, and f of Figure 1. Some samples in Phillips County were found to be free from quartz. Fired color ranged from buff to red, depending upon the iron and caliche contents.
Ives (1960) reported that Kansas calcium montmorillonite could easily be converted to sodium montmorillonite by boiling in a saturated sodium chloride (NaCl) solution. A DTA curve prepared after boiling showed the thermal decomposition to be that of a normal montmorillonite. This was difficult to accept. Why should a reaction essentially affecting only the base-exchange cations on the outside of the clay mineral particle affect the manner in which the interior OH ions were bonded into the structure?
The experiment was repeated selecting a sample that showed only the "abnormal" 525°C endothermic peak. The sample was sodium saturated by boiling in a saturated sodium chloride solution for one-half hour, then the DTA curve was produced. The sodiumproduced montmorillonite was then boiled in a saturated calcium chloride solution for one-half hour to return it to the calcium-saturated condition and a DTA curve was produced. Results are shown in Figure 2.
Figure 2--DTA curves showing the effect of exchangeable ions. DTA parameters same as Figure 1. (a) Untreated montmorillonite, Phillips County, O'Neill ranch. (b) Boiled onehalf hour in saturated sodium chloride. Second dehydration peak gone at 150°C. (c) Sodium saturated montmorillonite of curve b boiled one-half hour in saturated calcium chloride. Note the return of the 150°C endothermic inflection. The remainder of the curve beyond 200°C was unaffected by the boiling in either saturated solution.
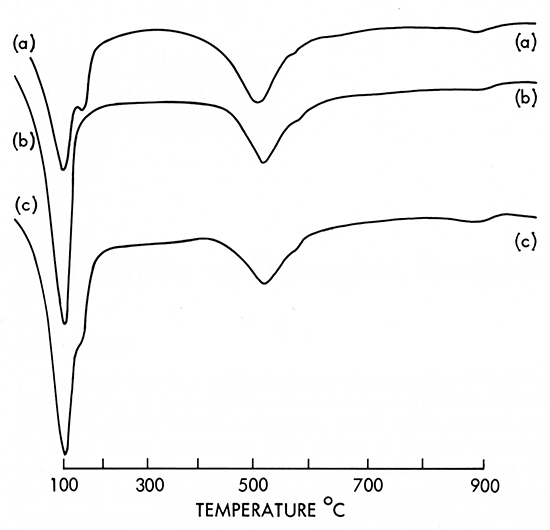
The only alteration in the sodium-saturation curve was the elimination of the second dehydration peak, producing the single-peak geometry typical of montmorillonite. An inflection point typical of calcium montmorillonite returned to the curve after resaturation with the calcium ions. There was no shift of the 525°C endothermic reaction peak.
Hill, R. D., 1953, Dehydration of clay minerals: Trans. British Ceramic Soc., v. 52, p. 589-613.
Ives, William, and Hill, W. E., Jr., 1960, Occurrence and bleaching properties of some Kansas montmorillonite clays: Kansas Ceol. Survey, Bull. 142, pt. 4, p. 149-188. [available online]
Mackenzie, R. C., 1964, The thermal investigation of soil clays; in, Soil clay mineralogy symposium, C. I. Rich and C. W. Kunze, eds.: Univ. North Carolina Press, Chapel Hill, N. C., p. 230-233.
Tien, Pei-lin, and Bauleke, M. P., 1970, A method of preparing pelleted clay samples of semi-micro quantity for differential thermal analysis: Clays and Clay Minerals, v. 18, no. 3, p. 179-181.
Kansas Geological Survey, Differential Thermal Analysis Study of Kansas Montmorillonitic Clays
Placed on web May 7, 2009; originally published in May 1971.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/202_1D/index.html