
Kansas Geological Survey, Bulletin 194, pt. 1, originally published in 1969

Originally published in 1969 as part of "Short Papers on Research in 1968," Kansas Geological Survey Bulletin 194, part 1, p. 9-11. This is, in general, the original text as published. The information has not been updated.
Several well-resolved major and minor bands are present in the far infrared spectra (500-30 cm-1) of the plagioclase feldspars. The major lattice mode vibrations appear to be diagnostic for the respective members of the plagioclase series; shifts of the absorption bands of certain vibrations appear related to the major element composition of the feldspars. A linear plot of the Na2O content versus wave number indicates a shift of the 190 cm-1 albite peak to 215 cm-1 in anorthite (i.e., as Na2O decreases, the absorption shifts to shorter wave lengths). A strong log correlation between the same peak and the Al/(Al + Si) ratio is also indicated. With a decrease in Al/(Al + Si) there is a shift toward longer wave lengths, which is expected from theory. The occurrence of major absorption bands within reasonably narrow wave-length limits helps in characterizing the spectra as a group.
Infrared spectra of plagioclase feldspars have been used for qualitative identification and to study the relation between absorption bands and chemical composition (Milkey, 1960; Thompson and Wadsworth, 1957, and others). These investigations, however, were limited to the intermediate (conventional) infrared region. The far infrared spectra of some of the more common minerals have been presented in only a few papers (Schaefer et al., 1934; Angino, 1964, 1967; Karr et al., 1967; Estep et al., 1968; Dorsey, 1968). The purpose of this paper is to present the far infrared spectra (70-340 cm-1) of some of the plagioclase feldspars. Owing to incomplete knowledge of the far infrared spectra of such complex inorganic materials as the silicates, a detailed discussion of these spectra is presently impossible. No attempt, therefore, is made to assign any of the absorption bands.
Major plagioclase absorption bands in the far region (70-400 cm-1) can be of four origins: (1) interval vibrations of a polyatomic ion; (2) torsional vibrations of water molecules in the sample, (3) sum and difference modes, and (4) lattice vibration modes. The latter possibility is the most likely origin for some of the major absorption bands observed.
The major absorption bands in this region are probably diagnostic for the specific minerals and it is possible that with further detailed studies shifts in the frequency of certain lattice-mode vibrations may be related to mass and radius differences of cationic substitution (calcium for sodium, etc.) through the plagioclase series. Such effects will surely be complicated in the plagioclases owing to their particular mineralogic phase problems.
The plagloclase specimens studied (Table 1) were obtained from Wards Scientific House. The far infrared spectra were obtained through the courtesy of Allan Budd and the Lincolnwood Laboratory of Beckman Instruments, Inc. All samples were examined by x-ray diffraction and optical methods to verify their identification. No good oligoclase sample was available. High temperature specimens were not used in the study.
Table 1--Far infrared absorption bands of plagioclase minerals.
| Mineral | Location | Absorption bands (cm-1) |
|---|---|---|
| 1 Albite | Bancroft, Ontario | 93W,* 116W, 165W, 187M, 252, 300W, 334W, 375W, 400W, 430W, 465W, 475, 500W, 590W |
| 3 Albite (variety cleavelandite) |
Keystone, South Dakota | 93W, 100S, 115S, 148S, 166W, 187M, 200W, 217W, 266S, 300W, 375S, 400S, 460S, 510W, 528W, 620W |
| 4 Andesine (in monzanite porphyry) |
Judith Basin, Montana | 89W, 101W, 144M, 170W, 181W, 200M, 244S, 285W, 311M, 320W, 384M, 479W, 540W, 575W, 622M |
| 5 Labradorite | Nain, Labrador | 85W, 89W, 95W, 138M, 163W, 182W, 210M, 234S, 290W, 317S, 390S, 470W, 626M |
| 6 Bytownite | Crystal Bay, Minnesota | 92W, 130W, 151W, 163W, 214S, 328W, 392M, 470W, 635M |
| 7 Anorthite | Miakejima, Japan | 93W, 173W, 217S, 265M, 336M, 376, 395, 465W, 540W |
| * W = Weak; M = Medium; S = Strong. | ||
Samples were prepared and run on a Beckman IR-11 spectrophotometer according to the methods described by Angino (1967). The resolution of the IR-11 over the range covered (33-800 cm-1) was better than 5 cm-1 and in most cases was between 2 and 3 cm-1. The chemical composition of the samples studied (Table 2) was obtained by atomic absorption spectrometry, using methods modified from those described by Angino and Billings (1967). Sodium and potassium concentrations were determined by standard flame photometric techniques.
Table 2--Chemical composition (percent) of plagloclase samples studied.
| Albite | Labradorite | Bytownite | Anorthite | |
|---|---|---|---|---|
| SiO2 | 63.56 | 54.85 | 48.83 | 43.92 |
| Al2O3 | 20.89 | 28.19 | 31.96 | 34.19 |
| Fe2O2* | 0.17 | 0.77 | 0.53 | 2.30 |
| TiO2 | 0.36 | 0.22 | 0.43 | 1.20 |
| CaO | 1.75 | 8.65 | 14.51 | 16.35 |
| MgO | 0.02 | 0.03 | 0.10 | 1.00 |
| K2O | 0.70 | 0.48 | Nil | Nil |
| Na2O | 11.47 | 6.12 | 2.62 | 0.53 |
| SrO | 0.22 | 0.02 | 0.06 | 0.06 |
| MnO | Nil | 0.02 | Nil | 0.02 |
| NiO | Nil | Nil | Nil | 0.04 |
| CaO | 0.07 | 0.07 | 0.05 | 0.10 |
| Li2O | Nil | Nil | Nil | Nil |
| LOI** | 1.18 | 0.38 | 0.66 | 0.05 |
| S | 0.02 | 0.04 | T | T |
| SO2 | 0.01 | 0.01 | T | 0.02 |
| Total | 100.38 | 99.81 | 99.75 | 99.78 |
| *Total Fe. ** LOI = Loss on ignition. T = Trace. |
||||
Representative spectra are shown in Figure 1. These illustrate the relative intensities of the various absorption bands whose wave number positions are listed in Table 1. The absorption peak at 72 cm-1 is due to the high-density polyethelene plates used in obtaining the spectra and appears on all the spectra. Peaks present in the range interval 340-800 cm-1 have been reported by Milkey (1960), Schaefer et al. (1934), and Thompson and Wadsworth (1957). To the best of my knowledge, no data for the range 70-340 cm-1 have been presented in the literature on the plagloclase feldspars.
Figure 1--Far infrared spectra of plagioclase feldspars. Identity of the numbered spectra is given in Table 1.
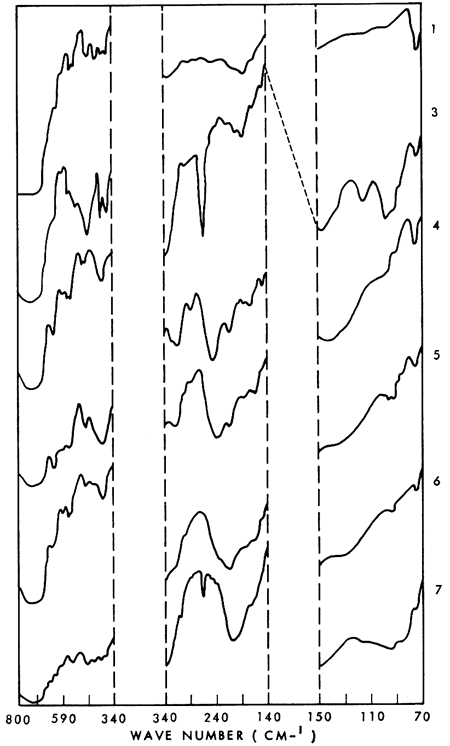
A linear plot (not shown) of the Na2O content versus wave number suggests a shift in the 190 cm-1 albite peak to 215 cm-1 in anorthite (i.e. as Na2O decreases, the absorption shifts to shorter wave lengths). Another trend for the same peak is indicated by a log plot of Al / (Al + Si) versus wave number. With a decrease in Al /(Al + Si) there is a shift toward longer wave lengths, which is expected from theory (Milkey, 1960). These trends do not include peaks resulting from additive combinations. To speculate at length on the significance and meaning (if any) of these trends would be premature, although it is possible that the Na2O shift is related to the substitution of the Ca ion for the Na ion in going from the albite to anorthite end of the plagioclase series.
Definite absorption bands for the plagioclase feldspars occur in the far infrared region, and it is possible that these spectra can be related to the chemical composition of each mineral. It is considered likely that the several well resolved major and minor absorption bands present in the far infrared spectra of the plagioclases are diagnostic for each mineral. These spectra obtained as part of another study are presented in the hope that they may stimulate additional studies of the far infrared absorption phenomena of the plagioclase feldspars and thereby extend the usefulness of the infrared method which is becoming commonplace in mineralogical studies.
Angino, E. E., 1964, Far infrared spectra of montmorillonite, kaolinite and illite: Nature, v. 204, no. 4958, p. 569-571.
Angino, E. E., 1967, Far infrared (500-30 cm-1) spectra of some carbonate minerals: Am. Mineral., v. 52, p. 137-148.
Angino, E. E., and Billings, G. K., 1967, Atomic absorption spectrometry in geology: Elsevier Publishing Co., Amsterdam, The Netherlands, 154 p.
Dorsey, G. A., Jr., 1968, Far infrared absorption of hydrous and anhydrous aluminas: Analytical Chem., v. 40, p. 971-972.
Estep, P. A., Kovach, J. J., and Karr, Clarence, Jr., 1968, Quantitative infrared multicomponent determination of minerals occurring in coal: Analytical Chem., v. 40, p. 358-363.
Karr, Clarence, Jr., Estep, P. A., and Kovach, J. J., 1967, Low-frequency infrared region aids coal minerals research: Instrument News (Perkin-Elmer Corp.), v. 18, no. 2, p. 1.
Milkey, R. G., 1960, Infrared spectra of some tectosilicates: Am. Mineral., v. 45, p. 990-1007.
Schaefer, C., Matossi, F. and Wirtz, K., 1934, Das ultrarote Reflexionsspektrum von Silikaten: Zeits. Physik., Band 89, p. 210-233.
Thompson, C. S., and Wadsworth, M. E., 1957, Determination of the composition of plagioclase feldspars by means of infrared spectroscopy: Am. Mineral., v. 42, p. 334-341.
Kansas Geological Survey, Short Papers on Research in 1968
Placed on web July 26, 2011; originally published in Feb. 1969.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/194_1B/index.html