
Kansas Geological Survey, Bulletin 191, pt. 1, originally published in 1968

Originally published in 1968 as part of "Short Papers on Research in 1967," Kansas Geological Survey Bulletin 191, part 1, p. 13-15. This is, in general, the original text as published. The information has not been updated.
Thermal expansion data are presented for eight siliceous clays from the Dakota Formation in Kansas. In clays that contain high percentages of free quartz, the negative thermal expansion behavior of the β-quartz exerts a controlling influence on the thermal expansion of the fired clay.
Difficulties were found in glazing test bricks made from siliceous clays from the Dakota Formation in a one-fire glazing operation requiring a temperature of 2300°F (cone 9). The matured glaze would pop off curved edges and even come loose from flat surfaces. Only a large mismatch in the thermal expansion of the glaze and the fired clay could cause such a condition. It was decided to measure the thermal expansion of eight selected clays from the Dakota Formation (Table 1).
X-ray diffraction methods identified the major clay mineral as kaolinite and the major impurity as quartz (SiO2) in each clay sample. Thermal expansion is defined as the ΔL/L per degree temperature change. ΔL is the change in length per degree of temperature change. L is the original length. Units of thermal expansion are in/in/°F or cm/cm/°C or percent length change. Percent length change is preferred as it does not require a conversion factor in order to compare data measured by two different temperature scales. All data in this study are reported as percent change of length.Table 1--Chemical analyses of clays from the Dakota Formation.
| Code | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | L.O.I. |
|---|---|---|---|---|---|---|---|---|---|
| C-52 | 70.15 | 19.35 | 1.46 | 1.22 | 0.22 | 0.06 | 0.75 | 0.09 | 6.57 |
| C-53 | 69.05 | 20.09 | 1.67 | 1.00 | 0.30 | 0.16 | 0.92 | 0.07 | 6.68 |
| C-77-B | 77.55 | 14.99 | 1.59 | 1.48 | 0.18 | 0.14 | 0.61 | 0.04 | 5.30 |
| C-77-C | 72.42 | 15.93 | 3.56 | 1.30 | 0.20 | 0.16 | 0.80 | 0.06 | 5.44 |
| El-60-6 | 73.26 | 17.86 | 0.65 | 1.25 | 0.14 | 0.15 | 0.06 | 0.05 | 6.48 |
| O-5-6 | 62.98 | 23.15 | 3.05 | 1.35 | 0.30 | 0.16 | 0.92 | 0.11 | 7.94 |
| O-40-3 | 76.43 | 14.37 | 1.38 | 1.57 | 0.26 | 0.22 | 0.58 | 0.14 | 4.83 |
| El-TC-2-10 | 76.71 | 13.82 | 1.43 | 1.27 | 0.30 | 0.29 | 0.93 | 0.09 | 4.84 |
| theoretical kaolinite |
45.5 | 39.6 | 13.9 | ||||||
| Analyses were done by the Geochemistry section of the State Geological Survey of Kansas. C--Cloud County; El-Ellsworth County; O-Ottawa County. |
|||||||||
Eight clays from the Dakota Formation of central Kansas were extruded into 1/2-inch rods, dried, and fired to appropriate temperatures. Numerous 2-inch test specimens were cut. Expansion data were taken using a Gaertner Scientific Corporation quartz-tube dilatometer model D-1200. A specimen was placed in a fused quartz tube surrounded by a heater and heated at 5°C per minute. The expansion of the specimen was transferred outside the hot zone by a fused quartz rod and was measured by a dial gauge to the nearest 0.0001 inch. No correction was made for the slight expansion of the quartz rod.
A thermal expansion measurement was considered satisfactory if the dial gauge returned to the original zero point setting after the measured specimen had cooled to room temperature. If the dial did not return to zero, the specimen was remounted and the test repeated.
An average thermal expansion value for a wide temperature range is convenient for structural design calculations but is of little use in designing a thermal expansion match for glazes or other coatings. The presence of any crystallographic inversions are obscured in averaged data unless care is used in selecting the averaging range. It is usually desirable to report thermal expansion data graphically for the entire curve or as average values for several temperature increments so that any nonlinearity of the expansion behavior may be detected. Because the shape of the thermal expansion curve is similar for all the clays, only one sample is presented in Figure 1. A thermal expansion curve for Kentucky kaolinite is shown for comparison. In Table 2 the data are divided into two sections, one below 1157°F (625°C) and the other above. The lower temperature range reflects the effects of the quartz (α-β) inversion reaction. The higher temperature range reflects the effects of the negative thermal expansion of the β-quartz phase. Note the low values for the thermal expansion.
Figure 1--Typical thermal expansion curve of a siliceous clay from the Dakota Formation compared with a sample of kaolin from Kentucky.
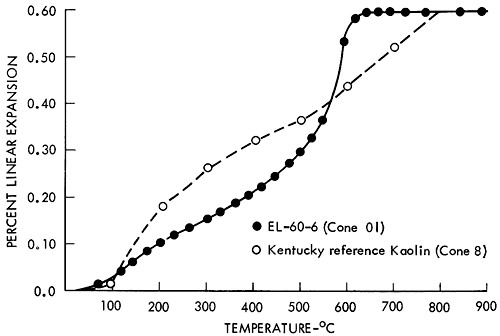
Table 2--Percent linear thermal expansion of fired siliceous clays from the Dakota Formation in Kansas.
| Clay code no. |
1118°C (2043°F) Cone 01 |
1200°C (2300°F) Cone 9 |
||
|---|---|---|---|---|
| (625°C) RT to 1157°F* |
(625-950°C) 1157-1742°F |
(625°C) RT to 1157°F* |
(625-950°C) 1157-1742°F |
|
| C-52 | 0.37 | 0.07 | 0.43 | 0.11 |
| C-53 | 0.37 | 0.04 | 0.57 | 0.06 |
| C-77-B | 0.45 | 0.04 | 0.56 | 0.07 |
| C-77-C | 0.48 | 0.03 | 0.56 | 0.06 |
| EI-60-6 | 0.59 | 0.01 | 0.56 | 0.02 |
| 0-5-6 | 0.32 | 0.12 | 0.51 | 0.13 |
| 0-40-3 | 0.53 | 0.05 | 0.53 | 0.13 |
| El-TC-2-10 | 0.44 | 0.03 | 0.63 | 0.06 |
| Kentucky kaolin | 0.46 | 0.20 | ||
| * RT = room temperature. | ||||
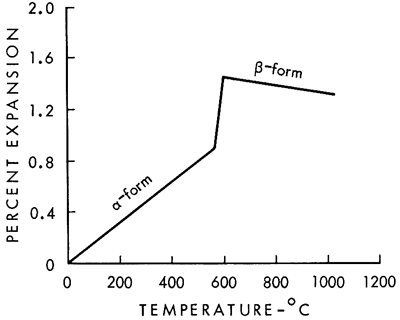
The thermal expansion curve clearly shows why there was trouble with a high-temperature glaze adhering to the fired siliceous clays from the Dakota Formation. The problem was caused by excessive free quartz. Quartz is an unusual mineral in that it undergoes a change in crystal structure at 575°C. A change in the size of the quartz grain accompanies the change in crystal structure, as shown in the thermal expansion curve for quartz (Fig. 2). But what is more significant is that the new β-quartz structure has a negative thermal expansion; that is, it shrinks as the temperature increases. X-ray diffraction analysis of the clay after firing showed that a large amount of free quartz was present. The combined expansion of the kaolinitic clay mineral and the shrinkage of the β-quartz mineral above 625°C produce a net result that is almost flat on the thermal expansion curve.
Figure 2--Thermal expansion of quartz (after Searle and Grimshaw, 1959, p. 723).
It is impossible to apply a glaze to such a material on which the glaze becomes rigidly attached to the clay at temperatures above 650°C. The severe mismatch caused by the shrinking of the glaze and the nonshrinking of the clay can be severe enough to cause the glaze to come loose from the fired clay.
Only a low-temperature glaze, one that is still plastic or slightly fluid at temperatures above 625°C, should be used on such clays. The expansion curve below 625°C can easily be matched to that of a glaze.
The thermal expansion behavior of fired siliceous clays from the Dakota Formation is influenced by the amount of free quartz present. At temperatures above the inversion of α- to β-quartz, the overall rate of increase in thermal expansion is small compared with the rate of expansion below the inversion temperature. Two distinct thermal expansion regions exist. A glaze designed for matching the expansion of one region will be completely unsatisfactory for the other region.
Searle, A. B., and Grimshaw, R. W., 1959, The chemistry and physics of clays: Interscience Publishers, Inc., New York, 942 p.
Kansas Geological Survey, Short Papers on Research in 1967
Placed on web Aug. 16, 2011; originally published in April 1968.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/191_1C/index.html