
Kansas Geological Survey, Bulletin 187, part 4, originally published in 1967

Originally published in 1967 as Kansas Geological Survey Bulletin 187, part 4. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (4 MB) is also available.
Increased demands for phosphate plant nutrients gives impetus to studying the feasibility of extracting the phosphate-bearing nodules from Kansas shales. Preliminary tests on Cabaniss and Pleasanton shales include crushing and screening, weathering, calcining and screening, froth flotation and heavy media separation. Results of tests suggest that roasting plus heavy media separation would be a practical method for separating phosphate rock from the shale. U.S. consumption of phosphate rock is mostly in fertilizers with use in the West North-Central States having a growth rate of over 41 percent per year since 1945.
The expanding use of and changes in fertilizers in the United States promises to keep the agri-mineral industry agitated for years to come. Consider that: from 1950 to 1960 U.S. consumption of major plant nutrients (N, P2O5, K2O) has nearly doubled; in specific geographical areas, the rate of fertilizer use has risen even faster. In the corn-growing West North-Central States annual consumption of the three plant nutrients leaped to almost 1.2 million tons in 1960 up from some 312,000 tons in 1950. Over the same period, tonnage of end-product mixed fertilizer rose from 666,200 tons per year to 1,562,600 tons per year.
In view of this growth in fertilizer use, the development of the phosphate-bearing shales of Kansas was investigated as a source of nutrients. Phosphate occurs in nodules that are scattered profusely throughout the shales containing them. These nodules can occur as spheres ranging in size from 3/4 inch down to 3/16 inch in diameter, or as "pancakes" an inch or more across and up to 1/4 inch in thickness. The shales in which these nodules occur contain considerable organic matter and might be classified as oil shales. In fact, as much as 9 gallons of oil per ton have been removed from some samples.
It is difficult to recognize the phosphate concretions in freshly mined shale, but after a short exposure the shales begin to air slake and color differences show up, making selection of unattached nodules possible.
Early work performed by Runnels on a number of shales (Table 1) gave the results that are shown in Table 2. [Runnels, R. T., 1949, Preliminary report on phosphate-bearing shales in eastern Kansas: Kansas Geol. Survey, Bull. 82, pt. 2, p. 42, available online] The P2O5 content of the shales averaged 2-3 percent. The nodules alone, from the shale, had P2O5 contents of 28-35 percent (Tables 3 and 4). However, attempts to concentrate the phosphate nodules in shales occurring in Kansas have not been successful up to the present.
Table 1--Localities where phosphate-bearing shales were sampled. (From Runnels, R. T., 1949, Bull. 82, Pt. 2, State Geol. Survey of Kansas, p. 42, available online.)
| Lab. No. | County | Location | Stratigraphic horizon |
Thickness of bed, ft. |
Type of sample |
||
|---|---|---|---|---|---|---|---|
| Sec. | T. | R. | |||||
| 48-98 | Wyandotte | 12 | 11 | 24E | Muncie Creek shale | 3 | Composite upper part |
| 48-99 | Wyandotte | 12 | 11 | 24E | Quivira shale | 3 | Lower half--composite |
| 48-219 | Labette | SE 17 | 32 | 19E | Pleasanton shale | 29 | Spot 15 ft from top |
| 48-220 | Labette | NE 16 | 33 | 21E | Little Anna shale | 2 | Composite |
| 48-224 | Labette | SE 17 | 32 | 19E | Pleasanton shale | 29 | Top 6 ft of 12 ft 18 ft from top |
| 48-295 Stark Quarry |
Crawford | SE 12 | 30 | 22E | Little Osage | Composite | |
| 48-296 | Crawford | SW SW 34 | 38 | 25E | Shale above Croweburg coal | 4 | Composite |
| 48-297 | Bourbon | SW SE 18 | 27 | 25E | Shale above Bevier coal | 6 | Composite |
| 48-298 | Bourbon | NE NW 27 | 26 | 25E | Shale above Mulky coal | 2 | Composite |
| 48-311 Ross Quarry |
Franklin | SW SW 6 | 17 | 19E | Eudora shale | 8 | Composite |
| 49-173 | Labette | SE 17 | 32 | 19E | Pleasanton shale | 29 | 16 in below 48-224 |
| 49-174 | Labette | SE 17 | 32 | 19E | Pleasanton shale | 29 | 10 in below 49-173 |
Table 2--Chemical analyses of samples. (From Runnels, R. T., 1949, Bull. 82, Pt. 2, State Geol. Survey of Kansas, p. 44, available online,] )
| Element | Sample No. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 48-98 | 48-99 | 48-219 | 48-220 | 48-224 | 48-295 | 48-296 | 48-297 | 48-298 | 48-311 | |
| Silicon dioxide | 48.78 | 61.21 | 55.97 | 47.79 | 52.96 | 44.13 | 45.58 | 51.03 | 53.41 | 53.61 |
| Alumina (Al2O3) | 14.54 | 18.91 | 15.72 | 14.44 | 12.43 | 13.79 | 14.10 | 21.63 | 11.65 | 16.86 |
| Iron oxide (Fe2O3) | 5.40 | 3.98 | 4.30 | 3.37 | 4.19 | 4.29 | 4.30 | 7.61 | 5.44 | 4.66 |
| Titanium oxide (TiO2)* | 1.03 | 0.86 | 1.18 | 1.00 | 0.85 | 0.29 | 0.29 | 1.20 | 1.96 | 1.12 |
| Calcium oxide (CaO) | 2.31 | 2.50 | 6.19 | 3.03 | 4.29 | 5.06 | 5.46 | 2.44 | 4.33 | 3.32 |
| Magnesium oxide (MgO) | 2.65 | 2.19 | 1.74 | 1.64 | 1.55 | 1.87 | 0.99 | 2.25 | 1.83 | 2.41 |
| Phosphorus pentoxide (P2O5) | 0.92 | 1.65 | 0.09 | 1.29 | 3.20 | 2.08 | 2.24 | Trace | 2.30 | 0.86 |
| Sulfur trioxide (SO3) | 0.49 | 0.13 | 0.96 | 0.39 | 0.35 | 1.24 | Trace | 0.84 | 0.12 | 1.95 |
| Potassium oxide (K2O) | N.D. | 3.87 | N.D. | N.D. | 2.07 | N.D. | N.D. | N.D. | N.D. | 3.46 |
| Sodium oxide (Na2O) | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Total nitrogen (N) | N.D. | N.D. | N.D. | N.D. | 0.20 | N.D. | N.D. | N.D. | N.D. | 0.10 |
| Loss on ignition at 1000° C | 20.12 | 5.46 | 10.74 | 21.42 | 18.00 | 20.22 | 21.77 | 8.45 | 16.10 | 11.41 |
| Dried at 140° C | ||||||||||
| Obtained by gravimetric method used by Runnels and Dubbins (1949, p. 16). | ||||||||||
Table 3--Chemical analyses of phosphate nodules. (Modified from Runnels, R. T., 1953, Bull. 102, Pt. 3, State Geol. Survey of Kansas, p. 99.)
| Sample code no. | 5023 | 50546 | 50547 | 50548 | 50549 |
|---|---|---|---|---|---|
| Silica (SiO2) | 10.79 | 10.37 | 6.93 | 8.86 | 4.07 |
| Alumina (Al2O3) | 6.18 | 6.03 | 2.64 | 2.59 | |
| Iron oxide (Fe2)3) | 1.04 | 1.77 | 2.38 | 2.47 | |
| Titanium oxide (TiO2) | 1.72 | 0.37 | 0.54 | 0.43 | 0.37 |
| Calcium oxide (CaO) | 44.27 | 44.12 | 44.71 | 43.74 | 47.10 |
| Magnesium oxide (MgO) | 0.81 | 0.08 | 0.32 | 0.20 | 0.11 |
| Phosphorus pentoxide (P2O5) | 28.23 | 29.01 | 30.88 | 30.44 | 34.48 |
| Sulfur trioxide (SO3) | nil | 0.13 | 0.15 | 0.12 | nil |
| Potassium oxide (K2O) | 0.03 | 0.31 | 0.03 | 0.03 | |
| Sodium oxide (Na2O) | 0.05 | 0.21 | 0.04 | 0.05 | |
| Uranium oxide (U3O8) | 0.007 | 0.010 | 0.020 | ||
| Fluorine (F) | 3.51 | 2.99 | 3.40 | 3.43 | 3.62 |
| Sulfur (S) | 0.21 | 0.09 | 0.63 | 0.70 | |
| Loss on ignition at 1000°C | 7.19 | 9.71 | 11.14 | 10.39 | 6.81 |
| Total | 99.75 | 99.34 | 99.65 | 99.72 | 99.13 |
Table 4--Location and description of phosphate nodules. (Modified from Runnels, R. T.,1953, Bull. 102, Pt. 3, State Geol. Survey of Kansas, p. 97.)
| Code no. | County | Location Sec., T, R |
Stratigraphic horizon |
Shale bed, thickness |
Type of sample | Topographic position |
|---|---|---|---|---|---|---|
| 5023 | Linn | 8, T. 22 S., R. 24 E. | Lake Neosho shale | ±3 ft | composite | highly weathered railway cut |
| 50546 | Labette | 2, T. 35 S, R. 20 E. | Mulky coal | ±3 ft | composite | |
| 50547 | Labette | 9, T. 34 S., R. 20 E. | Little Osage shale | ±4 ft | composite | outcrop |
| 50548 | Crawford | 30, T. 29 S., R. 21 E. | Lake Neosho shale | composite | outcrop | |
| 50549 | Labette | 3, T. 33 S., R. 20 E. | Little Anna shale |
Although Runnels' work indicated that raw shale might have some value as a source of trace minerals and small amounts of phosphorus when used directly as fertilizer, it seemed imperative that some way be found to enrich the phosphate content if the shales are to be commercially attractive. In such situations, the cost of concentrating a low-grade material must be compared with the savings effected in bagging and transportation.
The tendency for shales to fracture as thin platelets, in contrast to the more compact, equiaxis nature of the nodules, suggested that it might be possible to crush the raw shale to some optimum degree, then sift the chunky phosphate through screens, leaving the shale behind. While some encouraging results were obtained on shale which had been aged and air dried for several days, there was little evidence that the process would be successful on freshly quarried shale. The plate-cleavage tendency of the shales is not strong in the fresh shales; neither is there any strong tendency for the nodules to separate from the shale. Fresh shale, when crushed in a jaw crusher or a gyrating crusher, appeared to be a mixture of blocky particles of shale and nodules. A further study of the phosphate distribution in crushed shale was carried out by crushing a sample of shale until it passed a 1-inch-mesh screen, then sizing this product and analyzing the various size fractions for P2O5. The results of this test on the Cabaniss shale appear in Table 5.
Table 5--P2O5 distribution in various size-fractions of minus 1-inch Cabaniss shale.
| Size | Weight | P2O5 content | ||
|---|---|---|---|---|
| Mesh | Grams | Percent of total | This fraction | Percent of total P2O5 |
| -1" + 1/2" | 4675 | 39.5 | 4.7 | 47.6 |
| -1/2" + 1/4" | 3145 | 26.6 | 4.3 | 29.2 |
| -1/4"+ 6 | 1220 | 10.3 | 2.8 | 7.4 |
| -6 + 10 | 1045 | 8.8 | 2.6 | 5.9 |
| -10 + 30 | 866 | 7.3 | 2.4 | 4.6 |
| -30 + 50 | 232 | 2.0 | 2.3 | 1.1 |
| -50 + 100 | 329 | 2.8 | 2.6 | 2.0 |
| -100 + 200 | 167 | 1.4 | 2.9 | 1.1 |
| -200 | 154 | 1.3 | 3.2 | 1.1 |
| Calculated analysis of composite sample--3.9% P2O5 | ||||
These data seem to confirm the earlier observation that there is a natural tendency for the phosphate to concentrate in the larger particle sizes. A similar pattern was found upon repeating the test with Pleasanton shale.
The natural concentration of phosphatic nodules near an outcrop shows that weathering separates them from the shale. Several tests were made to investigate this.
Ten-pound (4540-gram) samples of fresh Pleasanton and Cabaniss shale were placed in large pans and exposed to July sunshine. The sample of Pleasanton shale is identified by lab number 48-224 in Table 1. The second sample (Cabaniss) is from the black fissile shale overlying the Croweburg coal bed and identified by lab number 48-296 of Table 1. It is a shale in the Cabaniss Formation. Both air-slaked considerably during three day's exposure, and a heavy rain at the end of the third day further assisted in the disintegration of the shale. After six days of weathering, 95 grams of nodules were picked from the Pleasanton sample and 48 grams from the Cabaniss. The Pleasanton shale appeared to be well freed of nodules and the remaining lumps of shale crumbled easily. Hard concretions of nodules and shale still remained in the Cabaniss shale.
After ten days, the samples were repicked. The total weights of nodules recovered from weathering and the analysis of the concentrates were:
| Sample | Weight of nodules recovered |
Weight percent removed |
P2O5 in nodules, % |
Total P2O5 recovered in nodules, % |
|---|---|---|---|---|
| Pleasanton Shale | 110 g | 2.4 | 11.0 | 26.7 |
| Cabaniss Formation | 99 g | 2.0 | 20.5 | 16.4 |
The weathered material remaining after all visible nodules had been removed was screened on a 1/4-inch-mesh screen. In both samples approximately two-thirds of the material passed through the screen. The material on the 1/4-inch-mesh screen (oversize) from the Pleasanton shale assayed 1.21 percent P2O5, the material passing the 1/4-inch-mesh screen (undersize) only 0.48 percent. In the Cabaniss sample, the oversize contained 3.0 percent P2O5, as compared to 1.46 percent in the undersize. Thus, it would appear that the material richer in phosphate is more resistant to weathering, but only a relatively small percentage of the phosphatic material is recoverable as visibly recognizable nodules.
In a second weathering test employing samples of approximately 19 pounds, raw shale was spread in a thin layer outdoors for 10 days, dried at 100° C, and screened on a 1/4-inch-mesh screen. Slightly less than half of the material passed through the screen. Weights and analyses for this test were:
| Sample | Passing 1/4-inch screen | Retained on 1/4-inch screen | ||
|---|---|---|---|---|
| Weight | P2O5, % | Weight | P2O5, % | |
| Pleasanton shale | 3800 | 0.34 | 4330 | 0.93* |
| Cabaniss shale | 3750 | 1.53 | 4790 | 4.5* |
| * Analysis calculated from P2O5 in original samples | ||||
This indicates an upgrading in the oversize material of approximately 50 percent above the P2O5 analysis of the original samples, and recoveries of slightly more than three-fourths of the P2O5 in the shale. Screening at sizes other than 1/4-inch would undoubtedly affect the results, but no tests were made to investigate this factor.
A third test combined the effects of weathering with mild abrasion. Twenty-pound samples of each shale were placed in the main traffic way of a parking lot so that the material was worked by the tires of passing automobiles. After one day of exposure, both shales were pretty well disintegrated, although there were still a few lumps of phosphatic aggregates in the Cabaniss shale sample. Although the hand-picked nodules were of somewhat higher grade (29 percent P2O5 for the Cabaniss and 28 percent for the Pleasanton) the yield was less than one-fifth of the total phosphate in the original sample.
The first and third tests may be taken to represent an optimum situation in which all visible nodules are hand-picked. The second test represents a typical commercial sizing operation. It appears that mechanical sizing not only is simpler from an operating standpoint, but is more effective in recovering the phosphatic material as well. However, the product is still low-grade compared with commercial phosphates.
The effects of natural weathering on the shales suggest that artificial weathering by drying and heating might accelerate decomposition of the shale. There are three temperature ranges which could assist in breaking down the shale structure: (1) Simple drying, in the vicinity of 212° F to drive off natural moisture; (2) Dehydration at 1000 to 1200° F to decompose the chemically combined water of hydration; and (3) Calcination and decarbonization at temperatures of 1500° F and higher. Weighed samples were placed in ovens and furnaces held at predetermined temperatures for several hours. The weight losses resulting from this treatment were:
| Sample | Weight loss (percent) upon heating to | |||
|---|---|---|---|---|
| 215° F | 1000° F | 1500° F | 1900° F | |
| Pleasanton shale | 2.1 | 17 | 21 | 21.2 |
| Cabaniss shale | 4.7 | 13 | 23 | 23.0 |
Oven drying had little effect on either shale except to induce fracturing in the larger pieces. At 1000° F, the larger lumps decrepitated and shattered into platelets. When air reached the hot shale there was some burning. The outside surfaces which had been burned were red or gray and easily pulverized, but the centers were still hard and black. Nodules were not easily recognized in either shale. Both samples burned vigorously with a yellow flame when heated to 1500° F was screened on a 1/4-inch-mesh screen, easily identified. Thin platelets of shale were very friable, but lumps of unburned material remained, particularly in the sample of Cabaniss shale. The nodules were quite hard and tough compared to the burned shale. Neither sample showed evidence of fusion after being heated to 1900° F.
The material which had been heated to 1500° F was screened on a 1/4-inch-mesh screen, and analyses were made on the oversize and undersize portions. No significant difference in P2O5 content was found between the coarser and the finer particles, the oversize being approximately 0.3 percent richer than the undersize. Some up-grading resulted from the reduction in weight of the sample, owing to the elimination of volatile or combustible constituents.
Another set of samples was heated in a gas-fired rotary kiln to see if agitation of the material during heating would be beneficial. After the shale cooled it was screened on 1/4-inch-mesh screen and the fractions were analyzed for P2O5. In each sample approximately 60 percent of the material was finer than the screen openings. Aggregates of nodules were still present in the Cabaniss sample, and unburned particles were abundant. Analyses of these samples were:
| Sample | Percent P2O5 | |||
|---|---|---|---|---|
| Through 1/4" mesh screen |
Retained on 1/4" mesh screen |
|||
| Pleasanton shale | 0.46 | 5.66 | ||
| Cabaniss shale | 2.06 | 7.02 | ||
These figures show that the Pleasanton shale is affected more by heating than is the Cabaniss shale, but heating and screening does not appear to be an effective method of concentrating the phosphates.
The heating tests previously described showed that (1) neither shale had much of a tendency to fuse and stick together upon being heated, and (2) both shales contain enough organic material to be combustible. A down-draft sintering hearth was prepared for autogenous roasting tests.
The shale was crushed in a jaw crusher set for 1-inch discharge and was used without further sizing. A bed of shale 3 inches deep was placed over the grate of the hearth, this was covered with a layer of wood and paper which, when lighted, served to ignite the shale. The draft was held to a minimum value which would maintain combustion. The shale decrepitated violently and as combustion proceeded, the accumulation of fines blocked the flow of air through the bed, but a considerable amount of the shale was burned satisfactorily. A porous sinter-cake was produced, but it could be easily broken for screening. The heat did not appear to affect the nodules adversely.
In one modification of this procedure, fine coal was added to the shale at a rate of 50 pounds of coal per ton of shale to provide more heat. In another modification, the shale was crushed to a smaller size before firing. After the sinter cake cooled, it was removed, broken, and screened on 1/4-inch-mesh screen. The results are shown in Table 6.
Table 6--Results of sinter-roasting and screening on Pleasanton and Cabaniss shales.
| Percent of P2O5 in product | ||
|---|---|---|
| Pleasanton | Cabaniss | |
| Shale crushed to 1 inch burned and screened | ||
| Passing 1/4"-mesh | 0.94 | 2.59 |
| Retained on 1/4"-mesh | 5.54 | 8.74 |
| Same as above with 50#/ton of coal | ||
| Passing 1/4"-mesh | 0.95 | |
| Retained on 1/4"-mesh | 6.27 | |
| Crushed to 3/4", burned and screened | ||
| Passing 1/4"-mesh | 1.55 | |
| Retained on 1/4"-mesh | 2.61 | |
| Crushed to 1/4", burned and screened | ||
| Passing 1/4"-mesh | 1.81 | |
| Retained on 1/4"-mesh | 2.51 | |
These data suggest that a coarse feed is much more effective, possibly because the phosphate nodules are retained and the waste shale breaks into fine particles.
Different species of finely ground minerals can often be separated from one another by employing reagents which alter the surface of the minerals and affect the wetability of the various constituents when pulped with water. Separation is effected by inducing certain constituents to become attached to air bubbles which rise to the surface of the pulp and are removed (froth flotation) or by agglomerating certain constituents into curdlike "rafts" which are swept away from the other minerals in a flowing film of water (agglomeration tabling). Under ideal conditions, either process can be highly effective.
Earlier attempts to recover the phosphatic material from crude shales by froth flotation were in vain. It was believed that the organic material in the shale nullified the selective action of the fuel oil used as a reagent; therefore, subsequent tests utilized shale which had been burned to remove as much of the organic material as possible.
The procedure for flotation tests followed one devised by Fine and Frommer for beneficiating Brazilian phosphate rock. [Fine, M. M., and Frommer, D. W., 1954, Beneficiation of a Brazilian Phosphate Rock: Research Invest. 5078, U.S. Bureau Mines, 9 p.] Shale was burned on the sintering hearth, then crushed and screened on 10-mesh, 40-mesh, and 200-mesh screens. Samples of 48- to 200-mesh shale were blunged for 10 minutes with enough water to make a pulp of 65 percent solids. The scrubbed particles were washed in water and screened again to remove slimes (200-mesh). The pulp was then stirred for 10 minutes with tall oil which was added in the proportion of 3.3 pounds of tall oil per ton of shale. At the end of the conditioning period air was introduced to produce a froth and begin flotation. Two products were saved for analysis: a concentrate from the froth, and tailings from the material remaining in the pulp. Results of the flotation tests appear in Table 7.
Table 7--Results of froth flotation tests on Pleasanton and Cabaniss shales.
| Type of Sample | Pleasanton | Cabaniss |
|---|---|---|
| Concentrate, % P2O5 | 16.01 | 26.01 |
| Tailings, % P2O5 | 0.08 | 3.86 |
| Concentration ratio, feed: concentrate |
15.4 to 1 | 7.8 to 1 |
| P2O5 recovery in concentrate, % |
94* | 59* |
| *This figure does not allow for P2O5 in slimes, but the amount was small. | ||
The procedure for agglomeration tabling also followed the one employed by Fine and Frommer. Slime-free 10- to 48-mesh burned shale was blunged at 70 percent solids in tap water with 2 pounds of tall oil soap (American Cyanamid Reagent 608) and 4 pounds of fuel oil per ton of shale. Blunging was continued until the pulp showed some tendency to stick together. The pulp was then washed into a beaker and was violently agitated by a small stream of water. The film which formed on the surface of the pulp was removed by decantation. This procedure was repeated until filming became negligible. The rough concentrate which was removed in the film was re-pulped and cleaned again by the same procedure. The products collected were tailings left from the first operation, finished concentrate from the reclaimed rough concentrate, and middlings, which were the equivalent of tailings from the reclaiming operation. These were recovered, dried, and analyzed for P2O5 content. The results are tabulated in Table 8.
Table 8--Results of agglomeration tabling of Pleasanton and Cabaniss shales.
| Pleasanton shale, 80-gram sample | |||
|---|---|---|---|
| Product | Weight, percent | P2O5 percent | Distribution of P2O5 percent |
| Concentrate | 8.2 | 6.47 | 53.5 |
| Middling | 33.8 | 0.96 | 32.5 |
| Tailing | 58.5 | 0.24 | 14.0 |
| Composite | 100.0 | 0.94 | 100.0 |
| Cabaniss shale, 100-gram sample | |||
| Product | Weight, percent | P2O5 percent | Distribution of P2O5 percent |
| Concentrate | 4.9 | 23.5 | 41.2 |
| Middling | 4.4 | 6.00 | 9.5 |
| Tailing | 80.7 | 1.71 | 49.4 |
| Composite | 100.0 | 2.8 | 100.1 |
The results of these tests indicate that froth flotation gives somewhat better recovery than agglomeration tabling. Neither method produced a satisfactory grade, i.e., 30 percent or more P2O5. Unfortunately time did not permit further testing, but the results offer encouragement. It is believed that further improvements and refinements are quite feasible.
Heavy-media concentration or "sink-float" utilizes differences in specific gravity to separate one mineral species from another in a fluid medium whose specific gravity lies between that of the two mineral species. For example, it would be a simple matter to separate sand from sawdust by stirring the mixture in water, whereupon the sand will sink and the sawdust will float. When both mineral species are heavier than water, some other medium must be used or the water must be densified by adding enough finely ground heavy material to make a quicksand or a slurry. Ferrosilicon or magnetite are often used because of their low cost, ease of grinding, and ease of removal from the minerals being separated.
Several tests were made with crushed crude shale, using a small laboratory cone-type heavy media concentrator with a slurry of water and ferrosilicon. In order to facilitate control of specific gravity in the medium (a difficult problem in small concentrating units), all material finer than 10 mesh was removed from the shale samples, which had previously been crushed to pass a 3/8-inch screen.
In making the tests, the air lifts and stirrer were started and adjusted to maintain the ferrosilicon in suspension, then the specific gravity was adjusted to the desired value by adding water or ferrosilicon as needed. Specific gravity was determined by means of a special hydrometer which floated in the medium. There was an appreciable difference in specific gravity from top to bottom despite efforts to minimize variations, and stoppages on the discharge screens sometimes held back enough ferrosilicon to lower the specific gravity, but generally there was no more than plus or minus 0.1 unit from the intended value during a test run.
The crushed shale was fed slowly and uniformly by means of a vibrating feeder. The sink products were conveyed from the bottom of the cone drainage screen by means of an air lift; the float product overflowed to a second drainage screen. When the feed was gone and the system appeared to have reached equilibrium, the test was stopped. The sink and float fractions were washed, dried, weighed and analyzed for P2O5 content. Some of the shale was always retained in the suspension, unable to sink or to float because of the vertical differences in specific gravity of the medium; this material was screened out and discarded.
Results of the tests in the heavy media concentrator appear in Table 9.
Although the recovery of phosphate is fairly good, especially with the Pleasanton shale, the grade is only about half the value desired.
Table 9--Results of tests in heavy media separator.
| Sample | Specific gravity of medium |
Weight, g |
Weight, % |
P2O5,% separated |
Percent of total P2O5 possible |
|---|---|---|---|---|---|
| Pleasanton shale | |||||
| Test 1. | 2.2 | ||||
| Sink | 173 | 14.85 | |||
| Float | 324 | 1.48 | |||
| Test 2. | 2.2-2.3 | 500 | 100 | ||
| Sink | 166 | 33 | 13.73 | 70. | |
| Float | 170 | 34 | 1.99 | 10. | |
| Test 3. | 2.1-2.3 | 500 | 100 | ||
| Sink | 152 | 16.97 | 80. | ||
| Float | 289 | 0.20 | 2. | ||
| Test 4--250 g Pleasanton nodules 250 g Pleasanton shale (barren) |
|||||
| Sink | 145 | 29 | 31.90 | 58. | |
| Float | 290 | 58 | 9.20 | 33. | |
| Cabaniss shale | |||||
| Test 5. | 2.2-2.3 | 500 | |||
| Sink | 37 | 7.4 | 15.33 | 28.5 | |
| Float | 421 | 84.2 | 1.79 | 37.8 | |
| Test 6. | 2.0-2.1 | 500 | |||
| Sink | 117 | 23.4 | 11.18 | 65.5 | |
| Float | 341 | 68.2 | 1.92 | 32.8 | |
"Phosphate rock" is a commercial term for a rock containing one or more phosphate minerals, usually calcium phosphate of sufficient grade and suitable composition to permit its use, either directly or after concentration in manufacturing commercial products. The term "phosphate rock" includes phosphatized limestones, sandstones, shales, and igneous rocks.
Chemical analyses usually are reported as percent P2O5 or tricalcium phosphate (Ca3(PO4)2) also known as B.P.L. (bone phosphate of lime). 1.0 percent tricalcium phosphate (B.P.L.) = 0.458 percent P2O5.
Phosphate rock does not have a definite chemical composition. The major phosphorus minerals of most phosphate rock are in the apatite group and can be represented by the formula Ca5(PO4)3(F, Cl, OH). Phosphate rock occurs as nodular phosphates, residual weathered phosphatic limestones, and consolidated and unconsolidated phosphatic sediments.
The United States has ample reserves to supply domestic requirements at a reasonably projected rate of consumption for several hundred years. The increase in production over the past 15 years has been at an average annual rate of about 5 percent.
U. S. production and reserves of phosphate rock as of 1963 are given in Table 10.
Table 10--Phosphate rock produced and reserves in principal producing areas (in millions long tons).
| Source | Production, rock | 1963 P2O5 | Total marketable reserves |
Potential resources |
||
|---|---|---|---|---|---|---|
| Rock | P2O5 | Rock | P2O5 | |||
| Florida | 14,592 | 4,818 | 2,040 | 660 | 23,350 | 4,932 |
| Western states | 2,891 | 785 | 3,000 | 870 | 20,000 | 5,800 |
| Tennessee | 2,352 | 612 | 80 | 12 | 5,398 | 1,129 |
| North Carolina | 2,000 | 600 | 15,000 | 4,650 | ||
| Arkansas | 20 | 5 | ||||
| South Carolina | 9 | 2 | ||||
| Total U.S. | 19,835 | 6,215 | 7,100 | 2,100 | ||
| Data from Chemical Economics Handbook, 1965, Stanford Research Institute (used by permission of copyright holder). |
||||||
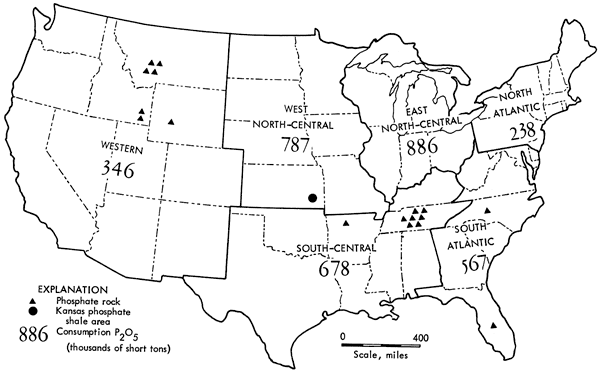
Current "phosphate rock" producing companies are given in Table 11, with approximate locations shown in Figure 1.
Table 11--Companies producing phosphate rock in 1963.
| Company | Operations in | ||
|---|---|---|---|
| Florida | Tennessee | Western states | |
| American Cyanamid Co. | X | ||
| Armour & Co. (Armour Agricultural Chemical Co. subsidiary) | X | X | |
| Cities Service Co. (Tennessee Corp. subsidiary) | X | ||
| Consolidated Mining & Smelting Co. (Montana Phosphate Products Co. subsidiary) | X | ||
| Continental Oil Co. (American Agricultural Chemical Co. subsidiary) | X | ||
| El Paso Natural Gas Co. (recent purchase of Central Farmers Fertilizer Co.'s Idaho operations) | X | ||
| W. R. Grace & Co. | X | ||
| International Minerals & Chemical Corp. | X | X | |
| San Francisco Chemical Co. (joint venture of Stauffer Chemical Co. and Mountain Copper Co., Ltd., of England) | X | ||
| J. R. Simplot Co. | X | ||
| Smith-Douglass Co. | X | ||
| Socony-Mobil Oil Co. (Virginia-Carolina Chemical Corp. subsidiary) | X | X | |
| Swift & Co. | X | ||
| Tennessee Valley Authority | X | ||
| Data from Chemical Economics Handbook, 1965, Stanford Research Institute (used by permission of copyright holder). | |||
Figure 1--Map of the United States showing phosphate-rock processing areas and phosphate fertilizer consumption by region, 1965 (data from 1965 Minerals Year-book, U.S. Bureau of Mines, vol. 1, 1966, p. 713: U.S. Govt. Printing Office, Washington, D.C.).
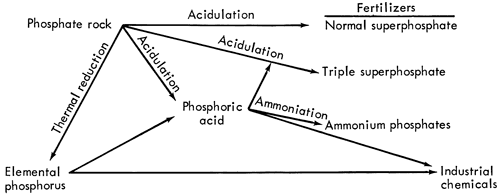
Most phosphate rocks require some chemical processing in order to convert the contained phosphorus into some usable form. The major processes and derivatives are shown in Figure 2.
Figure 2--Major steps in the processing of phosphate rock. (From Chemical Economics Handbook, 1965, Stanford Research Institute; used by permission of copyright holder).
Generally, phosphate rock is moved from mining areas to market areas and then processed.
Prices for phosphate rock are based upon the percentage of tricalcium phosphate (B.P.L.) present. Maximum limits of allowable iron and aluminium oxide are specified. Bonuses are paid and penalties assessed for variations above and below the base grade. Price quotations for Florida land-pebble phosphate are given in Table 12.
Table 12--Prices per short ton of Florida land-pebble, unground, washed, and dried phosphate rock, in bulk carlot, at mine in 1963.
| Grade | Dec. 30, 1963 | |
|---|---|---|
| Percent B.P.L. | Percent P2O5 | |
| 66-68 | .302-.312 | $5.38 |
| 68-70 | .312-.321 | $6.24 |
| 70-72 | .321-.330 | $6.82 |
| 74-75 | .339-.344 | $7.72 |
| 76-78 | .348-.358 | $8.61 |
| Data from Chemical Economics Handbook, 1965, Stanford Research
Institute (used by permission of copyright holder). |
||
Since 1930 almost 80 percent of the phosphate rock consumed in the United States was used to make phosphorus-nutrient fertilizers (Fig. 2). Use is primarily in mixed fertilizers with the balance in direct application. Most of the remaining phosphate rock is used for industrial chemicals starting with elemental phosphorus.
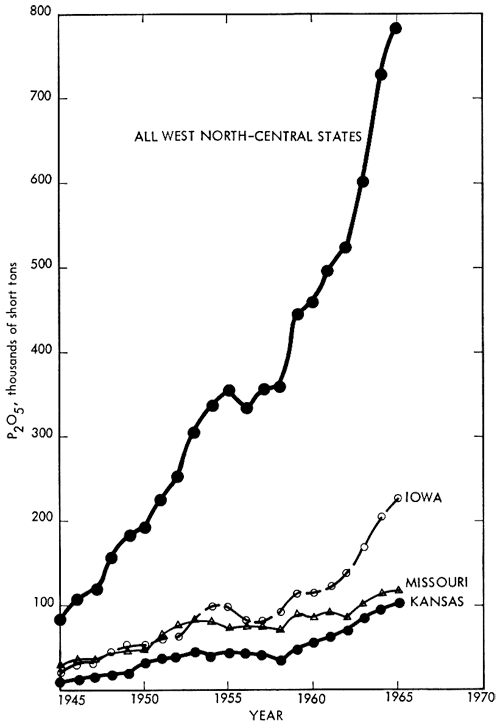
Consumption of phosphate rock in the West North-Central states is shown in Figure 3. Overall average growth has been over 41 percent per year since 1945. The potential P2O5 consumption for this area is calculated to be about 3 1/2 times the 1965 consumption of 787,000 short tons.
Figure 3--Consumption of phosphate fertilizer, West North-Central States, 1945-1965 (total potential consumption in 1965 was 2,541,000 short tons). (From Chemical Economics Handbook, 1965, Stanford Research Institute; used by permission of copyright holder.)
In view of the needed and impending obvious growth of plant nutrients to help in supplying the growing population's food requirements, there might be a role for Kansas phosphates in supplying a localized market carved out of the north-central states region. Work on Kansas phosphates has reached a point where pilot plant tests using commercial types of beneficiating equipment to separate phosphatic nodules from Kansas shales is required.
The shale in question has a specific gravity of approximately 2.0; that of the phosphatic nodules themselves is 2.4. Laboratory tests have shown that it is possible to recover about 80 percent of the phosphate of a grade of 15 to 20 percent P2O5 (the crude shale runs 1 to 3 percent P2O5) by sink-float, using a ferrosilicon pulp. Froth flotation has given similar results. However, a grade of 25 to 30 percent P2O5 would be much more desirable commercially.
The all-important question pertaining to Kansas phosphatic shales is whether or not the phosphate can be extracted and further treated and remain competitive with ores from the southeast and western sources. At some potential use points, a Kansas product should have shipping cost advantages. The cost of preparing a 3-percent P2O5 ore plus some shipping costs would need to be equated with preparing 15-percent ore from Florida plus shipping or 20-30-percent ore from Idaho-Montana plus shipping.
Kansas Geological Survey, Geology
Placed on web Nov. 5, 2008; originally published in Sept. 1967.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/187_4/index.html