
Kansas Geological Survey, Bulletin 187, pt. 1, originally published in 1967

Originally published in 1967 as part of "Short Papers on Research in 1966," Kansas Geological Survey Bulletin 187, part 1, p. 9-11. This is, in general, the original text as published. The information has not been updated.
Two wet chemical methods for the determination of calcium and magnesium were adapted to atomic absorption analysis. Experimental work was carried out with four National Bureau of Standard samples and reagent-grade calcium carbonate. Results from the wet chemical methods and the instrumental method were compared and found to be in reasonable agreement. The effect of four common acids (HClO4, HCl, HNO3, and H2SO4) on the analytical sensitivity of Ca and Mg by atomic absorption was also tested. Perchloric acid provides the greatest sensitivity and H2SO4 the least. Nitrate was found to interfere with the determination of Ca and Mg, but it can be eliminated by the addition of Sr or NH4Cl.
The method for putting carbonate and silicate samples into solution so that determinations of other elements can be made in addition to Ca and Mg is relatively simple and straightforward and involves the adaptation of two separate methods described by Hillebrand et al. (1955). One method consists of treating a weighed and dried sample with 48 percent HF, evaporating to dryness, putting the residue into solution with HCl, separating the R2O3 group by precipitation with NH4OH, and determining the Ca and Mg on the filtrate. This is a standard method for treatment of silicate samples. The other method consists of an initial step of separating the silica by dehydration with HClO4 and then precipitating the R2O3 from the filtrate of the silica separation. The R2O3 precipitation and the Ca and Mg determination are the same from this step on. The second method is used routinely on all carbonate samples in the analytical laboratories of the Kansas State Geological Survey and has an advantage over the first method in that it allows an SiO2 determination on the same sample on which all of the major elements are determined. If a silica determination is not desired as a preliminary step on carbonate samples, they may be treated with HF as described in the first method.
The filtrate and washings from the R2O3 precipitation are evaporated so that appropriate dilutions can be made and the Ca and Mg determined by atomic absorption. The dilutions used depend upon the size of the original sample (for silicates, a 1.0-g sample is a minimum amount) and the amount of Ca and Mg present. The R2O3 filtrate from a silicate sample is evaporated down to 250 ml. A 25-ml aliquot is then taken and diluted four times. If the Ca and Mg content is extremely low (less than 0.5 percent), no dilution is necessary. In the case of carbonates where large amounts of Ca and Mg are present, the original sample size is of some importance. If Si, Fe, and Al are to be determined on the same sample, a 2-g sample is needed because of the small amounts of these elements normally present in carbonates. If Ca and Mg are the only determinations to be made, a smaller sample size may be used and the later dilutions adjusted accordingly. For a 2-g sample, the R2O3 filtrate is put into a 1-liter volumetric flask and made up to the mark. A 10-mL aliquot is then taken and diluted 50 times. The above dilutions were adequate to allow readings to be taken on the middle part of the calibration curve. However, dilutions can be adjusted for individual samples or to meet the specific needs of a particular laboratory.
All determinations were made using the 4226Å and 2853Å lines of Ca and Mg respectively. It was found that the best concentration range for Ca analysis was 1-25 ppm. Because of the extreme sensitivity of Mg to atomic absorption analysis, the concentration range used was 0.1-3.0 ppm.
The instrument used for this work was a Jarrell-Ash Model 82-516 atomic-absorption flame-emission spectrophotometer equipped with a "Hetco" (high efficiency, total consumption) burner. The width of the entrance and exit slits on the Ebert-mount monochromator were 100 and 150 μ respectively. A Westinghouse multielement hollow-cathode tube containing Ca, Mg, Al, and Li was used. The tube was operated at a current of 5 ma; the photomultiplier, an R106, was operated at 480 v. An air-hydrogen flame was used with the best sensitivities obtained at air and fuel pressures of 12 and 10 psi respectively.
A series of National Bureau of Standard samples were run along with reagent-grade calcium carbonate and a dolomite sample supplied by the G. Fredrick Smith Chemical Company. Table 1 shows a comparison of results obtained by atomic absorption with values given on analysis certificates for each NBS standard. The results obtained with the atomic absorption method were close enough to the listed values to allow a reasonable confidence in the method.
Table 1--Comparison of values obtained by atomic absorption method with values given for several standards. An agreement of 0.20 percent was determined to be acceptable.
| Sample no. |
Ca, % | Mg, % | ||
|---|---|---|---|---|
| NBS Value |
At. Abs. |
NBS Value |
At. Abs. |
|
| National Bureau of Standards 76 |
0.19 | 0.14 | 0.35 | 0.19 |
| National Bureau of Standards 81 |
0.04 | 0.02 | 0.01 | 0.01 |
| National Bureau of Standards 102 |
1.64 | 1.88 | 0.13 | 0.05 |
| National Bureau of Standards 1A |
29.53 | 29.95 | 1.32 | 1.44 |
| G. Fredrick Smith 400 |
21.79 | 21.70 | 12.98 | 13.01 |
| CaCO3* | 40.04 | 39.96 | 0.00† | <0.10ppm |
| * Reagent-grade CaCO3. † Some Mg present, but listed as sulfate. Therefore an exact value is not known. |
||||
Interferences due to Al, SiO2, PO4-3, and SO4-2 are well documented in the literature (Trent and Slavin, 1964; Sprague, 1963). The treatment of silicate samples with HF and the separation of silica with HClO4 in the carbonate samples was used to eliminate silica as a possible interfering element. The R2O3 precipitation was used to minimize any aluminum and phosphate interference. Sulfate was present in very small quantities in the samples, but it was felt that it would cause no interference problems. With one exception, no problems with interferences were noted when the same acid was used to put the standards and the samples into solution.
Commercial atomic absorption standards supplied by the Fisher Scientific Company were used in the initial phases of the work on the methods discussed. Working curves made from these standards were used to obtain the first Ca and Mg results from the NBS standards. The initial values were approximately twice those listed on the analysis certificate. A check for the common interferences was made but no interferences were found. The standards used for the working curves were made up by dissolving CaCO3 and Mg-metal in dilute HNO3. The effect of acid upon the sensitivity of Ca and Mg was observed next. Standards were made up from CaCO3 and Mg-metal using HCl as the dissolving agent. The NBS samples were rerun, and the results compared with those listed on the analysis certificate and found to be acceptable.
The next step was to note the effect of different acids upon the sensitivity of Ca and Mg to atomic absorption analysis. A series of Ca and Mg standards were made up using four different acids (HClO4, HCl, HNO3, and H2SO4) and separate working curves plotted for each acid. The results for Mg do not show as wide a difference between curves as does Ca, but the same effect is present (Figs. 1, 2). Because of the known effect of sulfate ions on Ca and Mg, a check for nitrate interference seemed reasonable. To determine whether we were actually getting nitrate interference, three sets of Ca standards were made up. One set of standards was made up from the HNO3 solution. Another was made up from an HCl solution of CaCO3 to which 100 ppm nitrate had been added. The nitrate was obtained from a solution of primary standard KNO3. A third set of standards was made up using a solution of Ca(NO3)2 in water. The results showed that nitrate reduced the sensitivity of Ca to atomic absorption in terms of percent absorption by 2.5 percent at 1 ppm to 10 percent at 25 ppm when compared with an HCl solution of Ca. Figure 3 shows a comparison of Ca and Ca with nitrate added. The curves of the standards made up from Ca(NO3)2 and CaCO3 in HNO3 are essentially the same as the curve shown in Figure 3, but for the sake of simplicity in this presentation only the curve with 100 ppm nitrate is shown. The curves shown were run with the recorder set on the 0-10-mv scale. It is apparent from the curves that to determine Ca in amounts less than 1 ppm with nitrate present a scale expansion technique is necessary. However, with an HCl solution of Ca, it was possible to detect Ca in amounts of 0.1 ppm without scale expansion. Figure 3 also shows a comparison of the absorption of Mg and Mg with 100 ppm nitrate added. Again, the difference is not as great as with Ca, but the loss of sensitivity is apparent. Additional work showed that nitrate present in amounts as little as 10 ppm produced some interference effects. The interference effects of nitrate were not difficult to eliminate. Strontium added in the amount of 1500 ppm and NH4Cl in concentrations of 1000 ppm were effective in eliminating nitrate interference.
Figure 1--Comparison of calibration curves for calcium made up from four different acids. A, HClO4; B, HCl; C, HNO3; and D, H2SO4.
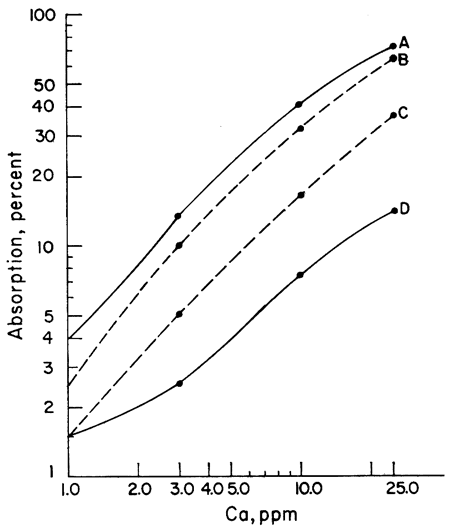
Figure 2--Comparison of calibration curves for magnesium made up from four different acids. A, HClO4; B, HCl; C, HNO3; D, H2SO4.
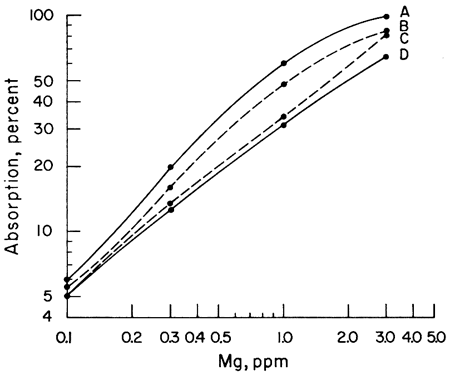
Figure 3--Comparison of calibration curves for calcium and magnesium. A, Mg; B, Mg + 100 ppm NO3; C, Ca; D, Ca + 100 ppm NO3.
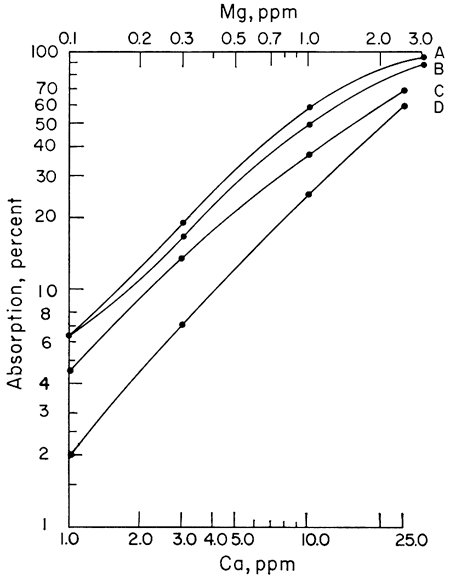
It is not known at this time why nitrate interferes with the determination of Ca and Mg. Other workers have reported nitrate interference, but presently no explanation for this interference is known. Although nitrates do interfere, the interference is not difficult to overcome and will not cause any great analytical problems. It is also apparent that even though several types of acid may be used to put Ca and Mg into solution, if the ultimate sensitivity is desired, the choice is limited to HClO4.
Hillebrand, W. F., Lundell, G. E. F., Bright, H. A., and Hoffman, J. I., 1955, Applied inorganic analysis: John Wiley and Sons, Inc., New York, 1,034 p.
Sprague, Sabina, 1963, Cement analysis: Atomic Absorption Newsletter, no. 14, p. 9-14.
Trent, D. J., and Slavin, W., 1964, Determination of the major metals in granitic and diabasic rocks by atomic absorption spectrophotometry: Atomic Absorption Newsletter, no. 19, p. 1-6.
Kansas Geological Survey, Short Papers on Research in 1966
Placed on web July 25, 2011; originally published in Feb. 1967.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/187_1C/index.html