
Kansas Geological Survey, Bulletin 187, pt. 1, originally published in 1967

Originally published in 1967 as part of "Short Papers on Research in 1966," Kansas Geological Survey Bulletin 187, part 1, p. 3-5. This is, in general, the original text as published. The information has not been updated.
Iron showed a strong covariance with insoluble (in H2O2, HC2H3O2, and H2O) material present in Recent carbonate sediments from Vieques Passage, Puerto Rico. The insoluble fraction, making up about 11 percent (range 8-17) of the sediment, carries approximately 83 percent (range 70-98) of the total iron present in the sediment. The mean Fe concentration of the samples was 0.48 percent with a range of 0.08 to 1.0 percent and a σ of 0.16.
The concentrations of trace and minor elements found in carbonate rocks are a function of the element concentrations present in (1) solid solution in the carbonate minerals (2) detrital minerals, (3) accessory authigenic precipitates, (4) noncarbonate skeletal material, (5) organic matter, (6) phases formed during diagenesis, and (7) elements absorbed upon all these materials. A given trace or minor element present in a carbonate rock is frequently distributed among components formed at different times in the history of the sediment by differing mechanisms. Evaluation of the minor element content of each of the components is difficult and the attempt has not often been made. The percentage distribution of the respective fractions of the trace and minor elements in carbonate rocks is imperfectly known at present. If we hope to understand the distribution patterns of trace and minor elements in ancient sediments, we must start with an understanding of those processes at work in Recent sediments. It was the purpose of this study to examine the distribution of Fe in Recent carbonate sediments taken from the Vieques Passage area of eastern Puerto Rico (Fig. 1).
Figure 1--Location map of study area. Dots represent sample locations. The 3 and 5 fathom lines (3F and 5F) are indicated.
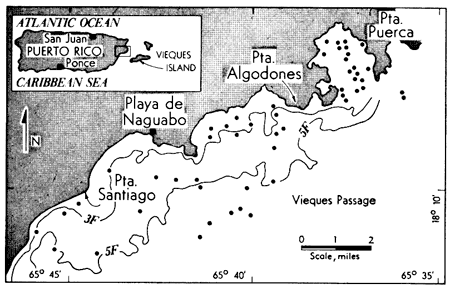
Vieques Passage lies between Puerto Rico on the west and Vieques Island on the east. The area of investigation lies between 65°36' and 65°46'W and 18°08' and 18°14'N. Vieques Passage is a relatively shallow body of water ranging up to 15 fathoms in depth. All of the samples used in this investigation were from depths of less than 12 fathoms (Fig. 1).
Excepting tidal currents to the south of Playa de Naguabo, coastal and nearshore currents flow in a general northeasterly direction under the influence of the general shoreline configuration and the north Equatorial Current (Kaye, 1959). Representative physical properties of water in the area are: Salinity, 35.39‰; Cl, 1959 mg/L; Ca, 402 mg/L; water temperature, 28.5°C; and pH, 8.05.
The major portion of the Recent carbonate sediments consists of the remains of carbonate-secreting organisms. Carbonate precipitation from sea water plays a minor part. Skeletal debris is largely composed of aragonite, low Mg-calcite, and high Mg-calcite. Sediment size distribution with depth shows a strong bimodal pattern indicating poor sorting. Mean grain size generally increases down the core. The bimodal pattern is thought to be the result of chemical solution of the fine-grained portion causing the sediments to be skewed towards the coarse fraction (Sommer et al., 1964). Montmorillonite is present in significant amounts only in those sediments containing less than 90 percent CaCO3 and was found only in the nearshore samples; kaolinite is more common in deeper water samples. Iron was found to vary with both the clay and insoluble fractions. A weak covariance between iron content and water depth was noted.
The association of iron with the clay minerals clearly affects the distribution of iron in these sediments. Montmorillonite has greater adsorptive properties than kaolinite. Montmorillonite might be adsorbed by or adsorb some part of any iron hydroxide and be forced out of suspension. The chemical state of iron necessary for ionic substitution into clays is incompatible with the forms bonded to hydroxide. Iron apparently follows the clay in essentially constant proportions. Most of the iron is probably brought to the site of clay mineral deposition as part of an Fe(OH)3 sol. It could be returned to the ionic state at or near the sediment interface by the acid effect of large amounts of organic material, and subsequently could enter the clay mineral lattice.
As has been noted, in most studies of the trace, minor, or major element concentrations in carbonate rocks, little attention has been paid to the relative concentrations of the elements in the various components that make up a carbonate sediment. This problem has been discussed by Hirst and Nicholls (1958), Hirst (1962a, b), and Angino (1964), but generally the study of element fractionation has been avoided. If the minor and trace element content of a sediment is ever to be of real value in reconstructing paleoecologic conditions, then consideration must be given to differentiating between the elemental content of the respective fractions making up the sample (i.e. water soluble, H2O2 soluble, acetic acid soluble, carbonate, and insoluble). However before this possibility can be attained, it will be necessary to give greater attention to a study of the trace element content of the various components making up a rock unit. With such analyses we may then be able to delineate more distinctly some of the detailed environmental conditions prevailing during the deposition of carbonate sediments.
The major carbonate minerals, calcite and aragonite, take few trace elements into solid solutions; only Sr, Mn, Ba, Pb, and Fe are likely to be present in significant amounts.
Four fractions were examined in this study. A water-soluble fraction was obtained by washing 1-2 g of sediment (ground to pass a 150-μ screen) in a 150-mL beaker with 100 mL of doubly distilled deionized water with constant stirring for 24 hours. The solution was then allowed to stand for 12 hours and the supernatant separated by centrifugation (or vacuum-filtration) and analyzed by atomic absorption spectrometry for iron.
For the organic (H2O2-soluble) fraction, 75 mL of 10 percent H2O2 was added to 1 to 2 g of crushed sample (150-μ), placed in a 150-mL beaker and allowed to set for 4 hours with frequent stirring. This solution was separated by centrifugation and analysed for Fe.
In a study of the acid soluble fraction a 9:1 v/v water-acetic acid solution was used to dissolve the carbonate and the sample allowed to set for 2 to 4 hours prior to separation of the supernatant.
A total analysis for iron was obtained by dissolving a 1- to 2-g sample in 5-mL 48 percent HF and 5 drops concentrated H2SO4, and heating at 100°C to dryness. This step was repeated three times and the solution transferred to a 250-mL erlenmeyer flask using 200 mL of hot 9:1 v/v water/HCl. If a black residue remained, it was taken up in H2O2 and analysed for Fe. The sum of the Fe content of the other three fractions subtracted from the total gives the Fe content in the insoluble fraction. A total determination was also made for manganese. All iron and manganese determinations were made on a Perkin-Elmer 303 atomic absorption spectrometer using the 2483Å and 2803Å lines of Fe and Mn respectively and an air-acetylene flame. Except for a slight interference caused by Ca molecular absorption, no other interferences were noted.
The well known strong covariance of iron and manganese is shown by the insoluble content of the Recent carbonate sediments from the Vieques Passage area (Fig. 2). As might be expected, the Fe/Mn ratio is relatively constant (range 7-44). Further evidence of the pronounced concentration of Fe in the acetic acid insoluble fraction is shown in Fig. 3. Although making up only about 10.9 percent of the sample, the insoluble fraction carries approximately 83 percent of the Fe present in the sediment. Similar data for the other fractions are summarized in Table 1.
Figure 2--Covariance of iron and manganese with percent insoluble component in the sample. The trend lines were visually fitted. Data from Sommer et al. (1964).
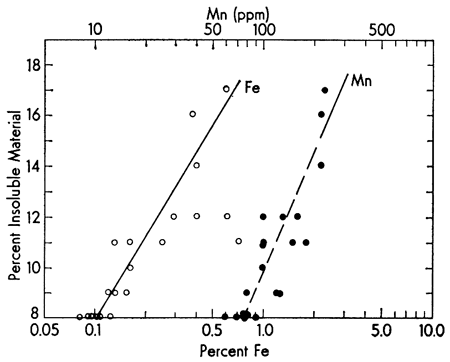
Figure 3--Ternary diagram showing extreme fractionation and concentration of iron in the insoluble component of the sample. I, C, and O indicate insoluble, carbonate, and organic fraction. Large dots represent those few data that did not fall within the indicated field.
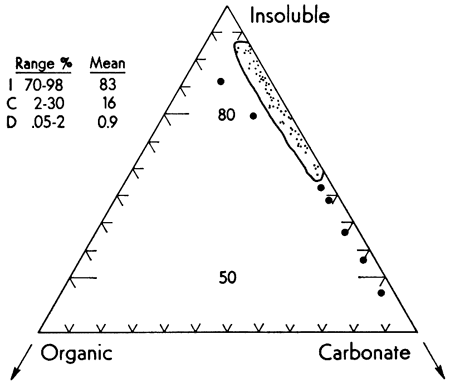
Table 1--Percent iron in each fraction of carbonate samples from Vieques Passage.
| Fraction | Percent of sample (range) |
Fe content as percent of total sample |
Mean Fe, % |
|---|---|---|---|
| Insoluble | 8-17 | 70-98 | 83 |
| Acetic acid soluble (carbonate) | 83-92 | 2-30 | 15.8 |
| H2O2 soluble (organic) | 0-< 1 | 0.02-2 | 0.86 |
| Water soluble | 0-<0.1 | 0.06-1.2 | 0.45 |
The mean iron content, based on a total Fe analysis of all the samples was 0.48 percent (4800 ppm) with a range of 0.08 to 1.0 percent and a σ of 0.16.
It is obvious that although the insoluble fraction makes up no more than 8-17 percent of the carbonate sediment, it carries 70-98 percent of the Fe. Although aragonite comprised 89-97 percent of the carbonate fraction, no association of Fe content with either aragonite or calcite was noted.
To use the figures for the absolute concentration of Fe in these carbonate sediments to make any predictions about environmental conditions prevailing during the deposition of these sediments would be difficult. To attempt to make predictions about the paleoenvironment of ancient carbonate sediments based solely on the absolute concentration of Fe (or any other trace element) in those sediments would be meaningless with the present state of our knowledge of those factors controlling trace element distribution in carbonate sediments.
Angino, E. E., 1964 [1966], Trace elements and cyclic deposition; in, Symposium on Cyclic Sedimentation, D. F. Merriam, ed.: Kansas Geol. Survey, Bull. 169, p. 21-30. [available online]
Hirst, D. M., and Nicholls, G. D., 1958, Techniques in sedimentary geochemistry: (1) separation of the detrital and nondetrital fractions of limestones: Jour. Sed. Petrol., v. 28, p. 468-81.
Hirst, D. M., 1962a, The geochemistry of modern sediments from the Gulf of Paria--I. The relationship between the mineralogy and the distribution of major elements: Geochim. et Cosmochim. Acta, v. 26, p. 309-34.
Hirst, D. M., 1962b, The geochemistry of modern sediments from the Gulf of Paria--II. The location and distribution of trace elements: Geochim. et Cosmochim. Acta, v. 26, p. 1,147-1,187.
Kaye, C. A., 1959, Shoreline features and Quaternary shoreline changes, Puerto Rico: U.S. Geol. Survey, Prof. Paper 317-B.
Sommer, S. E., Angino, E. E., and Hood, D. W., 1964, Mineralogy and geochemistry of Recent sediments, Vieques Passage, Puerto Rico: Texas A&M Research Foundation, Ref. 64-12T, 59 p.
Kansas Geological Survey, Short Papers on Research in 1966
Placed on web July 25, 2011; originally published in Feb. 1967.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/187_1A/index.html