Kansas Geological Survey, Bulletin 180, pt. 1, originally published in 1966
Originally published in 1966 as Kansas Geological Survey Bulletin 180, pt. 1. This is, in general, the original text as published. The information has not been updated.
The compositional variance between samples of the Plattsmouth Limestone Member of the Oread Limestone, Shawnee Group (Late Pennsylvanian age) was studied with respect to the distance between sample locations . Nine samples spaced approximately 150 to 200 feet apart were collected from an underground quarry in Atchison County, Kansas, analyzed, and the compositional variance compared with that of 11 outcrop samples collected on a spacing of approximately 6 miles. Results indicate that at the 95-percent confidence level, the variances are equal. It is concluded that chemical data can be used to depict trends within the Plattsmouth Limestone Member when sample locations are spaced as much as 6 miles apart. The study also demonstrates that, because of the variance, a series of samples should be collected from a given area and analyzed before economic development is begun. It is also concluded that the results of this study may then be generally applied to many of the limestones of Pennsylvanian age from the Midcontinent area.
A comparison of the compositional variance between limestone samples taken on a 6-mile interval and a 200-foot interval can be helpful in establishing the spacing of locations for the collection of geologic samples for chemical analysis. A convenient spread is necessary to provide meaningful chemical results at a minimum of time and expense. Previous work by the Geochemistry Division of the State Geological Survey of Kansas indicated that chemical trends can be established when samples are collected on a regular pattern of 6 miles along the outcrop (Galle, et al., unpublished data). However, because of the variance in analyses from sample to sample, the accuracy of these trends has been subject to question. Collection of samples at intervals of a few hundred feet gives rise to the possibility of less sample-to-sample variation.
If true, then a greater degree of control could be exercised in a given area, allowing more accurate predictions of chemical trends within a limestone unit. However, if the compositional variance between limestones collected on a wide spacing is the same as that of limestones collected on a close spacing, the accuracy of any predicted chemical trends should be the same. Thus, it would be possible to do a chemical study of a limestone unit extending several hundred miles along an outcrop with no need for the collection of an extremely large number of samples. Hence a study of compositional variance would be of value if the proper samples could be obtained.
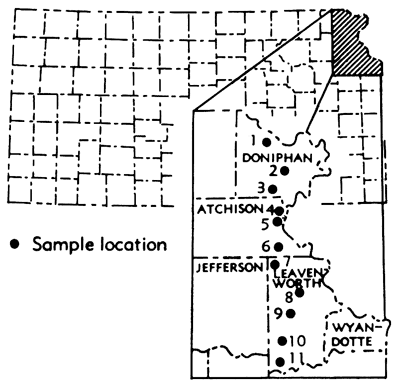
The opportunity to conduct a study of the type described above became a reality when the Kerford Quarry Company opened a new underground quarry at the northeast edge of Atchison, Kansas. As a result, it was possible to collect samples of the Plattsmouth Limestone Member of the Oread Limestone at a spacing of 150 to 200 feet within the quarry. The samples were analyzed for major chemical constituents and the variation between samples compared with the variation in the analyses of the Plattsmouth Limestone taken from outcrop locations at a spacing of approximately 6 miles along a line from northern Doniphan County to southern Leavenworth County.
The Plattsmouth Limestone was chosen for this study because of the availability of the large underground quarry and previously analyzed samples that are a part of a geochemical study (in progress) of the Oread Limestone (Galle, et al., unpublished data).
The authors are grateful to George Kerford, of Atchison, Kansas, for help and cooperation in obtaining samples from the quarry. Gratitude is also expressed to W. E. Hill, Jr., of the Geochemistry Division, who helped collect some of the samples and map part of the quarry.
Composite channel samples of the Plattsmouth Limestone were taken from 11 outcrop locations including the outside face of the underground quarry (Fig. 1; Table 1). Six miles, approximately the width of one township, was chosen as the distance between samples.
Figure 1--Map of Kansas showing locations where samples were collected from the outcrop.

Table 1--KGS sample numbers corresponding to outcrop location numbers.
| Sample Number |
Location Number |
|---|---|
| 6130 | 1 |
| 58163 | 2 |
| 6129 | 3 |
| 604 | 4 |
| 63117 | 5 |
| 6170 | 6 |
| 58252 | 7 |
| 58254 | 8 |
| 6166 | 9 |
| 58251 | 10 |
| 6167 | 11 |
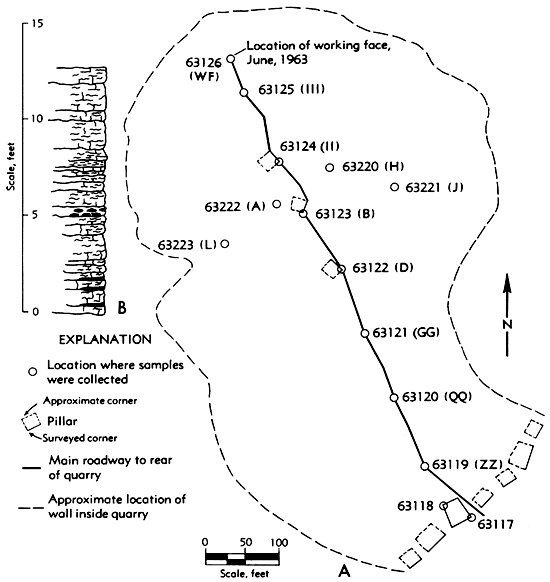
Samples from the underground quarry were taken from every other pillar on a line from the front to the rear of the quarry. Additional samples were taken from pillars on both sides of this line to give some indication of the major constituents of the limestone from other locations within the quarry. A map of the quarry and the location of the pillars from which the samples were taken is shown in Figure 2, A.
All samples were collected in the manner outlined by (Galle (1964). The sample size ranged from 10 to 20 pounds. Each sample was passed through a jaw crusher, thoroughly mixed, and split down to a size of approximately 50 grams. The 50-gram portion was then ground to a grain size small enough to pass a 60-mesh screen, mixed once more, and then analyzed using the techniques described by Hill, et al. (1961).
Figure 2--Kerford Quarry (SE sec. 30, T. 5 S., R. 21 E.), Atchison County, Kansas. A, Map of underground quarry showing locations where samples were taken. Numbers are laboratory designations of samples. Pillar identifications within quarry are shown in parentheses. B, Measured section of Plattsmouth Limestone Member of the Oread Limestone (pillar D inside quarry). (Map showing size and shape of quarry was supplied by George Kerford; sample locations were mapped by members of the Geochemistry Division.)
The Plattsmouth Limestone is composed of a series of wavy beds of limestone 4 to 8 inches in thickness separated by thin shale partings, which locally thicken to several inches. The base of the Member in the area studied is a massive bed of limestone containing dark, wavy bands of shale and carbonaceous material. Black, brown, and grey chert spotted with white or grey siliceous replacements of fossils is abundant throughout the Member in this area. The chert nodules are 1 to 8 inches in diameter, and contain discontinuous thin black shale partings which extend into the limestone. The color of the limestone on a fresh surface is characteristically light grey, but on weathering it is light yellow to light grey.
The Plattsmouth Limestone ranges in thickness from 19.8 feet in Doniphan County to 11.0 feet in Leavenworth County, a distance of approximately 70 miles. The thickness of the limestone in the quarry ranged from 11 feet to 13.5 feet. A representative stratigraphic section of the limestone from the quarry is shown in Figure 2, B.
The results of the analyses of the sample taken from outcrop location were arranged in a progression from north to south across the area studied (Table 2). Included in the list of outcrop samples is the sample taken from the outside face of the underground quarry. It should be noted that this sample location (5) is approximately midway between location 4 on the north and location 6 on the south (Fig. 1). It is significant that although these samples are closely spaced (about 3 miles) the same trends can be noted if only the samples from locations 4 and 6 are compared. Analyses of samples collected from pillars along the main roadway from the front to the rear of the underground quarry (Fig. 2) are shown in Table 3. The samples were arranged in this manner to provide a line of sampling similar to that along the outcrop. In this manner a close sample spacing was obtained for comparison with those samples taken along the outcrop. The mean, variance (S2), and the standard deviation of each constituent are also shown in Tables 2 and 3.
Table 2--Chemical analyses (in weight percent) of samples taken from outcrop locations. (Arranged in progression from north to south across the area studied.)
| Location number |
Sample number |
County* | Locality | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | P2O5 | SO3 | K2O | Na2O | S† | Differential loss on ignition | Total | Calculated CaCO3 |
Calculated MgCO3 |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sec. | T. South |
R. East |
Noncarbonate | Carbonate | |||||||||||||||||
| 1 | 6130 | Dp | NW 24 | 2 | 21 | 14.44 | 1.34 | 1.89 | 0.16 | 41.07 | 3.43 | 0.09 | 0.16 | 0.21 | 0.11 | 0.11 | 1.03 | 35.43 | 99.36 | 72.89 | 6.48 |
| 2 | 58163 | Dp | SW 21 | 3 | 22 | 9.46 | 1.12 | 1.14 | 0.08 | 48.41 | 0.66 | 0.06 | Trace | 0.09 | 0.02 | 0.01 | 0.86 | 38.09 | 99.99 | 86.26 | 0.31 |
| 3 | 6129 | Dp | SE 26 | 4 | 21 | 14.11 | 1.19 | 1.04 | 0.13 | 45.66 | 0.79 | 0.05 | Trace | 0.09 | 0.03 | 0.02 | 0.81 | 36.05 | 99.95 | 81.37 | 0.52 |
| 4 | 604 | At | SW 18 | 5 | 21 | 12.77 | 1.40 | 1.60 | 0.05 | 45.08 | 1.31 | 0.06 | 0.09 | 0.10 | 0.03 | 0.06 | 1.11 | 36.17 | 99.77 | 80.21 | 1.72 |
| 5 | 63117 | At | SW 30 | 5 | 21 | 10.90 | 1.03 | 1.33 | 0.06 | 45.12 | 2.25 | 0.09 | 0.23 | 0.19 | 0.04 | 0.07 | 0.91 | 37.40 | 99.55 | 80.03 | 4.23 |
| 6 | 6170 | At | NW 32 | 6 | 21 | 9.30 | 1.28 | 1.50 | 0.01 | 45.27 | 3.17 | 0.03 | 0.14 | 0.15 | 0.09 | 0.12 | 0.98 | 38.42 | 100.34 | 80.55 | 5.75 |
| 7 | 58252 | Lv | SW 25 | 7 | 21 | 15.32 | 1.11 | 1.18 | 0.16 | 44.09 | 1.01 | 0.13 | Trace | 0.05 | 0.01 | Trace | 0.89 | 35.25 | 99.20 | 78.39 | 1.49 |
| 8 | 58254 | Lv | E 33 | 8 | 22 | 7.97 | 0.86 | 1.12 | 0.10 | 49.17 | 0.57 | 0.08 | Trace | 0.02 | 0.01 | 0.01 | 0.74 | 39.01 | 99.65 | 87.56 | 0.98 |
| 9 | 6166 | Lv | SE 14 | 9 | 21 | 10.74 | 0.86 | 1.28 | 0.01 | 47.55 | 1.14 | 0.08 | Trace | 0.07 | 0.03 | 0.02 | 0.68 | 37.67 | 100.11 | 84.67 | 0.84 |
| 10 | 58251 | Lv | NW 7 | 10 | 21 | 11.47 | 1.28 | 1.87 | 0.14 | 43.82 | 2.57 | 0.06 | Trace | 0.11 | 0.05 | 0.16 | 0.81 | 37.04 | 99.40 | 78.08 | 5.17 |
| 11 | 6167 | Lv | NW 7 | 11 | 21 | 11.45 | 1.21 | 1.80 | 0.07 | 43.40 | 3.01 | 0.08 | 0.20 | 0.18 | 0.09 | 0.20 | 0.99 | 37.04 | 99.72 | 77.01 | 6.09 |
| Mean | 11.63 | 1.15 | 1.43 | 0.09 | 45.33 | 1.89 | 0.07 | 0.07 | 0.11 | ||||||||||||
| S2 | 5.3870 | 0.0323 | 0.1003 | 0.0030 | 5.5071 | 1.1710 | 0.0007 | 0.0037 | |||||||||||||
| Standard Deviation |
2.3200 | 0.1797 | 0.3167 | 0.0548 | 2.3470 | 1.0820 | 0.0264 | 0.0608 | |||||||||||||
| *County designations are as follows: Dp=Doniphan; At=Atchison; Lv=Leavenworth. † Not included in total. |
|||||||||||||||||||||
Table 3--Chemical analyses (in weight percent) of samples taken from points along the main roadway inside the quarry. (Arranged in a progression from the front to the rear of the quarry.*)
| Pillar id |
Sample number |
SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | P2O5 | SO3 | K2O | Na2O | S† | Differential loss on ignition | Total | Calculated CaCO3 |
Calculated MgCO3 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noncarbonate | Carbonate | ||||||||||||||||
| 63118 | 7.02 | 0.77 | 0.87 | 0.02 | 48.84 | 1.56 | 0.02 | 0.30 | 0.17 | 0.02 | 0.09 | 0.90 | 39.10 | 99.62 | 86.67 | 1.94 | |
| ZZ | 63119 | 7.51 | 0.64 | 0.90 | 0.03 | 48.97 | 1.53 | 0.05 | 0.26 | 0.18 | 0.02 | 0.08 | 0.79 | 39.28 | 100.16 | 87.08 | 1.78 |
| 63120 | 6.74 | 1.11 | 0.67 | 0.03 | 48.78 | 1.39 | 0.01 | 0.18 | 0.18 | 0.03 | 0.14 | 1.02 | 39.15 | 99.29 | 86.76 | 1.92 | |
| GG | 63121 | 7.55 | 0.84 | 1.24 | 0.04 | 47.71 | 1.99 | 0.06 | 0.11 | 0.25 | 0.06 | 0.11 | 0.92 | 38.80 | 99.57 | 84.72 | 2.97 |
| D | 63122 | 9.46 | 1.16 | 1.49 | 0.03 | 45.83 | 2.57 | 0.06 | 0.09 | 0.29 | 0.05 | 0.14 | 1.08 | 37.48 | 99.59 | 81.62 | 3.05 |
| B | 63123 | 8.70 | 1.15 | 1.22 | 0.02 | 46.39 | 2.35 | 0.04 | 0.17 | 0.26 | 0.07 | 0.13 | 1.16 | 38.23 | 99.76 | 82.51 | 3.74 |
| II | 63124 | 12.33 | 1.60 | 1.43 | 0.05 | 43.41 | 3.15 | 0.06 | 0.20 | 0.35 | 0.12 | 0.18 | 1.19 | 36.01 | 99.90 | 77.08 | 4.06 |
| III | 63125 | 8.93 | 1.30 | 1.34 | 0.04 | 45.77 | 2.51 | 0.04 | 0.13 | 0.30 | 0.08 | 0.19 | 1.24 | 37.63 | 99.31 | 81.53 | 3.41 |
| WF | 63126 | 12.25 | 1.25 | 1.39 | 0.04 | 43.86 | 3.06 | 0.05 | 0.04 | 0.13 | 0.30 | 0.08 | 1.17 | 36.43 | 99.91 | 78.10 | 3.81 |
| Mean | 8.94 | 1.09 | 1.17 | 0.03 | 46.62 | 2.23 | 0.04 | 0.16 | 0.25 | 0.06 | 0.14 | ||||||
| S2 | 4.4100 | 0.0883 | 0.0835 | 0.0001 | 4.4050 | 0.4307 | 0.0003 | 0.0041 | 0.0010 | ||||||||
| Standard Deviation |
2.1000 | 0.2970 | 0.2890 | 0.0100 | 2.0980 | 0.6563 | 0.0170 | 0.0640 | 0.0316 | ||||||||
| *All samples except no. 63118 were taken from the NE corner of each pillar sampled. Sample no. 63118 was collected from the SW corner of pillar 1. † Not included in total. |
|||||||||||||||||
The results of the analyses of all the composite samples taken from the underground quarry are shown in Table 4. Included in this table is the standard deviation of each constituent.
Table 4--Chemical analyses (in weight percent) of all samples taken from inside the quarry.*
| Pillar id |
Sample number |
SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | P2O5 | SO3 | K2O | Na2O | S† | Differential loss on ignition | Total | Calculated CaCO3 |
Calculated MgCO3 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noncarbonate | Carbonate | ||||||||||||||||
| 63118 | 7.02 | 0.77 | 0.87 | 0.02 | 48.84 | 1.56 | 0.02 | 0.30 | 0.17 | 0.02 | 0.09 | 0.90 | 39.10 | 99.62 | 86.67 | 1.94 | |
| ZZ | 63119 | 7.51 | 0.64 | 0.90 | 0.03 | 48.97 | 1.53 | 0.05 | 0.26 | 0.18 | 0.02 | 0.08 | 0.79 | 39.28 | 100.16 | 87.08 | 1.78 |
| 63120 | 6.74 | 1.11 | 0.67 | 0.03 | 48.78 | 1.39 | 0.01 | 0.18 | 0.18 | 0.03 | 0.14 | 1.02 | 39.15 | 99.29 | 86.76 | 1.92 | |
| GG | 63121 | 7.55 | 0.84 | 1.24 | 0.04 | 47.71 | 1.99 | 0.06 | 0.11 | 0.25 | 0.06 | 0.11 | 0.92 | 38.80 | 99.57 | 84.72 | 2.97 |
| D | 63122 | 9.46 | 1.16 | 1.49 | 0.03 | 45.83 | 2.57 | 0.06 | 0.09 | 0.29 | 0.05 | 0.14 | 1.08 | 37.48 | 99.59 | 81.62 | 3.05 |
| L | 63223 | 10.27 | 1.07 | 1.15 | 0.08 | 45.83 | 2.04 | 0.08 | 0.12 | 0.14 | 0.07 | 0.13 | 1.04 | 37.51 | 99.40 | 81.46 | 3.24 |
| J | 63221 | 8.94 | 1.16 | 1.06 | 0.10 | 46.54 | 2.21 | 0.05 | 0.10 | 0.18 | 0.07 | 0.13 | 1.05 | 38.34 | 99.90 | 82.71 | 3.77 |
| B | 63123 | 8.70 | 1.15 | 1.22 | 0.02 | 46.39 | 2.35 | 0.04 | 0.17 | 0.26 | 0.07 | 0.13 | 1.16 | 38.23 | 99.76 | 82.51 | 3.74 |
| A | 63222 | 10.46 | 1.14 | 1.25 | 0.05 | 44.97 | 2.49 | 0.09 | 0.13 | 0.21 | 0.08 | 0.12 | 1.10 | 37.31 | 99.28 | 79.98 | 4.10 |
| H | 63220 | 7.54 | 1.04 | 0.95 | 0.06 | 47.82 | 1.62 | 0.06 | 0.12 | 0.17 | 0.07 | 0.15 | 1.14 | 38.77 | 99.36 | 85.06 | 2.63 |
| II | 63124 | 12.33 | 1.60 | 1.43 | 0.05 | 43.41 | 3.15 | 0.06 | 0.20 | 0.35 | 0.12 | 1.18 | 1.19 | 36.01 | 99.90 | 77.08 | 4.06 |
| III | 63125 | 8.93 | 1.30 | 1.34 | 0.04 | 45.77 | 2.51 | 0.04 | 0.13 | 0.30 | 0.08 | 0.19 | 1.24 | 37.63 | 99.31 | 81.53 | 3.41 |
| WF | 63126 | 12.25 | 1.25 | 1.39 | 0.04 | 43.86 | 3.06 | 0.05 | 0.04 | 0.30 | 0.07 | 0.17 | 1.17 | 36.43 | 99.91 | 78.10 | 3.81 |
| Mean | 9.05 | 1.09 | 1.15 | 0.05 | 46.52 | 2.19 | 0.05 | 0.15 | 0.23 | 0.06 | 0.13 | 1.06 | |||||
| Standard Deviation |
1.782 | 0.235 | 0.235 | 0.023 | 1.769 | 0.570 | 0.022 | 0.071 | 0.066 | 0.015 | 0.032 | 0.129 | |||||
| *All samples were collected from the NE corner of each pillar sampled except sample no. 63118 which was collected from the SW corner of the pillar next to the entrance of the quarry as shown in Figure 2. † Not included in total. |
|||||||||||||||||
Comparison of the variation between samples taken inside the quarry (Table 3) and the variation between samples taken from outcrop locations was done by using an hypothesis which tests the variance of two populations as outlined by Dixon and Massey (1951, Chap. 8). The method uses an F distribution to test the hypothesis that the variances σ12 and σ22 of two normally distributed populations are equal. The two populations need not have equal means. The procedure used in testing this hypothesis is to take a random sample from each population and compute the ratio of the sample variances F = (S12/S22). In this report S12 is the variance in the samples from within the quarry and S22 is the variance in the samples from the outcrop location. S2 is calculated using the following equation:
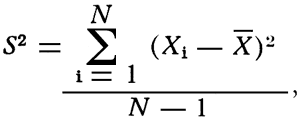
where S2 = variance
Xi = individual deviation from the mean
![]() = mean
= mean
N = number of observations.
If the ratio F = (S12/S22) is larger or smaller than expected by chance 95 percent of the time, the hypothesis that σ12 = σ22 must be rejected. The hypothesis of equal variance was rejected if F > 3.85 or if F < 0.260. F-distribution tables from Dixon and Massey (1951, p. 311) were used to determine the upper and lower limits of rejection. Half of the 5-percent rejection region was assigned to the large values of F and half to the small values of F. For example, this report is concerned with populations that have 9 and 11 samples respectively. There is then, a 2.5-percent chance that S12/S22 > 3.85 and a 2.5-percent chance that S12/S22 < 0.260. The following calculation will illustrate how all calculations were carried out in order to test the hypothesis. SiO2 values from the two sample case are used in the example.
The problem is to determine if σ12 = σ22, where σ1 = variance in the quarry and σ2 = variance along the outcrop. First, a level of significance is chosen. For these calculations the 5-percent level was chosen. Then, using the statistic F = (S12/S22), the hypothesis is tested. The calculated value of S12 for SiO2 is 4.410 (Table 3) and the S22 value for SiO2 is 5.387 (Table 2). The values are then used in the following way: F = (S12/S22) 4.410/5.387 = 0.8186. The hypothesis if rejected is F > 3.85 or if F < 0.260. The calculation has indicated that F is not in the critical region, so the hypothesis that σ12 = σ22 may be accepted.
The results of the calculations of F (S12/S22) for all of the major constituents as well as some of the minor constituents are given in Table 5. It should be noted that in every case, the values of F are within the prescribed upper and lower limits, indicating that at the 5-percent level of significance, the variances are equal.
Table 5--Values of F as calculated to test the hypothesis concerning two variances. The hypothesis is rejected if F > 3.85 or < 0.260.
| Chemical constituent | F |
|---|---|
| SiO2 | 0.8186 |
| Al2O3 | 2.734 |
| Fe2O3 | 0.8325 |
| CaO | 0.8939 |
| MgO | 0.3678 |
| P2O5 | 0.4286 |
| K2O | 1.108 |
| Na2O | 0.8333 |
| CO2 | 0.9900 |
From the foregoing discussion it is apparent that, within the prescribed limits, the variation between samples collected on the close spacing within the quarry and the variation between samples taken on a 6-mile spacing are the same. Thus it can be concluded that where chemical data are used to depict trends within the Plattsmouth Limestone, a spacing of approximately 6 miles would be just as effective as a spacing of several hundred feet. Although the analysis of variance has not been applied to other limestone units, experience in the geochemistry laboratory seems to indicate that the conclusions arrived at in this study would hold true for other limestones of the Pennsylvanian System in the Midcontinent region, particularly those limestones in the Shawnee Group.
Even though the results indicate equal variance within a 5-percent level of significance, difference in absolute composition exists from location to location and conclusions drawn from chemical analyses of a series of samples must be carefully evaluated. Interpretations of chemical data obtained from rock analyses must, as always, be prefaced by an evaluation of the sampling technique used. It should be evident that considerable care and judgment should be exercised when samples are collected for any project. A geochemical sampling program should be flexible enough so that the spacing between samples can be varied to include areas where unconformities or physical changes are obvious. If such areas are not sampled because they do not fall within the prescribed sampling grid, it is possible that significant differences in the chemistry of the rock will be missed. The wider sample spacing is an excellent means of determining chemical trends and establishing the general chemical composition of a limestone unit. However, an investigator should always return and collect additional samples in those areas where initial chemical analyses indicate unique changes in chemical content.
It should also be evident that, although the sample spacing does not prohibit the detection of chemical trends, the distance between samples, whether large or small, does not necessarily insure accurate prediction. Incorrect conclusions concerning chemical trends may be drawn from the analyses of samples collected on a close sample spacing as well as on a wide spacing if poor sampling techniques are employed. Careful chemical analysis of limestone samples is no guarantee of an accurate representation of chemical trends. It might be said that a chemical analysis does not start in the laboratory, it starts at the location where the sample is collected.
The values shown in Tables 2 and 3 indicate that general chemical trends can be depicted regardless of the spacing between samples. As seen in Table 2, the CaO and CO2 show a general increase from north to south along the outcrop, the SiO2 and Al2O3 decrease from north to south, and the Fe2O3 and MgO increase slightly in the central part of the area. The samples from inside the quarry indicate a decrease in CaO and CO2 from front to back (Table 3). It is of interest to note that the specimens from the quarry were also sampled on a general north to south grid (Fig. 2), as were the outcrop samples (Fig. 1), and the same general trends can be noted for both sets of samples.
The analyses shown in Table 4 indicate that samples of a single limestone unit collected from various locations within an area give differing results. It would be difficult to select one analysis from Table 4 that is representative of the entire area. Several examples of the wide variation in results can be seen in the results obtained for SiO2, CaO, and MgO. The values for SiO2 range from 6.9 percent to 12.33 percent and the values for CaO range from 48.97 percent to 43.41 percent. MgO values show a range of 1.39 percent to 3.15 percent. The standard deviation also has a wide range of values. They include 1.782 percent and 1.769 percent for SiO2, and CaO respectively to 0.023 percent for SO3. When these and the other values listed in Table 4 are considered it is evident that an initial part of any economic study of the development of any deposit should be the collection and analysis of a series of samples from a series of locations over the entire area under consideration. If one analysis that is representative of the entire deposit is desired, it is possible to take the analyses of all samples from the area in question and average the results as suggested by Wager and Brown (1960). However, if the average of several samples is used, the range in the composition and the standard deviations must also be noted to give an accurate representation of the chemical composition of the deposit. All of these factors should be evaluated and presented as an integral part of the economic appraisal of any limestone deposit.
Dixon, Wilfrid J., and Massey, Frank J., Jr., 1951, Introduction to Statistical Analysis: McGraw-Hill Book Company, Inc., New York, 370 p.
Galle, O. K., 1964, Comparison of chemical analysis based upon two sampling procedures and two sample preparation methods: Trans. Kansas Acad. Sci., vol. 67, no. 1, p. 100- 110.
Galle, O. K., Waugh, W. N., and Hill, W. E., Jr., 1965, The geochemistry of the limestones of the Shawnee Group in Kansas, Part 1, Oread Formation: State Geol. Survey of Kansas, Open-file report.
Hill, W. E., Jr., Waugh, W. N., Galle, O. K. and Runnels, R. T., 1961, Methods of chemical analysis for carbonate and silicate rocks: Kansas Geol. Survey, Bull. 152, pt. 1, p. 1-130.
Wager, L. R. and Brown, G. M., 1960, Chapter II, Collection and preparation of material for analysis; in, Methods in Geochemistry, H.A. Smales and L. R. Wager, eds.: Interscience Publishers, New York, 64 p.
Kansas Geological Survey, Plattsmouth Limestone Member (Pennsylvanian) in Kansas
Placed on web May 7, 2009; originally published in Feb. 1966.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/180_1/index.html