
Kansas Geological Survey, Bulletin 175, part 3, originally published in 1965

Originally published in 1965 as Kansas Geological Survey Bulletin 175, part 3. This is, in general, the original text as published. The information has not been updated. An Acrobat PDF version (5 MB) is also available.
Solubility experiments were conducted on twenty carbonate and non-carbonate minerals using selected Versene solutions and acids. Two sets of experiments were run simultaneously. The first set contained only the mineral fragment and Versene solution; the second set contained another fragment of the same mineral and a block of limestone. For comparison, experiments were carried out with 10- and 25-percent solutions of hydrochloric and acetic acids.
The results of the solubility tests using Versene were found to be similar to the mineral solution results obtained with 10-percent acetic acid. Five of the twenty minerals, gypsum, anhydrite, calcite, aragonite, and witherite, were consistently affected by the Versene solutions. However, in the presence of a reacting block of limestone the rates of solution of these five minerals were altered. The gypsum was dissolved more rapidly in the presence of a limestone block and the other four minerals were dissolved more slowly. Techniques using Versene solution show little advantage over acetic acid techniques in the extraction from limestones of the twenty accessory minerals which were used in these experiments. However, a better recovery of carbonate minerals other than calcite and aragonite can be expected with Versene residue methods.
This study of the solubility of twenty minerals in Versene solutions is based in part upon investigations of the chelating properties of Versene (ethylenediaminetetraacetic acid or EDTA) by Hill and Runnels (1960), Glover (1961), and Hill and Goebel (1963), which have shown that the variously substituted Versenes can effect a separation of calcite from many accessory minerals, and, although all carbonate minerals are not attacked equally, calcite is preferentially dissolved.
Limestone is composed primarily of the carbonates of calcium and magnesium. However, many other minerals are found in limestones in varying quantities. Of these minerals some are relatively common and others rare, but the accessory mineral assemblage of a limestone can be important in studies of source of sediment, environment of deposition, and changes related to diagenesis. Many of the minerals present in a limestone are below the detection limits of X-ray diffraction methods, unless they can be concentrated by removal of the calcite matrix. The recovery of accessory minerals with a minimum of alteration during the extraction process is necessary for an accurate evaluation and analysis of the secondary mineral suite.
A detailed study of the geochemistry of carbonates requires the development of newer methods of separating the mineral components, some of which cannot be successfully separated by existing methods.
To date, acid insoluble residue techniques have been the principal method used to separate the component parts of a limestone for further study. These techniques produce the partial breakdown of a carbonate rock into an acid insoluble residue and a solution of the acid soluble fraction. The various acid residue methods result in a rapid solution of some of the carbonate minerals and represent the fastest way of obtaining a residue for examination.
A resume of recent studies in this field is presented by Ellingboe and Wilson (1964) who discuss the effects of acid on a number of minerals and the effects of acid leaching of clay minerals.
The effects of Versene solutions on carbonates other than calcite and upon clays and non-carbonate minerals are little known. Glover (1961) reported the recovery of glauconitic fragments from carbonate rocks. Hill and Goebel (1963), in a study of the chelating effects of selected Versene solutions, reported a visually apparent alteration of part of the iron minerals of the limestone to a brown, gelatinous residue, probably ferric hydroxide.
The chemical effects of Versene on accessory minerals and clays had to be determined in order to predict accurately the results of using Versene insoluble residue techniques on limestone and other calcareous sediments. The purpose of this investigation was to discover what minerals could be recovered, what the relative solution rates of the various minerals were, and what acid-soluble minerals, if any, could be recovered. It was hoped that the results obtained by the Versene solution techniques for insoluble residues would complement the information obtained by acid insoluble residue methods through the recovery of minerals normally dissolved or altered by conventional acid techniques.
Fragments of twenty carbonate and non-carbonate minerals present in some limestones as accessory minerals (Table 1) were chosen for the preliminary investigation of the solubility of minerals in Versene. The minerals comprised three groups: (1) Carbonate minerals (acid-soluble); (2) Non-carbonate calcium minerals (water-soluble to acid-insoluble); and (3) R2O3 minerals (acid-soluble and insoluble).
Table 1--Samples and their properties.
| Mineral | Specific gravity | Hardness | Crystal form | Color |
|---|---|---|---|---|
| 1. Calcite (CaCO3) | 2.71 | 3 | Single crystal | Yellow |
| 2. Magnesite (MgCO3) | 3.00 | 3-4 1/4 | Coarsely crystalline | White |
| 3. Siderite (FeCO3) | 3.96 | 3-4 1/4 | Coarsely crystalline | Yellow |
| 4. Rhodochrosite (MnCO3) | 3.70 | 3 1/2-4 | Coarsely crystalline | Pink |
| 5. Aragonite (CaCO3) | 2.95 | 3 1/2-4 | Pseudohexogonal crystal | Yellow |
| 6. Witherite (BaCO3) | 4.29 | 3-3 1/2 | Coarsely crystalline | White-buff |
| 7. Strontianite (SrCO3) | 3.76 | 3 1/2 | Coarsely crystalline | White |
| 8. Dolomite [CaMg(CO3)2] | 2.85 | 3 1/2-4 | Coarsely crystalline | White |
| 9. Anhydrite (CaSO4) | 2.98 | 3 1/2 | Cryptocrystalline | Gray |
| 10. Gypsum (CaSO4·2H2O) | 2.31 | 2 | Cryptocrystalline | White |
| 11. Fluorite (CaF2) | 3.18 | 4 | Single crystal | Yellow |
| 12. Apatite [Ca5(PO4)3F] | 3.1-3.2 | 5 | Coarsely crystalline | Green |
| 13. Marcasite (FeS2) | 4.89 | 6-6 1/2 | Coarsely crystalline (cockscomb) | Brassy Yellow |
| 14. Pyrrhotite [Fe(1-x)S] | 4.58-4.65 | 3 1/2 -4 1/2 | Cryptocrystalline | Yellow |
| 15. Hematite (Fe2O3) | 5.26 | 5-6 | Cryptocrystalline | Black |
| 16. Ilmenite (FeTiO3) | 4.72 | 5-6 | Cryptocrystalline | Black |
| 17. Magnetite (Fe3O4) | 5.18 | 5 1/2-6 1/2 | Cryptocrystalline | Black |
| 18. Limonite (Hydrous Fe2O3) | 2.7-43 | 4-5 1/2 | Amorphous | Yellow-brown |
| 19. Bauxite (Hydrous Al2O3) | Amorphous | Yellow | ||
| 20. Pyrolusite (MnO2) | 4.4-5.0 | 6-6 1/2 | Cryptocrystalline | Black |
Several non-carbonate minerals of calcium were included to ascertain the extent to which the preferential chelation of calcium carried over into these minerals. The iron, manganese, and aluminum minerals (R2O3-group minerals) were included because it was necessary to determine the solution of iron minerals (or minerals of other trivalent elements) in Versene.
The twenty mineral samples ranged in crystallinity from cryptocrystalline to coarsely crystalline, and included single crystals and pieces of single crystals. In working with a wide range of crystallinities, porosities, and slightly different sample sizes, no control over exposed surface area was possible. The mineral fragments used were approximately the same size (1/2" x 1" x 1") but were randomly shaped. To minimize any variation between blocks, wherever possible, the same mineral fragments were washed, dried, and reused in consecutive tests in the different Versenes of the series. The weights of each mineral fragment before immersion and after immersion for 24 and 48 hours were recorded.
X-ray diffractograms confirmed the mineralogic composition and revealed no significant amounts of impurities in any of the samples used in the test series. The limestone blocks used in the second series of Versene experiments were cut from the same limestone block used as an arbitrary standard by Hill and Goebel (1963). Recent unpublished work on the conodont assemblage of this limestone indicates that it is probably Keokuk Limestone and not Warsaw Limestone as originally identified (Thompson, 1965).
Hydrogen-, and sodium-substituted Versenes are commercially available. Potassium-, and ammonium-substituted Versenes were synthesized in the Geochemistry Laboratory of the State Geological Survey of Kansas. Commercially available technical grade tetra-sodium ethylenediamine tetra acetic acid was used as the basic reagent in these syntheses. The low cost of this form of Versene (approximately 43 cents per pound [price as of October, 1964]) permits bulk usage of it for the solution of large quantities of calcareous materials much more economically than analytical reagent-grade Versenes, which range from $6.00 to $8.00 per pound.
To make up the three different Versenes used in the tests it was necessary first to convert the tetra-sodium Versene to the Versene acid salt and resubstitute the alkali metals or other compounds desired. This conversion and resubstitution was also the basic method of reclaiming spent Versene for reuse. The tetra-sodium Versene was dissolved in distilled water and precipitated in the tetra-hydrogen Versene acid form by the addition of hydrochloric acid in small increments until a pH of 1 was attained. The solution and precipitation were carried out in 20-quart polyethylene wastebaskets placed on a magnetic stirrer. In an inert-reaction vessel of this size, 3-5 kg of Versene was converted at one time. The supernatant liquid was decanted and vacuum-filtered through Munktells 11 cm OK filter paper in a buchner funnel. The filtrate (acidic sodium chloride solution) was discarded and the precipitate was washed with 10-percent hydrochloric acid and vacuum-filtered. The resultant filter cakes of Versene acid were air dried and placed in a single, covered storage container. A spectrographic analysis of the precipitated Versene acid showed only trace amounts of sodium remaining, indicating an almost complete conversion to the tetra-hydrogen form.
The Versene acid from spent Versene solutions is easily recycled by addition of sufficient hydrochloric acid as described above and washing the precipitate. In this manner, the Versene acid may be reclaimed and reused many times, thus greatly reducing the cost of reagents. Because sulfuric acid co-precipitates calcium sulfate and complicates the separation of Versene acid, its use is not recommended.
One and 2.0 N, sodium-, potassium-, and ammonium-substituted Versenes were prepared. The normality of the Versene solutions was based upon the reaction
4 MOH + H4 (EDTA) → M4 (EDTA) + 4H2O,
where M is any univalent metal or positive radical.
Weighed amounts of technical-grade sodium hydroxide or potassium hydroxide were dissolved in distilled water; to these solutions weighed amounts of Versene acid (powdered form) were added to give the normalities desired (Table 2). Some adjustment of the amount of alkali metal hydroxide was necessary to attain the desired pH. The pH values of the solutions were adjusted to pH 8 for the tests by addition of small increments of solid alkali hydroxide, with continuous agitation of the solution by a magnetic stirrer. When pH 8 was attained, each wastebasket was covered with a plastic sheet and reserved for testing. Aliquots (200 ml) were withdrawn from this stock solution and placed in 250-ml beakers. Test solutions of ammonium Versene were prepared by titrating the appropriate amount of Versene acid for the desired normality (Table 2) with the ammonium hydroxide to pH 8, and adjusting to volume.
Table 2--Amounts of selected reagent required for conversion of Versene acid to desired concentrations of bialkali-substituted Versene. [In order to convert to bialkali Versene, use the indicated weight of Versene acid with the indicated weight of one of the alkali hydroxides for the concentration desired and make up to one liter. For quantities greater than one liter, use multiples of both weights. Trialkali Versene is manufactured by using weight of Versene acid and 1.5 times the indicated amount of alkali hydroxide. For quadri-alkali Versene, double the indicated amount of alkali hydroxide.]
| Conc. desired |
g/l Versene acid |
g/l of any one alkali hydroxide below |
Rated efficiency (will dissolve CaCO3 g/l)† |
|
|---|---|---|---|---|
| NaOH | KOH | |||
| 0.1 N | 73 | 2.0 | 2.8 | 25 |
| 05 N | 365 | 10.0 | 14.0 | 125 |
| 1.0 N | 73.1 | 20.0 | 28.0 | 25.0 |
| 2.0 N | 146.1 | 40.0 | 56.0 | 50.0 |
| 3.0 N | 219.2 | 60.0 | 84.0 | 75.0 |
| 4.0 N | 292.3 | 80.0 | 112.0 | 100.0 |
| 5.0 N | 365.3 | 100.0 | 140.2 | 125.0 |
| † Based upon the reaction M4 (EDTA) + CaCO3 → CaM2 (EDTA) + M2CO3, where M is hydrogen, alkali metal, or other substituted group. |
||||
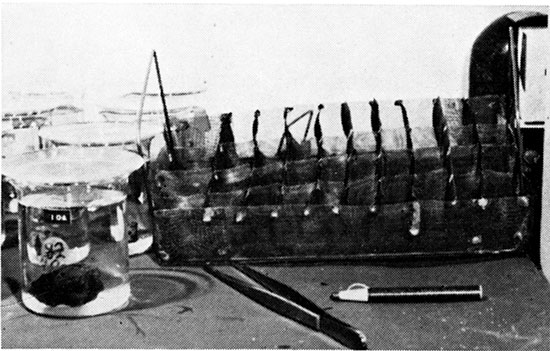
The mineral solution tests were run in serially numbered 250-ml beakers (Fig. 1). Each beaker contained 200 ml of the Versene test solution. Two sets of the twenty minerals were run simultaneously with each different Versene solution. The first set of twenty beakers contained only the mineral fragments and Versene solution. Each beaker of the second set contained a block of limestone in addition to the mineral fragment and Versene solution in order to determine the effect of the presence of limestone on the reaction of Versene with the mineral. The reaction of the limestone will cause a more rapid weakening of the Versene reagent, and the solution rate of the mineral in the presence of the limestone should be lower than the solution rate of the mineral alone. The mineral-limestone solution rate in Versene would more closely approximate that of an insoluble residue of a carbonate rock.
Figure 1--Serially numbered beakers showing mineral samples immersed in Versene solution. The beaker in front is from the second set of beakers and shows a mineral fragment with a block of limestone in Versene solution.

Before beginning the solution tests in Versene, mineral samples were washed for two hours in a circulating distilled-water bath and then dried for 1 hour at 140°C. After cooling the samples were weighed to the nearest 0.1 g and the original sample weights recorded. Each sample was then placed in the beaker of Versene solution with stainless steel tweezers. After 24 hours of immersion the samples were removed from solution and placed in a compartmented copper-screen tray (Fig. 2). The tray was immersed in a circulating distilled-water bath for two hours. The tray of samples was dried for 1 hour at 140°C and each sample weighed.
Figure 2--The compartmented copper-screen tray which was used to hold the samples while being washed and dried.
Before reimmersing the blocks, the Versene solutions were stirred to minimize any effect of density stratification in the partially spent solutions. No provision was made for additional agitation. Each block was returned to its beaker for a second 24-hour immersion period in the same 200 ml of Versene solution. After two 24-hour immersion periods in the same Versene solution the samples were removed, washed, dried, and weighed as before. This sequence was repeated in 1.0 and 2.0 N solutions of sodium, potassium, and ammonium Versene and the data recorded. Some surficial loss of fine mineral grains occurred; these grains were either undercut by solution along mineral planes of greater solubility, or abraded in handling and remained in the bottom of the beaker at the completion of the test series. The residues were filtered onto numbered, dried, and tared 11 cm Munktells OK filter papers. Any residual Versene solution in the paper was washed through with distilled water. Each filter paper and residue was dried at 140°C for one hour and weighed to the nearest 0.01 g and the residue weights were determined. These weights permit a correction of the weight loss value after 48 hours, where the residue is sufficiently large to necessitate a correction. If the residue weighed 0.1 g or more a correction was made.
Comparative solution-rate tests on the minerals were made with 10-percent acetic and hydrochloric acids for a 24-hour period as recommended by Ellingboe and Wilson (1964). Additional solution tests were made with 25-percent acetic and hydrochloric acids for a period of 4 hours.
The data and calculations obtained from the solution tests for each concentration of each Versene and acid are presented in the section Tables of Solution Data (Tables 3 to 22). The values reported as solution rate in grams per hour are empirical and, within the control limits of the method used, give a relative rate of solution of the minerals for comparison with the solution rate of limestone.
The mineral samples were run in duplicate. In one beaker a piece of the mineral was placed in 200 ml of the Versene reagent. In a second beaker a piece of the same mineral and a small block of limestone were placed in 200 ml of the Versene reagent. The effects of the blocks of limestone on the reaction of the Versene with the mineral tested are reported in Tables 9 through 14. Some solution effects could be seen. In the beakers containing the blocks of limestone, a thin layer of insoluble residue settled to the bottom of the beaker and the solutions were colored a pale yellow from the hydrolysis of the iron minerals. The solutions in the beakers containing blocks of the mineral marcasite we're colored a dark yellow after reaction with Versene solutions. Some effervescence was encountered with the gypsum samples in sodium Versene solutions. Attempts to duplicate the reaction with similar blocks of gypsum in the same and other Versene solutions were unsuccessful. Of the twenty minerals used in the study, gypsum, anhydrite, calcite, aragonite, and witherite were attacked by the Versene solution.
A summary of the effects of the different Versene solutions and acids on the twenty minerals used in this study is presented in Table 23. A coding of weight loss was used in this table for rapid comparison and the weight loss values for 0-48 hours were divided into three categories: (1) greater than 1.0 g loss in 48 hrs; (2) 0-0.9 g loss in 48 hrs; and (3) no apparent loss in weight, less than 0.1 g in 48 hrs.
The minerals which consistently exhibited the greatest weight losses in the Versene solutions were the calcium sulfate compounds, gypsum and anhydrite. The calcium carbonate samples, calcite and aragonite, were next highest in weight loss. Witherite (barium carbonate) was the fifth mineral whose original weight was consistently affected by the Versene solutions, and it was the least affected of the five minerals.
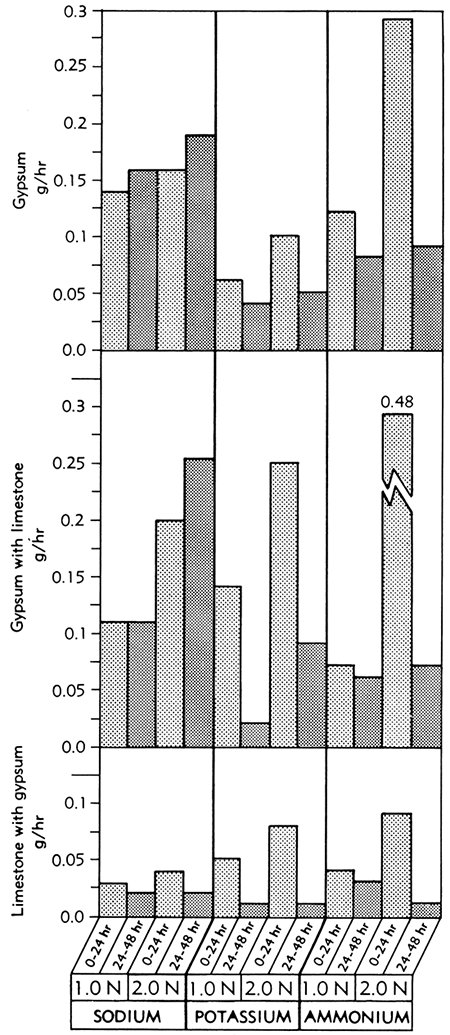
Solution rates (g/hr) of gypsum in the three Versenes at the two concentrations used are shown in Figure 3. Of the twenty minerals in the study, gypsum exhibited the highest rate of solution. In the presence of a block of limestone the solution rate of gypsum increased.
Figure 3--Bar graphs showing solution rates of gypsum, gypsum in the presence of limestone, and limestone in the presence of gypsum, averaged at the end of the first and second 24-hour periods of immersion in various concentrations of sodium, potassium, and ammonium Versene solutions at room temperature.
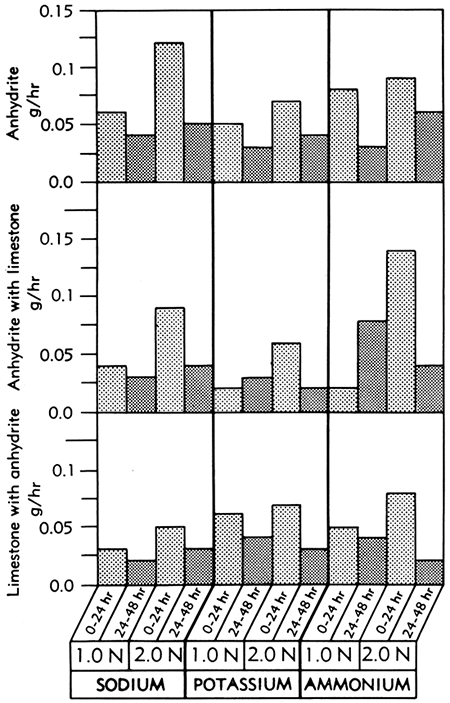
The anhydrite samples also dissolved in the Versene solutions, but their rate of solution was approximately one-half or less than that of the gypsum samples (Fig. 4). As expected, the solution rate of anhydrite decreased slightly upon the addition of the block of limestone.
Figure 4--Bar graphs showing solution rates of anhydrite, anhydrite in the presence of limestone, and limestone in the presence of anhydrite, averaged at the end of the first and second 24-hour periods of immersion in various concentrations of sodium, potassium, and ammonium Versene solutions at room temperature.
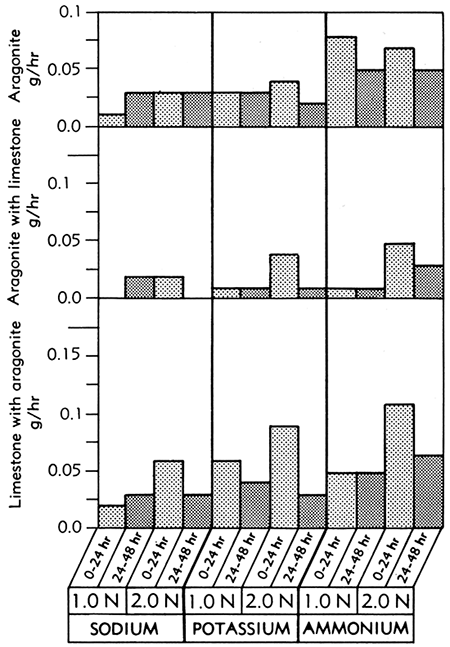
The aragonite samples showed a slightly faster rate of solution than the calcite samples and the rates of solution were significantly slowed by the addition of the limestone blocks (Fig. 5).
Figure 5--Bar graphs showing solution rates of aragonite, aragonite in the presence of limestone, and limestone in the presence of aragonite, averaged at the end of the first and second 24-hour periods of immersion in various concentrations of sodium, potassium, and ammonium Versene solutions at room temperature.
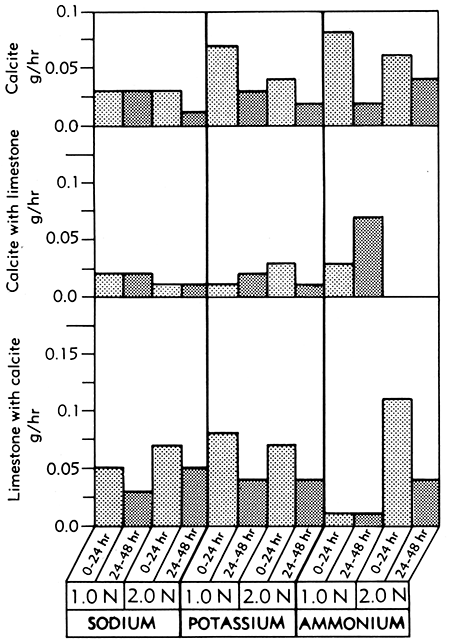
The solution rate of a single crystal of calcite (Fig. 6) decreased when the reaction took place with Versene in the presence of a block of fine-grained limestone. The finely crystalline limestone reacted much faster than the large, single crystal of calcite. This differential rate of solubility may be useful in making peels for petrofabric studies of carbonate rocks when a polished surface is etched by Versene.
Figure 6--Bar graphs showing solution rates of calcite, calcite in the presence of limestone, and limestone in the presence of calcite, averaged at the end of the first and second 24-hour periods of immersion in various concentrations of sodium, potassium, and ammonium Versene solutions at room temperature.
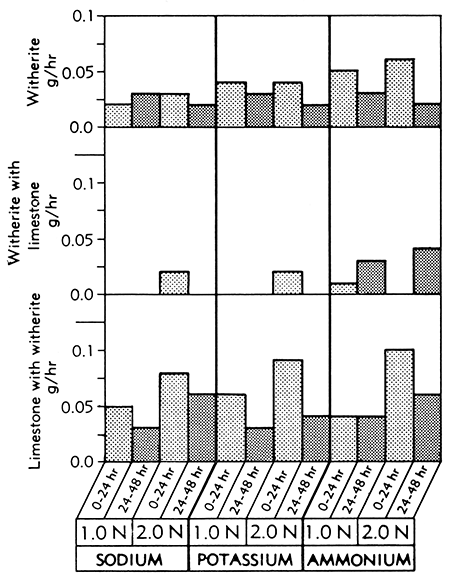
Witherite was dissolved by the Versene solution (Fig. 7), but reaction with Versene in the presence of limestone greatly decreased the rate of solution.
Figure 7--Bar graphs showing solution rates of witherite, witherite in the presence of limestone, and limestone in the presence of witherite, averaged at the end of the first and second 24-hour periods of immersion in various concentrations of sodium, potassium, and ammonium Versene solutions at room temperature.
Lower solution rates were noted for marcasite, pyrolusite, and strontianite, in the Versene solutions, but again the solution rates were decreased by the addition of the blocks of limestone.
Small solution effects were found on many of the other minerals in 1.0 N sodium Versene. In general, these effects were not as great in potassium Versene and ammonium Versene (Table 23).
Some of the minerals used in this study, reported to be soluble in acids, were discovered to be relatively insoluble upon examination of the hydrochloric and acetic acid solution rates derived from our experiments. The solubility of minerals in acid is relative, and the solution of many of the less soluble minerals is very dependent upon the type of acid used, dilution factor, time of immersion, and temperature, in addition to the crystallinity, grain size, and exposed surface area of the sample itself. Many of the minerals reported in mineralogy texts as acid-soluble showed very little loss in weight under the experimental conditions employed in our solution tests. In this study, because no control was possible over crystallinity, surface area, or sample size, only gross observations of the soluble or insoluble character of a reportedly soluble mineral could be made.
Comparison of results showed that a number of minerals were strongly attacked by 10-percent hydrochloric acid that were unaffected by Versene solutions. In general, 10-percent acetic acid attacked the same minerals at approximately the same rate as the Versene solutions but dissolved more strontianite. However, the acetic acid solution did not attack gypsum and some of the R2O3 minerals to the extent that the Versene solutions did. During a four-hour period the 25-percent solutions of hydrochloric and acetic acid dissolved the same minerals as the 10-percent acids did during 24 hours. The hydrochloric acid experiments resulted in greater amounts of solution of more minerals than did the acetic acid and Versene experiments. Insoluble residues from acetic acid solutions can provide a more complete and more representative acid residue than can be recovered from hydrochloric acid solutions.
Both acids and Versene solutions are capable of dissolving the calcite fraction of a limestone sample to yield an insoluble residue. Most of the acid techniques yield useable insoluble residues faster than the Versene techniques. The comparative acid data (Tables 21 and 22) present a strong case for the use of acetic acid rather than hydrochloric acid for obtaining insoluble residues because of better accessory mineral recovery. Within the limits of the test conditions used, acetic acid attacked fewer of the accessory minerals than did hydrochloric acid. The primary value of the slower Versene residue techniques over the acetic acid methods is a better recovery of dolomite and accessory carbonate minerals. Of all the minerals investigated, magnesite was found to be the least soluble carbonate mineral when immersed in hydrochloric acid. Magnesite, siderite, and rhodochrosite were relatively unaffected by acetic acid solutions. The R2O3 group of minerals is relatively insoluble in both the weaker acids and Versenes.
Apatite, magnesite, siderite, rhodochrosite, strontianite and dolomite were little effected by the Versene solutions. When blocks of fine-grained limestone were placed in beakers with the mineral samples, the attack upon the coarsely crystalline calcite, aragonite, and witherite samples was greatly decreased. This indicates that grain size is a significant factor in the Versene insoluble residue techniques.
The recovery of apatite in Versene residues permits the extraction of phosphatic fossils, as do the acetic acid techniques. Surface etching and pitting of the phosphatic microfossils in acetic acid and Versene solutions led to the development of a new separation technique (Welch, et al., 1964). This method of residue removal from a reacting medium should result in a better insoluble residue recovery with both acetic and Versene solutions.
Versene solutions furnish a method for the solution of calcium carbonate and some other minerals at a neutral or basic pH, eliminating the necessity of an extremely low pH for the extraction of insoluble residues. The reaction is odorless and does not release gas; therefore, no fume hood is needed and the reactions can be carried out in smaller beakers. The results of this preliminary study indicate that possibly a better recovery of accessory alkaline earth and R2O3 carbonates can be expected with Versene residue methods.
Ellingboe, John, and Wilson, James, 1964, A quantitative separation of non-carbonate minerals: Jour. Sed. Petrology, v. 34, no. 2, p, 412-418.
Glover, E. D., 1961, Method of solution of calcareous materials using the complexing agent, EDTA: Jour. Sed. Petrology, v. 31, no. 4, p. 622-626.
Hill, W. E., Jr., and Goebel, E. D., 1963, Rates of solution of limestone using the chelating properties of Versene (EDTA) compounds: Kansas Geol. Survey, Bull. 165, pt. 7, p. 1-15. [available online]
Hill, W. E., Jr., and Runnels, R. T., 1960, Versene, a new tool for the study of carbonate rocks: Am. Assoc. Petroleum Geologists, Bull., v, 44, no. 5, p. 632-633.
Palache, Charles, Berman, Harry, and Frondel, Clifford, 1944, The system of mineralogy of J. D. Dana and E. S. Dalla: John Wiley and Sons, Inc., New York, v. 2, ed. 7, 1124 p.,
Thompson, T. L., 1965, Conodonts from the Meramecian Stage (Upper Mississippian) of Kansas: Unpublished Ph.D. Dissertation, Univ, of Iowa, 205 p.
Welch, R. G., Hill, W. E., Jr., and Ireland, H. A., 1964, A contin[u]ous extraction technique for insoluble residues and phosphatic microfossils: Trans. Kan, Acad. Sci., v. 67, no. 3, p, 553-555.
Table 3--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N sodium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 18.9 | 0.8 | 0.7 | 0.03 | 0.03 | 0.03 | 7.9 | 0.05 |
| 2. Magnesite | 23.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 3. Siderite | 25.9 | 0.4 | 0.0 | 0.02 | 0.00 | 0.01 | 1.5 | 0.09 |
| 4. Rhodochrosite | 28.8 | 0.2 | 0.1 | 0.01 | 0.00 | 0.01 | 1.0 | 0.09 |
| 5. Aragonite | 16.9 | 0.3 | 0.7 | 0.01 | 0.03 | 0.02 | 5.9 | 0.04 |
| 6. Witherite | 20.0 | 0.4 | 0.6 | 0.02 | 0.03 | 0.03 | 5.0 | 0.09 |
| 7. Strontianite | 16.0 | 0.0 | 0.3 | 0.00 | 0.01 | 0.01 | 1.8 | 0.10 |
| 8. Dolomite | 21.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 9. Anhydrite | 22.4 | 1.4 | 1.0 | 0.06 | 0.04 | 0.05 | 10.7 | 0.05 |
| 10. Gypsum | 23.2 | 3.4 | 3.8 | 0.14 | 0.16 | 0.15 | 31.0 | 0.06 |
| 11. Fluorite | 26.5 | 0.2 | 0.1 | 0.01 | 0.00 | 0.01 | 0.1 | 0.06 |
| 12. Apatite | 15.1 | 0.2 | 0.0 | 0.0 | 0.00 | 0.01 | 1.3 | 0.05 |
| 13. Marcasite | 27.9 | 0.6 | 0.4 | 0.02 | 0.02 | 0.02 | 3.2 | 0.15 |
| 14. Pyrrhotite | 23.1 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.4 | 0.08 |
| 15. Hematite | 13.8 | 0.2 | 0.1 | 0.01 | 0.00 | 0.01 | 2.1 | 0.07 |
| 16. Ilmenite | 30.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 17. Magnetite | 37.9 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.2 | 0.08 |
| 18. Limonite | 34.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 19. Bauxite | 11.5 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.8 | 0.08 |
| 20. Pyrolusite | 25.6 | 0.0 | 0.2 | 0.00 | 0.01 | 0.01 | 0.7 | 0.09 |
Table 4--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N sodium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 17.4 | 0.7 | 0.2 | 0.03 | 0.01 | 0.02 | 5.1 | 0.03 |
| 2. Magnesite | 23.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.02 |
| 3. Siderite | 25.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.03 |
| 4. Rhodochrosite | 28.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 5. Aragonite | 15.9 | 0.7 | 0.8 | 0.03 | 0.03 | 0.03 | 9.4 | 0.05 |
| 6. Witherite | 19.0 | 0.8 | 0.5 | 0.03 | 0.02 | 0.03 | 6.8 | 0.08 |
| 7. Strontianite | 15.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 8. Dolomite | 21.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 9. Anhydrite | 20.0 | 2.8 | 1.3 | 0.12 | 0.05 | 0.09 | 20.5 | 0.09 |
| 10. Gypsum | 16.0 | 3.9 | 4.5 | 0.16 | 0.19 | 0.18 | 52.5 | 0.07 |
| 11. Fluorite | 26.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 12. Apatite | 14.9 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.6 | 0.00 |
| 13. Marcasite | 26.9 | 0.2 | 0.0 | 0.01 | 0.00 | 0.01 | 0.7 | 0.03 |
| 14. Pyrrhotite | 23.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 |
| 15. Hematite | 13.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.02 |
| 16. Ilmenite | 30.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 17. Magnetite | 37.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.00 |
| 18. Limonite | 34.4 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.2 | 0.02 |
| 19. Bauxite | 11.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 20. Pyrolusite | 25.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
Table 5--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N potassium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 16.5 | 1.7 | 0.6 | 0.07 | 0.03 | 0.05 | 13.9 | 0.05 |
| 2. Magnesite | 23.5 | 0.2 | 0.0 | 0.01 | 0.00 | 0.01 | 0.8 | 0.09 |
| 3. Siderite | 25.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 4. Rhodochrosite | 28.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.09 |
| 5. Aragonite | 16.4 | 0.7 | 0.7 | 0.03 | 0.03 | 0.03 | 85 | 0.07 |
| 6. Witherite | 17.7 | 1.0 | 0.7 | 0.04 | 0.03 | 0.04 | 9.6 | 0.09 |
| 7. Strontianite | 15.7 | 0.6 | 0.1 | 0.03 | 0.00 | 0.02 | 4.4 | 0.05 |
| 8. Dolomite | 21.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
| 9. Anhydrite | 15.9 | 1.3 | 0.8 | 0.05 | 0.03 | 0.04 | 13.2 | 0.05 |
| 10. Gypsum | 7.6 | 1.4 | 1.1 | 0.06 | 0.04 | 0.05 | 32.8 | 0.04 |
| 11. Fluorite | 26.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 12. Apatite | 14.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 13. Marcasite | 26.7 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.7 | 0.06 |
| 14. Pyrrhotite | 23.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 15. Hematite | 13.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 16. Ilmenite | 30.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 17. Magnetite | 37.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 18. Limonite | 34.3 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.5 | 0.07 |
| 19. Bauxite | 11.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
| 20. Pyrolusite | 25.4 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.7 | 0.06 |
Table 6--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N potassium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 14.2 | 1.1 | 0.5 | 0.04 | 0.02 | 0.03 | 11.2 | 0.06 |
| 2. Magnesite | 23.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 3. Siderite | 25.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 4. Rhodochrosite | 28.5 | 0.3 | 0.0 | 0.01 | 0.00 | 0.01 | 1.0 | 0.05 |
| 5. Aragonite | 15.0 | 1.1 | 0.6 | 0.04 | 0.02 | 0.03 | 11.3 | 0.06 |
| 6. Witherite | 16.0 | 1.1 | 0.5 | 0.04 | 0.02 | 0.03 | 10.0 | 0.09 |
| 7. Strontianite | 15.0 | 0.4 | 0.0 | 0.02 | 0.00 | 0.01 | 2.6 | 0.09 |
| 8. Dolomite | 21.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 9. Anhydrite | 13.8 | 1.7 | 1.1 | 0.07 | 0.04 | 0.06 | 20.2 | 0.05 |
| 10. Gypsum | 5.1 | 2.4 | 1.3 | 0.10 | 0.05 | 0.08 | 72.5 | 0.04 |
| 11. Fluorite | 26.2 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.3 | 0.06 |
| 12. Apatite | 14.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 13. Marcasite | 26.5 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.7 | 0.07 |
| 14. Pyrrhotite | 23.0 | 0.3 | 0.0 | 0.01 | 0.00 | 0.01 | 1.3 | 0.06 |
| 15. Hematite | 13.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 16. Ilmenite | 30.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 17. Magnetite | 37.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
| 18. Limonite | 34.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 19. Bauxite | 11.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 20. Pyrolusite | 25.2 | 0.0 | 0.4 | 0.00 | 0.02 | 0.01 | 1.5 | 0.05 |
Table 7--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N ammonium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 12.6 | 2.0 | 0.6 | 0.08 | 0.02 | 0.05 | 20.6 | 0.05 |
| 2. Magnesite | 23.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 3. Siderite | 25.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 4. Rhodochrosite | 28.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 5. Aragonite | 13.3 | 1.9 | 1.2 | 0.08 | 0.05 | 0.07 | 23.3 | 0.07 |
| 6. Witherite | 14.4 | 1.3 | 0.8 | 0.05 | 0.03 | 0.04 | 14.5 | 0.06 |
| 7. Strontianite | 14.6 | 0.6 | 0.4 | 0.02 | 0.02 | 0.02 | 6.8 | 0.05 |
| 8. Dolomite | 21.7 | 0.2 | 0.3 | 0.01 | 0.01 | 0.01 | 2.3 | 0.09 |
| 9. Anhydrite | 11.0 | 2.0 | 0.7 | 0.08 | 0.03 | 0.06 | 24.5 | 0.05 |
| 10. Gypsum | 11.7 | 2.9 | 2.0 | 0.12 | 0.08 | 0.10 | 41.8 | 0.07 |
| II. Fluorite | 26.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 12. Apatite | 14.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 13. Marcasite | 26.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 14. Pyrrhotite | 22.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.03 |
| 15. Hematite | 13.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 16. Ilmenite | 30.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 17. Magnetite | 37.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 18. Limonite | 34.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.03 |
| 19. Bauxite | 11.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 20. Pyrolusite | 24.8 | 0.3 | 0.0 | 0.01 | 0.00 | 0.01 | 1.2 | 0.09 |
Table 8--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N ammonium Versene at periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
Residue weight 0-48 hrs g |
|---|---|---|---|---|---|---|---|---|
| 1. Calcite | 13.2 | 1.4 | 1.0 | 0.06 | 0.04 | 0.05 | 18.1 | 0.05 |
| 2. Magnesite | 7.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.07 |
| 3. Siderite | 33.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 4. Rhodochrosite | 13.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.08 |
| 5. Aragonite | 26.0 | 1.8 | 1.3 | 0.07 | 0.05 | 0.06 | 11.9 | 0.02 |
| 6. Witherite | 23.0 | 1.4 | 0.5 | 0.06 | 0.02 | 0.04 | 8.2 | 0.04 |
| 7. Strontianite | 11.8 | 0.0 | 0.2 | 0.00 | 0.00 | 0.00 | 1.6 | 0.07 |
| 8. Dolomite | 14.3 | 0.2 | 0.3 | 0.01 | 0.01 | 0.01 | 3.4 | 0.04 |
| 9. Anhydrite | 10.0 | 2.3 | 1.6 | 0.09 | 0.06 | 0.08 | 39.0 | 0.06 |
| 10. Gypsum | 17.1 | 7.0 | 2.2 | 0.29 | 0.09 | 0.19 | 53.8 | 0.03 |
| 11. Fluorite | 13.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 12. Apatite | 9.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
| 13. Marcasite | 26.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.05 |
| 14. Pyrrhotite | 14.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.04 |
| 15. Hematite | 16.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.09 |
| 16. Ilmenite | 15.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 17. Magnetite | 16.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.09 |
| 18. Limonite | 9.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 | 0.06 |
| 19. Bauxite | 9.0 | 0.4 | 0.0 | 0.02 | 0.00 | 0.01 | 4.4 | 0.04 |
| 20. Pyrolusite | 16.3 | 0.0 | 0.6 | 0.00 | 0.02 | 0.01 | 3.6 | 0.04 |
Table 9--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N sodium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 22.4 | 0.4 | 0.5 | 0.01 | 0.02 | 0.02 | 4.0 |
| 2. Magnesite | 29.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 25.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 4. Rhodochrosite | 25.7 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.3 |
| 5. Aragonite | 18.0 | 0.1 | 0.6 | 0.00 | 0.02 | 0.01 | 3.8 |
| 6: Witherite | 23.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 7. Strontianite | 26.2 | 0.2 | 0.0 | 0.01 | 0.00 | 0.01 | 0.7 |
| 8. Dolomite | 15.0 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.6 |
| 9. Anhydrite | 14.6 | 1.0 | 0.7 | 0.04 | 0.03 | 0.04 | 11.6 |
| 10. Gypsum | 13.1 | 2.7 | 2.8 | 0.11 | 0.11 | 0.11 | 41.9 |
| 11. Fluorite | 26.6 | 0.1 | 0.1 | 0.00 | 0.00 | 0.00 | 0.7 |
| 12. Apatite | 11.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.9 | 0.3 | 0.2 | 0.01 | 0.01 | 0.01 | 4.2 |
| 14. Pyrrhotite | 34.7 | 0.1 | 0.3 | 0.00 | 0.01 | 0.01 | 1.1 |
| 15. Hematite | 17.4 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.5 |
| 16. Ilmenite | 22.4 | 0.1 | 0.1 | 0.00 | 0.00 | 0.00 | 0.8 |
| 17. Magnetite | 26.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 18. Limonite | 17.3 | 0.2 | 0.1 | 0.00 | 0.00 | 0.00 | 1.7 |
| 19. Bauxite | 19.0 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 1.0 |
| 20. Pyrolusite | 14.5 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.6 |
Table 10--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N sodium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 21.5 | 0.3 | 0.4 | 0.01 | 0.01 | 0.01 | 3.2 |
| 2. Magnesite | 29.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 25.4 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.7 |
| 4. Rhodochrosite | 25.6 | 0.3 | 0.4 | 0.01 | 0.01 | 0.01 | 2.7 |
| 5. Aragonite | 17.3 | 0.5 | 0.0 | 0.02 | 0.00 | 0.01 | 2.8 |
| 6. Witherite | 23.5 | 0.6 | 0.1 | 0.02 | 0.00 | 0.01 | 2.9 |
| 7. Strontianite | 26.0 | 0.0 | 0.2 | 0.00 | 0.00 | 0.00 | 0.7 |
| 8. Dolomite | 14.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 9. Anhydrite | 12.9 | 2.3 | 1.1 | 0.09 | 0.04 | 0.07 | 26.3 |
| 10. Gypsum | 22.4 | 5.0 | 6.8 | 0.20 | 0.28 | 0.24 | 52.6 |
| 11. Fluorite | 26.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 12. Apatite | 11.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.4 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 1.7 |
| 14. Pyrrhotite | 34.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15. Hematite | 17.3 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 1.1 |
| 16. Ilmenite | 22.2 | 0.0 | 0.1 | 0.00 | 0.00 | 0.00 | 0.4 |
| 17. Magnetite | 26.4 | 0.0 | 0.4 | 0.00 | 0.01 | 0.01 | 1.5 |
| 18. Limonite | 17.0 | 0.1 | 0.0 | 0.00 | 0.00 | 0.00 | 0.5 |
| 19. Bauxite | 18.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 20. Pyrolusite | 14.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
Table 11--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N potassium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 20.8 | 0.4 | 0.5 | 0.01 | 0.02 | 0.02 | 4.3 |
| 2. Magnesite | 29.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 25.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 4. Rhodochrosite | 24.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 5. Aragonite | 19.4 | 0.3 | 0.3 | 0.01 | 0.01 | 0.01 | 3.0 |
| 6. Witherite | 22.8 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.8 |
| 7. Strontianite | 25.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 8. Dolomite | 14.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 9. Anhydrite | 9.5 | 0.5 | 0.8 | 0.02 | 0.03 | 0.03 | 13.6 |
| 10. Gypsum | 10.6 | 3.5 | 0.5 | 0.14 | 0.02 | 0.08 | 37.7 |
| 11. Fluorite | 26.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 12. Apatite | 11.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 14. Pyrrhotite | 34.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15. Hematite | 17.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 16. Ilmenite | 22.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 17. Magnetite | 26.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 18. Limonite | 16.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 19, Bauxite | 18.8 | 0.0 | 0.1 | 0.00 | 0.00 | 0.00 | 0.5 |
| 20. Pyrolusite | 14.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
Table 12--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N potassium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 19.9 | 0.7 | 0.4 | 0.03 | 0.01 | 0.02 | 5.5 |
| 2. Magnesite | 29.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 25.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 4. Rhodochrosite | 24.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 5. Aragonite | 18.8 | 0.9 | 0.3 | 0.04 | 0.01 | 0.03 | 63 |
| 6. Witherite | 22.6 | 0.6 | 0.0 | 0.02 | 0.00 | 0.01 | 2.6 |
| 7. Strontianite | 25.8 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 0.7 |
| 8. Dolomite | 14.9 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 1.3 |
| 9. Anhydrite | 8.2 | 1.5 | 0.5 | 0.06 | 0.02 | 0.04 | 24.3 |
| 10. Gypsum | 6.6 | 6.0 | 1.9 | 0.25 | 0.09 | 0.17 | 43.1 |
| 11. Fluorite | 26.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 12. Apatite | 11.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.2 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 1.7 |
| 14. Pyrrhotite | 34.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15. Hematite | 17.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 16. Ilmenite | 22.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 17. Magnetite | 26.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 18. Limonite | 16.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 19. Bauxite | 18.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 20. Pyrolusite | 14.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
Table 13--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 1.0 N ammonium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 18.8 | 0.7 | 0.4 | 0.03 | 0.07 | 0.05 | 5.8 |
| 2. Magnesite | 29.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 25.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 4. Rhodochrosite | 24.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 5. Aragonite | 17.6 | 0.4 | 0.4 | 0.01 | 0.01 | 0.01 | 4.5 |
| 6. Witherite | 22.0 | 0.3 | 0.7 | 0.01 | 0.03 | 0.02 | 4.5 |
| 7. Strontianite | 25.6 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 8. Dolomite | 14.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 9. Anhydrite | 6.2 | 0.4 | 1.9 | 0.02 | 0.08 | 0.05 | 37.0 |
| 10. Gypsum | 7.6 | 1.7 | 1.6 | 0.07 | 0.06 | 0.07 | 43.4 |
| 11. Fluorite | 26.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 12. Apatite | 11.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 14. Pyrrhotite | 34.3 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15. Hematite | 17.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 16. Ilmenite | 22.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 17. Magnetite | 26.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 18. Limonite | 16.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 19. Bauxite | 18.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 20. Pyrolusite | 14.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
Table 14--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 2.0 N ammonium Versene when reacted in the presence of blocks of limestone for periods of 24 and 48 hours.
| Mineral no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1. Calcite | 6.7 | 0.2 | 0.0 | 0.00 | 0.00 | 0.00 | 2.9 |
| 2. Magnesite | 20.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 3. Siderite | 37.5 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 4. Rhodochrosite | 26.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 5. Aragonite | 27.2 | 13 | 0.8 | 0.05 | 0.03 | 0.04 | 7.7 |
| 6. Witherite | 15.1 | 0.0 | 1.1 | 0.00 | 0.04 | 0.02 | 7.2 |
| 7. Strontianite | 18.2 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 8. Dolomite | 12.4 | 0.3 | 0.0 | 0.01 | 0.00 | 0.01 | 2.4 |
| 9. Anhydrite | 11.7 | 0.4 | 1.9 | 0.14 | 0.04 | 0.09 | 36.7 |
| 10. Gypsum | 19.7 | 11.6 | 1.7 | 0.48 | 0.07 | 0.28 | 67.5 |
| 11. Fluorite | 9.8 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 12. Apatite | 9.1 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 13. Marcasite | 11.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 14. Pyrrhotite | 11.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 15. Hematite | 17.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 16. Ilmenite | 13.0 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 17. Magnetite | 16.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 18. Limonite | 10.9 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 19. Bauxite | 5.4 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
| 20. Pyrolusite | 30.7 | 0.0 | 0.0 | 0.00 | 0.00 | 0.00 | 0.0 |
Table 15--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 1.0 N sodium Versene for periods of 24 and 48 hours.
| Sample Limestone block no.* |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 22.6 | 1.2 | 0.8 | 0.05 | 0.03 | 0.04 | 8.8 |
| 2 | 23.9 | 1.6 | 0.8 | 0.07 | 0.03 | 0.05 | 10.0 |
| 3 | 22.8 | 1.3 | 0.9 | 0.05 | 0.03 | 0.04 | 9.6 |
| 4 | 21.9 | 1.2 | 0.8 | 0.05 | 0.03 | 0.04 | 9.1 |
| 5 | 17.2 | 0.5 | 0.8 | 0.02 | 0.03 | 0.03 | 7.5 |
| 6 | 24.2 | 1.2 | 0.8 | 0.05 | 0.03 | 0.04 | 8.2 |
| 7 | 20.3 | 1.1 | 0.8 | 0.04 | 0.03 | 0.04 | 9.3 |
| 8 | 29.7 | 1.5 | 0.9 | 0.06 | 0.04 | 0.05 | 8.5 |
| 9 | 20.7 | 0.7 | 0.6 | 0.03 | 0.02 | 0.03 | 6.2 |
| 10 | 20.0 | 0.8 | 0.4 | 0.03 | 0.02 | 0.03 | 6.0 |
| 11 | 15.6 | 1.2 | 0.8 | 0.05 | 0.03 | 0.04 | 12.8 |
| 12 | 22.2 | 1.2 | 1.2 | 0.05 | 0.05 | 0.05 | 10.8 |
| 13 | 17.2 | 1.1 | 1.0 | 0.04 | 0.04 | 0.04 | 12.2 |
| 14 | 20.6 | 1.2 | 0.9 | 0.05 | 0.04 | 0.05 | 10.1 |
| 15 | 20.9 | 1.0 | 1.0 | 0.04 | 0.04 | 0.04 | 9.5 |
| 16 | 15.4 | 1.1 | 0.5 | 0.04 | 0.02 | 0.03 | 10.3 |
| 17 | 20.0 | 1.5 | 0.5 | 0.06 | 0.02 | 0.04 | 10.0 |
| 18 | 20.0 | 1.2 | 0.8 | 0.05 | 0.03 | 0.04 | 10.0 |
| 19 | 17.4 | 1.3 | 0.6 | 0.05 | 0.03 | 0.04 | 10.9 |
| 20 | 20.3 | 1.2 | 0.7 | 0.05 | 0.03 | 0.04 | 9.3 |
| * Limestone block numbers correspond to mineral numbers listed in Table 1. | |||||||
Table 16--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 2.0 N sodium Versene for periods of 24 and 48 hours.
| Sample Limestone block no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 20.6 | 1.8 | 1.2 | 0.07 | 0.05 | 0.06 | 14.5 |
| 2 | 21.5 | 2.2 | 1.5 | 0.09 | 0.06 | 0.08 | 17.2 |
| 3 | 20.6 | 1.9 | 1.3 | 0.08 | 0.05 | 0.07 | 15.5 |
| 4 | 19.9 | 2.2 | 1.3 | 0.09 | 0.05 | 0.07 | 17.5 |
| 5 | 15.9 | 1.6 | 0.9 | 0.06 | 0.03 | 0.05 | 15.7 |
| 6 | 22.2 | 2.1 | 1.4 | 0.08 | 0.06 | 0.07 | 15.7 |
| 7 | 18.4 | 2.0 | 1.5 | 0.08 | 0.06 | 0.07 | 19.0 |
| 8 | 27.3 | 2.5 | 1.0 | 0.10 | 0.04 | 0.07 | 12.8 |
| 9 | 19.4 | 1.4 | 0.7 | 0.05 | 0.03 | 0.04 | 10.8 |
| 10 | 18.8 | 0.9 | 0.5 | 0.04 | 0.02 | 0.03 | 7.4 |
| 11 | 19.0 | 2.1 | 1.2 | 0.09 | 0.05 | 0.07 | 17.3 |
| 12 | 19.8 | 1.9 | 1.3 | 0.07 | 0.05 | 0.06 | 16.1 |
| 13 | 15.1 | 1.5 | 1.4 | 0.06 | 0.05 | 0.06 | 19.2 |
| 14 | 18.5 | 1.7 | 1.0 | 0.07 | 0.04 | 0.06 | 14.5 |
| 15 | 18.9 | 2.0 | 1.2 | 0.08 | 0.05 | 0.07 | 16.9 |
| 16 | 13.8 | 1.6 | 1.2 | 0.06 | 0.05 | 0.06 | 20.2 |
| 17 | 18.0 | 2.1 | 1.6 | 0.08 | 0.06 | 0.07 | 20.5 |
| 18 | 18.0 | 2.1 | 1.3 | 0.09 | 0.05 | 0.07 | 18.8 |
| 19 | 15.5 | 1.9 | 1.2 | 0.07 | 0.05 | 0.06 | 20.0 |
| 20 | 18.4 | 1.8 | 1.6 | 0.07 | 0.06 | 0.07 | 18.4 |
Table 17--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 1.0 N potassium Versene for periods of 24 and 48 hours.
| Sample Limestone block no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 24.2 | 2.0 | 0.9 | 0.08 | 0.04 | 0.06 | 1.9 |
| 2 | 28.3 | 1.5 | 1.1 | 0.06 | 0.04 | 0.05 | 9.1 |
| 3 | 21.8 | 1.5 | 1.0 | 0.06 | 0.04 | 0.05 | 11.4 |
| 4 | 20.6 | 1.7 | 0.9 | 0.07 | 0.03 | 0.05 | 12.6 |
| 5 | 22.9 | 1.5 | 0.9 | 0.06 | 0.04 | 0.05 | 10.4 |
| 6 | 22.2 | 1.5 | 0.7 | 0.06 | 0.03 | 0.05 | 9.9 |
| 7 | 23.2 | 2.0 | 0.8 | 0.08 | 0.03 | 0.06 | 12.0 |
| 8 | 14.2 | 1.3 | 1.0 | 0.05 | 0.04 | 0.05 | 16.1 |
| 9 | 20.7 | 1.4 | 0.9 | 0.06 | 0.04 | 0.05 | 11.1 |
| 10 | 23.7 | 1.3 | 0.4 | 0.05 | 0.01 | 0.03 | 7.1 |
| 11 | 21.3 | 1.4 | 1.0 | 0.06 | 0.04 | 0.05 | 11.2 |
| 12 | 16.0 | 1.6 | 1.0 | 0.06 | 0.04 | 0.05 | 16.2 |
| 13 | 21.8 | 1.6 | 1.1 | 0.06 | 0.04 | 0.05 | 12.3 |
| 14 | 21.7 | 1.5 | 1.0 | 0.06 | 0.04 | 0.05 | 11.5 |
| 15 | 19.7 | 1.4 | 1.1 | 0.06 | 0.04 | 0.05 | 12.6 |
| 16 | 24.8 | 1.4 | 1.1 | 0.06 | 0.04 | 0.05 | 10.0 |
| 17 | 25.5 | 1.6 | 1.0 | 0.06 | 0.04 | 0.05 | 10.1 |
| 18 | 22.3 | 1.6 | 1.0 | 0.06 | 0.04 | 0.05 | 11.6 |
| 19 | 18.9 | 1.3 | 0.9 | 0.05 | 0.03 | 0.04 | 11.6 |
| 20 | 21.8 | 1.5 | 1.0 | 0.06 | 0.04 | 0.05 | 11.4 |
Table 18--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 2.0 N potassium Versene for periods of 24 and 48 hours.
| Sample Limestone block no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 22.3 | 1.8 | 0.9 | 0.07 | 0.04 | 0.06 | 12.1 |
| 2 | 25.7 | 2.2 | 1.5 | 0.09 | 0.06 | 0.08 | 14.3 |
| 3 | 19.3 | 2.1 | 1.3 | 0.10 | 0.05 | 0.08 | 19.1 |
| 4 | 18.0 | 2.1 | 1.2 | 0.09 | 0.05 | 0.07 | 18.3 |
| 5 | 20.5 | 2.1 | 0.7 | 0.09 | 0.03 | 0.06 | 13.6 |
| 6 | 20.0 | 2.3 | 1.0 | 0.09 | 0.04 | 0.07 | 16.5 |
| 7 | 20.4 | 2.3 | 1.2 | 0.09 | 0.05 | 0.07 | 17.1 |
| S | 11.9 | 1.7 | 1.0 | 0.07 | 0.04 | 0.06 | 22.6 |
| 9 | 18.4 | 1.7 | 0.6 | 0.07 | 0.03 | 0.05 | 12.5 |
| 10 | 22.0 | 2.0 | 0.3 | 0.08 | 0.01 | 0.05 | 10.4 |
| 11 | 18.9 | 2.2 | 1.2 | 0.09 | 0.05 | 0.07 | 17.9 |
| 12 | 13.4 | 1.9 | 0.9 | 0.08 | 0.03 | 0.06 | 20.8 |
| 13 | 19.1 | 2.5 | 1.0 | 0.10 | 0.04 | 0.07 | 18.3 |
| 14 | 19.2 | 2.2 | 0.9 | 0.09 | 0.03 | 0.06 | 16.1 |
| 15 | 17.2 | 1.9 | 1.1 | 0.08 | 0.04 | 0.06 | 17.4 |
| 16 | 22.3 | 2.3 | 1.5 | 0.09 | 0.06 | 0.08 | 17.0 |
| 17 | 22.9 | 2.3 | 1.4 | 0.09 | 0.06 | 0.08 | 16.1 |
| 18 | 19.7 | 2.1 | 1.4 | 0.08 | 0.06 | 0.07 | 17.7 |
| 19 | 16.7 | 1.9 | 1.0 | 0.08 | 0.04 | 0.06 | 17.3 |
| 20 | 19.3 | 2.2 | 1.0 | 0.09 | 0.04 | 0.07 | 17.0 |
Table 19--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 1.0 N ammonium Versene for periods of 24 and 48 hours.
| Sample Limestone block no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 23.2 | 0.6 | 05 | 0.02 | 0.02 | 0.02 | 4.7 |
| 2 | 22.4 | 1.4 | 1.3 | 0.05 | 0.05 | 0.05 | 12.0 |
| 3 | 20.0 | 1.2 | 1.3 | 0.05 | 0.05 | 0.05 | 12.5 |
| 4 | 19.6 | 1.2 | 1.2 | 0.05 | 0.05 | 0.05 | 12.2 |
| 5 | 28.5 | 1.2 | 1.3 | 0.05 | 0.05 | 0.05 | 8.7 |
| 6 | 22.0 | 1.1 | 1.1 | 0.04 | 0.04 | 0.04 | 10.0 |
| 7 | 20.6 | 1.2 | 1.1 | 0.05 | 0.04 | 0.05 | 11.1 |
| 8 | 26.2 | 1.7 | 1.4 | 0.07 | 0.05 | 0.06 | 11.8 |
| 9 | 25.1 | 1.2 | 1.1 | 0.05 | 0.04 | 0.05 | 9.1 |
| 10 | 26.4 | 1.1 | 0.8 | 0.04 | 0.03 | 0.04 | 7.1 |
| 11 | 24.3 | 2.3 | 1.0 | 0.09 | 0.04 | 0.07 | 13.5 |
| 12 | 25.0 | 1.8 | 1.5 | 0.07 | 0.06 | 0.07 | 13.2 |
| 13 | 18.2 | 1.4 | 1.1 | 0.06 | 0.04 | 0.05 | 13.7 |
| 14 | 13.5 | 0.8 | 1.1 | 0.03 | 0.04 | 0.04 | 14.0 |
| 15 | 23.5 | 1.4 | 1.2 | 0.05 | 0.05 | 0.05 | 11.0 |
| 16 | 22.5 | 1.6 | 0.9 | 0.06 | 0.03 | 0.05 | 11.1 |
| 17 | 20.6 | 1.1 | 1.2 | 0.04 | 0.05 | 0.05 | 11.1 |
| 18 | 21.2 | 1.3 | 1.1 | 0.05 | 0.04 | 0.05 | 11.3 |
| 19 | 25.2 | 1.6 | 1.3 | 0.07 | 0.05 | 0.06 | 11.5 |
| 20 | 20.7 | 1.3 | 1.6 | 0.05 | 0.07 | 0.06 | 14.0 |
Table 20--Weight, loss in weight, and percent loss in weight of 20 blocks of limestone reacted with mineral blocks in 2.0 N ammonium Versene for periods of 24 and 48 hours.
| Sample Limestone block no. |
Sample weight g |
Loss in weight 0-24 hrs |
Loss in weight 24-48 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight 24-48 hrs g/hr |
Total loss in weight 0-48 hrs g/hr (averaged) |
Loss in weight 0-48 hrs % |
|---|---|---|---|---|---|---|---|
| 1 | 19.6 | 2.7 | 1.1 | 0.11 | 0.04 | 0.08 | 19.3 |
| 2 | 22.0 | 2.6 | 2.3 | 0.11 | 0.10 | 0.11 | 22.2 |
| 3 | 15.6 | 2.6 | 1.4 | 0.11 | 0.06 | 0.09 | 25.6 |
| 4 | 14.7 | 2.6 | 1.0 | 0.11 | 0.04 | 0.08 | 24.4 |
| 5 | 17.7 | 2.8 | 1.1 | 0.11 | 0.04 | 0.08 | 22.0 |
| 6 | 16.7 | 2.5 | 1.4 | 0.10 | 0.06 | 0.08 | 23.3 |
| 7 | 16.9 | 3.0 | 1.7 | 0.12 | 0.07 | 0.10 | 27.8 |
| 8 | 14.1 | 1.6 | 0.8 | 0.07 | 0.03 | 0.05 | 17.0 |
| 9 | 16.1 | 1.9 | 0.6 | 0.08 | 0.02 | 0.05 | 15.5 |
| 10 | 20.0 | 2.2 | 0.3 | 0.09 | 0.01 | 0.05 | 125 |
| 11 | 15.5 | 2.9 | 1.3 | 0.12 | 0.05 | 0.09 | 27.0 |
| 12 | 10.6 | 2.2 | 2.2 | 0.09 | 0.09 | 0.09 | 41.5 |
| 13 | 16.4 | 2.7 | 1.6 | 0.11 | 0.07 | 0.09 | 26.2 |
| 14 | 16.1 | 2.9 | 1.5 | 0.12 | 0.06 | 0.09 | 27.3 |
| 15 | 14.2 | 2.6 | 0.5 | 0.11 | 0.02 | 0.07 | 21.8 |
| 16 | 18.5 | 2.6 | 1.4 | 0.11 | 0.06 | 0.09 | 16.2 |
| 17 | 19.2 | 2.9 | 2.2 | 0.12 | 0.09 | 0.11 | 26.5 |
| 18 | 16.2 | 1.9 | 1.8 | 0.08 | 0.08 | 0.08 | 22.8 |
| 19 | 13.8 | 2.9 | 1.0 | 0.12 | 0.04 | 0.08 | 28.2 |
| 20 | 16.1 | 2.8 | 1.3 | 0.12 | 0.05 | 0.09 | 25.4 |
Table 21--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 10-perccnt hydrochloric and acetic acids for 24 hours.
| Mineral no. |
10% HCl | 10% Acetic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample weight g |
Sample weight @ 24 hrs |
Loss in weight 24 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight % |
Sample weight g |
Sample weight @ 24 hrs |
Loss in weight 24 hrs |
Loss in weight 0-24 hrs g/hr |
Loss in weight % |
|
| 1. Calcite | 10.0 | 0.0 | 9.9* | 0.41 | 99.0 | 17.7 | 9.7 | 8.0 | 0.33 | 45.1 |
| 2. Magnesite | 23.3 | 22.6 | 0.7 | 0.03 | 3.0 | 29.7 | 29.7 | 0.0 | 0.00 | 0.0 |
| 3. Siderite | 25.5 | 23.7 | 1.6 | 0.07 | 6.2 | 25.5 | 25.5 | 0.0 | 0.00 | 0.0 |
| 4. Rhodochrosite | 28.2 | 19.4 | 8.8 | 0.37 | 31.2 | 24.9 | 24.7 | 0.2 | 0.01 | 0.8 |
| 5. Aragonite | 10.2 | 0.4 | 9.8 | 0.41 | 96.0 | 16.8 | 11.4 | 5.4 | 0.22 | 32.1 |
| 6. Witherite | 12.3 | 0.0 | 12.1* | 0.55 | 98.3 | 21.0 | 11.8 | 9.0* | 0.37 | 42.8 |
| 7. Strontianite | 13.6 | 0.0 | 13.3* | 0.55 | 97.7 | 25.6 | 23.7 | 1.7* | 0.07 | 6.6 |
| 8. Dolomite | 21.2 | 8.2 | 12.7* | 0.53 | 38.6 | 13.7 | 13.0 | 0.7 | 0.03 | 5.1 |
| 9. Anhydrite | 8.3 | 7.0 | 1.3 | 0.05 | 15.6 | 6.2 | 5.0 | 1.2 | 0.05 | 19.3 |
| 1O. Gypsum | 6.8 | 4.9 | 1.9 | 0.07 | 27.9 | 4.3 | 3.6 | 0.7 | 0.03 | 16.2 |
| 11. Fluorite | 26.1 | 26.1 | 0.0 | 0.00 | 0.0 | 26.4 | 26.4 | 0.0 | 0.00 | 00.0 |
| 12. Apatite | 14.8 | 11.7 | 3.1 | 0.13 | 20.9 | 11.1 | 10.8 | 0.3 | 0.01 | 2.7 |
| 13. Marcasite | 26.3 | 25.9 | 0.4 | 0.02 | 1.5 | 11.0 | 11.0 | 0.0 | 0.00 | 0.0 |
| 14. Pyrrhotite | 22.7 | 21.7 | 1.0 | 0.04 | 4.4 | 34.3 | 34.3 | 0.0 | 0.00 | 0.0 |
| 15. Hematite | 13.5 | 13.2 | 0.3 | 0.01 | 2.2 | 15.8 | 15.8 | 0.0 | 0.00 | 0.0 |
| 16. Ilmenite | 30.0 | 30.0 | 0.0 | 0.00 | 0.0 | 22.1 | 22.1 | 0.0 | 0.00 | 0.0 |
| 17. Magnetite | 37.8 | 37.5 | 0.3 | 0.01 | 0.7 | 26.0 | 26.0 | 0.0 | 0.00 | 0.0 |
| 18. Limonite | 34.1 | 34.1 | 0.0 | 0.00 | 0.0 | 16.9 | 16.9 | 0.0 | 0.00 | 0.0 |
| 19. Bauxite | 11.4 | 11.4 | 0.0 | 0.00 | 0.0 | 18.7 | 18.7 | 0.0 | 0.00 | 0.0 |
| 20. Pyrolusite | 24.5 | 24.2 | 0.3 | 0.01 | 1.2 | 14.4 | 14.4 | 0.0 | 0.00 | 0.0 |
| * Weights corrected because of residue. | ||||||||||
Table 22--Weight, loss in weight, and percent loss in weight of 20 mineral samples in 25-percent hydrochloric and acetic acids for 4 hours.
| Mineral no. |
25% HCl | 25% Acetic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample weight g |
Sample weight @ 4 hrs |
Loss in weight 4 hrs |
Loss in weight 0-4 hrs g/hr |
Loss in weight % |
Sample weight g |
Sample weight @ 4 hrs |
Loss in weight 4 hrs |
Loss in weight 0-4 hrs g/hr |
Loss in weight % |
|
| 1. Calcite | 10.8 | 0.0 | 10.8 | 2.70 | 100.0 | 9.7 | 7.0 | 2.7 | 0.67 | 27.8 |
| 2. Magnesite | 22.6 | 22.5 | 0.1 | 0.02 | 0.4 | 29.7 | 29.7 | 0.0 | 0.00 | 0.0 |
| 3. Siderite | 23.7 | 22.2 | 1.5 | 0.37 | 6.3 | 25.2 | 25.2 | 0.0 | 0.00 | 0.0 |
| 4. Rhodochrosite | 19.4 | 13.2 | 5.3* | 1.32 | 27.3 | 24.7 | 24.7 | 0.0 | 0.00 | 0.0 |
| 5. Aragonite | 14.4 | 0.0 | 14.4 | 3.60 | 100.0 | 11.4 | 9.8 | 1.6 | 0.40 | 14.0 |
| 6. Witherite | 20.1 | 0.0 | 19.3* | 4.82 | 96.0 | 11.8 | 6.8 | 4.6* | 1.15 | 38.9 |
| 7. Strontianite | 11.6 | 0.0 | 11.6 | 2.90 | 100.0 | 23.7 | 23.2 | 0.5 | 0.12 | 2.1 |
| 8. Dolomite | 8.2 | 0.4 | 7.8 | 1.95 | 95.1 | 13.0 | 12.7 | 0.3 | 0.07 | 2.3 |
| 9. Anhydrite | 7.0 | 6.5 | 0.5 | 0.12 | 7.1 | 5.0 | 5.0 | 0.0 | 0.00 | 0.0 |
| 10. Gypsum | 4.9 | 3.8 | 1.1 | 0.27 | 22.4 | 3.6 | 3.3 | 0.3 | 0.07 | 8.3 |
| 11. Fluorite | 26.1 | 26.0 | 0.1 | 0.02 | 0.3 | 26.4 | 26.4 | 0.0 | 0.00 | 0.0 |
| 12. Apatite | 11.7 | 8.6 | 3.1 | 0.77 | 26.4 | 10.8 | 10.8 | 0.0 | 0.00 | 0.0 |
| 13. Marcasite | 25.9 | 25.9 | 0.0 | 0.00 | 0.0 | 11.0 | 11.0 | 0.0 | 0.00 | 0.0 |
| 14. Pyrrhotite | 21.7 | 21.7 | 0.0 | 0.00 | 0.0 | 34.3 | 34.3 | 0.0 | 0.00 | 0.0 |
| 15. Hematite | 13.2 | 13.2 | 0.0 | 0.00 | 0.0 | 15.8 | 15.8 | 0.0 | 0.00 | 0.0 |
| 16. Ilmenite | 30.0 | 30.0 | 0.0 | 0.00 | 0.0 | 22.1 | 22.1 | 0.0 | 0.00 | 0.0 |
| 17. Magnetite | 37.5 | 37.5 | 0.0 | 0.00 | 0.0 | 26.0 | 26.0 | 0.0 | 0.00 | 0.0 |
| 18. Limonite | 34.1 | 34.1 | 0.0 | 0.00 | 0.0 | 16.9 | 16.9 | 0.0 | 0.00 | 0.0 |
| 19. Bauxite | 11.4 | 11.4 | 0.0 | 0.00 | 0.0 | 18.7 | 18.7 | 0.0 | 0.00 | 0.0 |
| 20. Pyrolusite | 24.2 | 24.0 | 0.2 | 0.05 | 0.8 | 14.4 | 14.4 | 0.0 | 0.00 | 0.0 |
| * Weights corrected because of residue. | ||||||||||
Table 23--Summary of the effects of different Versene solutions and acids on 20 mineral samples.
| Mineral no. |
Mineral & Versene Solution | Mineral & Limestone & Versene Solution | Mineral & Acid | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 hrs 200 ml | 48 hrs 200 ml | 24 hrs 200 ml | 4 hrs 200 ml | |||||||||||||
| Na | K | NH3 | Na | K | NH3 | HCl | Acetic | HCl | Acetic | |||||||
| 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 1.0 | 2.0 | 10% | 10% | 25% | 25% | |
| 1. Calcite | X | 1 | X | X | X | X | 1 | 1 | 1 | X | X | 1 | X | X | X | X |
| 2. Magnesite | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 3. Siderite | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | X | 0 | X | 0 |
| 4. Rhodochrosite | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | X | 1 | X | 0 |
| 5. Aragonite | 1 | X | X | X | X | X | 1 | 1 | 1 | X | 1 | X | X | X | X | X |
| 6. Witherite | 1 | X | X | X | X | X | 0 | 1 | 1 | 1 | 1 | X | X | X | X | X |
| 7. Strontianite | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | X | X | X | 1 |
| 8. Dolomite | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | X | 1 | X | 1 |
| 9. Anhydrite | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 1 | 0 |
| 10. Gypsum | X | X | X | X | X | X | X | X | X | X | X | X | X | 1 | X | 1 |
| 11. Fluorite | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 12. Apatite | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | X | 1 | X | 0 |
| 13. Marcasite | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 14. Pyrrhotite | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 15. Hematite | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 16. Ilmenite | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17. Magnetite | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 18. Limonite | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19. Bauxite | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20. Pyrolusite | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| 0 = No observed attack. 1 = 0-1.0 g dissolved in 48 hrs. X=> g dissolved in 48 hrs. |
||||||||||||||||
Kansas Geological Survey
Placed on web April 6, 2015; originally published November 1965.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/175_3/index.html