Kansas Geological Survey, Bulletin 109, Part 8, originally published in 1954
Originally published in 1954 as Kansas Geological Survey Bulletin 109, Part 8. This is, in general, the original text as published. The information has not been updated.
A detailed spectrographic method for determining the germanium content of coal is described and the results of analyses of 6 Kansas coals at 24 different locations are reported. The 6 coals sampled were the Mulberry, Mulky, Bevier, Croweburg, Mineral, and Pilot. No definite conclusions are made as to geographic or stratigraphic variation in germanium content. The concentration of germanium in the coal ash ranges from 0.0036 to 0.0680 percent, and in the total coal from 0.00069 to 0.00480 percent.
The increasing demand for germanium in many phases of the electronic industry and its relatively short supply have prompted extensive research into possible sources of this element. Small amounts of germanium are found throughout the earth's crust but so far it has not been found in concentration sufficiently high to permit its direct recovery. The chief source of germanium in the past has been distillation of certain residues derived from the smelting of zinc ores. The uncertain economics of zinc mining and smelting, however, have led to the investigation of other source materials, especially coal. Several plants now in operation in Germany, Japan, and England recover germanium from the fly-ash and residual ash of coal that is being used in industrial quantities. The possibility that Kansas coals might be a source of germanium prompted the State Geological Survey to initiate a study, by spectrochemical analyses, of the germanium content of coals found in the State. Most of the Kansas coal seams are thin, a desirable factor in so far as germanium is concerned, for it has been found that the element is more abundant in thin coal seams than in the thicker coal beds. The chief purpose of this initial investigation was directed toward developing an accurate and rapid spectrographic method for determining the germanium content of coals and ascertaining, by this method, the germanium content of six Kansas coals.
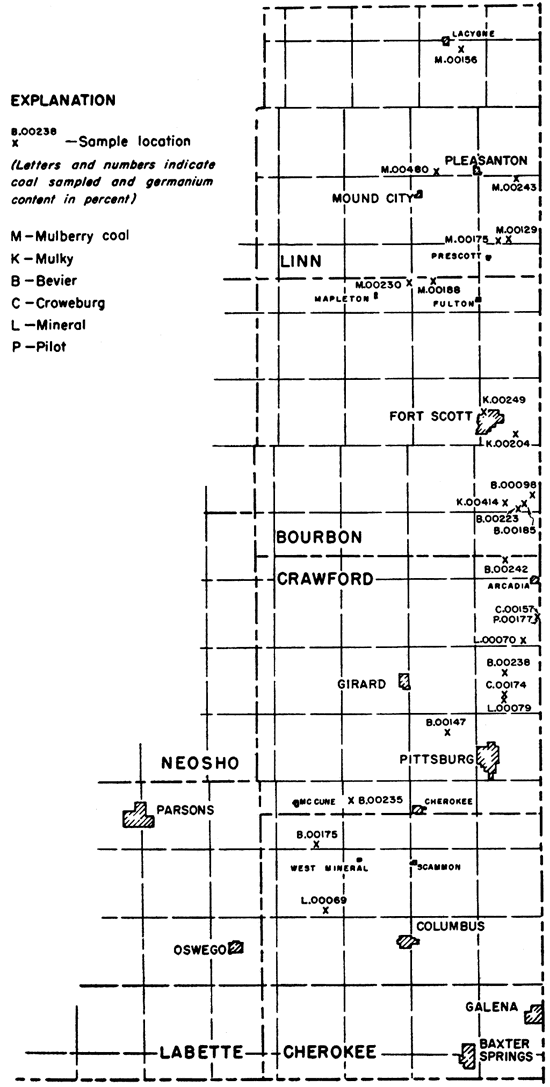
Coals from 6 seams--Mulberry, Mulky, Bevier, Croweburg, Mineral, and Pilot--sampled and collected from 24 localities were analyzed. Eleven of the samples were collected and previously studied by Hambleton (1953) and 11 were taken by Schoewe in 1953 in connection with his studies of the Marmaton (Mulberry) coal of Linn and Bourbon counties. All the coals studied are of Desmoinesian (middle Pennsylvanian) ace. Data pertaining to the location of the coal samples studied, name of coal seam, average thickness of the coal, and laboratory number of samples are presented in Table 1. Figure 1 shows the locations sampled.
Figure 1--Map showing location and germanium content of samples.

Table 1--Coals studied for germanium content, locations of samples collected, and laboratory identification
| Lab. no. | County | Location | Coal | |
|---|---|---|---|---|
| Seam | Thickness, inches |
|||
| 53305 | Bourbon | NW 34-25-25E | Mulky | 14 |
| 53306 | Bourbon | NW 19-25-25E | Mulky | 14 |
| 53307 | Bourbon | NE 33-26-25E | Mulky | 13 |
| 53308 | Bourbon | NE 20-23-24E | Mulberry | 22 |
| 53309 | Bourbon | NE 24-23-23E | Mulberry | 13 |
| 53310 | Linn | 33-22-25E | Mulberry | 22 |
| 53311 | Linn | SW 33-21-24E | Mulberry | 23 |
| 53312 | Bourbon | SE 34-26-25E | Bevier | 15 |
| 53313 | Linn | SE 3-20-24E | Mulberry | 40 |
| 53314 | Linn | NW 3-22-25E | Mulberry | 24 |
| 53315 | Linn | SE 32-22-25E | Mulberry | 24 |
| BN-2-B | Bourbon | NW 25-26-25E | Bevier | 24 |
| BN-3-B | Bourbon | NW 35-26-25E | Bevier | 24 |
| CR-9-B | Crawford | NE 28-27-25E | Bevier | 24 |
| CR-6-B | Crawford | NW 16-29-25E | Bevier | 24 |
| CR-14-B | Crawford | SW 10-30-24E | Bevier | 24 |
| CR-13-B | Crawford | SE 7-31-23E | Bevier | 24 |
| CK-6-B | Cherokee | SE 27-33-21E | Bevier | 24 |
| CK-4-M | Cherokee | NW 35-32-22E | Mineral | 20 |
| CR-8-M | Crawford | SW 28-29-25E | Mineral | 20 |
| CR-4-M | Crawford | SW 35-28-25E | Mineral | 20 |
| CR-1-C | Crawford | NW 28-29-25E | Croweburg | 12 |
| 53143 | Crawford | 24-28-25E | Croweburg | 12 |
| 53142 | Crawford | 24-28-25E | Pilot | 9 |
A sample of each coal sufficient in size, as calculated from the proximate analysis, to produce at least 150 mgm of ash was weighed and placed in a platinum dish of about 100 ml capacity. The dish was then covered with a tight pyrex watch glass and 25 to 35 ml of concentrated nitric acid was added through the pourout lip. The dish was heated on an electric hotplate in a fume hood at about 250° F. until all the nitric acid either had reacted with the coal or had been distilled out through the pourout lip. The tight watch glass was used to prevent undesirable rapid evolution of the acid, since refluxing seemed to promote more efficient use of the acid's oxidizing properties. When the sample was completely dried by this method it looked like coke. To this hot substance 15 ml of concentrated nitric acid was added and again permitted to fume off, with refluxing, as before. After complete dryness was again attained, the dish was placed in a cold muffle furnace and the temperature raised slowly (approximately 75° to 100° C. per hour) to 450° C. When the sample was completely ashed, the furnace was immediately shut off. The immediate turning off of the furnace at 450° C. is deemed desirable, as prolonged heating even at the low temperature of 450° C. might result in the loss of volatile oxides, including that of germanium. According to Tucker and Waring (1954), neither temperature (200° to 1000° C.) nor time of ignition (1 to 4 hours) affected the concentration of germanium in their coal samples, whereas Goldschmidt and Peters (1933, as cited by Ahrens, 1950, p. 215) have reported otherwise. For the purposes of this investigation the relatively "safe" temperature of 450° C. was chosen. The electric muffle furnace used was a Hoskins, equipped with a manually operated panel rheostat and controlled by a Brown recording potentiometer which automatically turned off the furnace when the desired 450° C. temperature was reached. The last 25° C. rise in temperature was accompanied by a large evolution of fumes both of organic material and inorganic acids which were dispersed by the use of an efficient exhaust fan. Upon cooling, the ash was weighed and percent "wet" ash calculated. In every case the percent "wet" ash calculated exceeded the actual ash value of the coal; the addition of the nitrate radical, the oxidation of sulfur to sulfate, and the lack of high-temperature ignition all tend to increase the weight of the ash fraction. The calculated percentage of "wet" ash was used later to calculate the concentration of germanium in the total coal.
Bismuth was chosen as the internal standard element (Rusanov, 1940, as reported by Ahrens, 1950, p. 216). Comparison of the properties of germanium and bismuth indicated the following similarities.
| Ge | Bi | |
|---|---|---|
| Ionization potential | 8.09 V | Ca 8.0 V |
| Excitation potential (For the lines chosen) |
4.94 V | 5.5 V |
The melting points and boiling points of both the elements and their oxides are relatively low, and, as nearly as one could judge from theoretical evidence, they seem to be well suited as an element pair. From the weight of ash it was possible to calculate, weigh, and add the amount of bismuth trioxide necessary to produce a concentration of 1 percent bismuth in the ash. The density of bismuth trioxide would introduce difficulties in the addition and thorough mixing of an amount smaller than 1 percent. The bismuth trioxide used was germanium-free Johnson, Matthey and Co., Ltd. "Specpure" grade, distributed by the Jarrell-Ash Company. The first samples of ash were ground and mixed with the bismuth trioxide for about 2 hours each in a 5.5 cm mullite mortar. In the interest of time-saving, seven subsequent samples were ground in a Fisher improved mortar grinder with a mullite mortar and pestle for only 30 minutes since after that time the particles had been reduced to a size where compaction in the mortar resulted. Scraping them off the mortar and further grinding resulted in immediate re-compaction. It should be noted that although the mechanical grinder was very efficient in reducing the particle size, its mixing action, at least on samples of only 150 mgm size, was not satisfactory (Table 2). During the grinding of all the hand-ground samples, any compaction of the sample in the mortar was frequently scraped loose and broken up with a small platinum spatula.
Samples 53142 and 53143 were obtained and prepared subsequent to the completion of all the others. In the case of these two, the hand mixing and grinding with the bismuth trioxide was cut to about 20 minutes per sample. The analytical results obtained by this method of mixing were satisfactory, as the first three determinations on each ash agreed with one another within the desired limit of reproducibility.
A matrix approximating closely the composition of an average coal ash was prepared by grinding and mixing together 9.14 gm silica, 2.76 gm calcium carbonate, 3.90 gm ferric oxide, and 3.54 gm alumina. As internal standard 0.2787 gm bismuth trioxide was added. Chemical analyses were used to determine the major constituents of the average coal "wet" ash, and the composition of the matrix was so arranged that 7.85 gm of the matrix was equivalent in composition to 10.00 gm of the average "wet" ash. The discrepancy in equivalence was due to the use of oxides and carbonates in the preparation of the matrix, which were available in high-purity form; in the wet ash, the major metallic constituents were present as sulfates. The effect of the presence of sulfate ion on the ignition of ash was compensated for in the prepared matrix by the use of lithium sulfate as buffer, which is standard practice for the Kansas Survey laboratory.
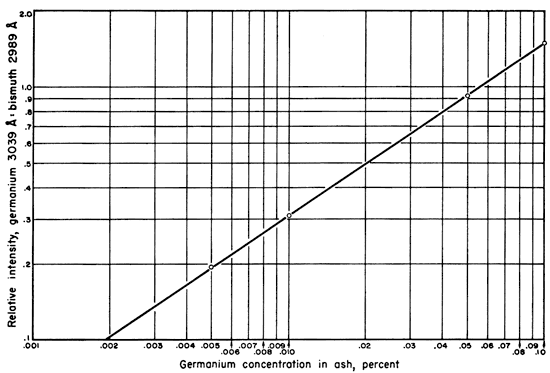
To 3.925 gm of the matrix plus bismuth was added 5 mgm of germanium as germanium dioxide. The resulting mixture contained the equivalent of 0.1 percent germanium. Successive dilutions were made with portions of the original matrix plus bismuth until standards containing 0.1 percent, 0.05 percent, 0.01 percent, 0.005 percent, and 0.001 percent germanium, in essentially identical matrices were available. These standards and the unknown coal ashes were ignited under identical conditions. Seven spectra were run for each standard, a total of 210 mgm equivalent for each standard, then averaged to give the points from which the working curve for the germanium-bismuth ratios was constructed (Fig. 2).
Figure 2--Graph illustrating relation of germanium content in coal ash to intensity ratio of spectrum lines used.
The spectrograph used was an Applied Research Laboratories 1.5 meter grating spectrograph powered by a D.C. arc source unit. The electrodes were National Carbon Company standard electrode-grade graphite rods cut to 5 cm in length and formed as an undercut crater electrode similar to the standard Harvey electrode but with thinner wall. The crater is 3.0 mm deep and 5.25 mm inside diameter. The counterelectrode is the standard ARL platform electrode with centerpost, selected because the concave platform seems to increase the arc sensitivity by reflective increase of the temperature of the sample. The arc was stabilized by a rotating magnet of the type suggested by Meyers and Brunstetter (1947). The rotating sector was set at 10 percent, the grating doors closed to a setting of 4.7 or about 67 percent of the maximum opening, and the electrodes at the beginning of ignition were 4 mm apart with the lower electrode containing the positive charge. No attempt was made to keep the electrode distance constant throughout the ignition. The arc strike was made each time with a rubber-handled sharpened graphite rod from the centerpost of the counterelectrode to the crater edge below, which assisted the stability of the arc by preventing it from striking from the rim of the platform and thus being, able to wander around the outside of the counterelectrode. The film used was Eastman spectrum analysis No. 1.
Several moving film spectra were made to determine persistence of both the germanium and bismuth lines in the samples and their sensitivity at various current ratings and with varying amounts of buffer. Optimum results were obtained using an 8 ampere arc for an exposure of 60 seconds. The buffer, lithium sulfate, was added experimentally in varying amounts. The combination of 10 mgm of ash and 5 mgm of lithium sulfate gave the greatest buffering action, a reasonable cyanogen-band suppression, a higher sensitivity than lesser amounts of buffer, and a more rapid evolution of the element pair than that afforded by a larger proportion of buffer. The ash and buffer were mixed perfunctorily and introduced into the craters of the electrodes after which the mixture was firmly compacted with a flat-faced glass rod of the same diameter as the electrode crater.
After exposure, the film was developed for 3 minutes in D-19, shortstopped for 10 seconds in 3 percent acetic acid, and fixed for 1 minute in Kodak rapid liquid fixer with hardener. After a 1-minute tap-water rinse and a 30-second distilled-water rinse the film was sponged and dried on an infra-red forced-air film dryer. Density measurements were then read on an A.R.L. densitometer-comparitor.
The lines chosen for density measurements were the germanium line at 3039.0 A. and the bismuth line at 2989 A. (Harrison, 1946). This particular bismuth line was chosen because of its nearness to the germanium line. The relatively high (1 percent) bismuth concentration caused more sensitive and more commonly used lines in this region to be too intense. The results of the spectral intensity ratios as plotted on the working curve, their averages, the mean deviation, the standard deviation, and the percent mean deviation are shown in Table 2.
As a further elimination of the effect of arc instability three samples of each ash were ignited consecutively and superimposed as one spectrum, producing a type of "internal average" of the three samples. Three or four spectra of each sample were obtained in this way, and the results averaged. If the first three spectra agreed closely, their average was taken as the final result. If the first three spectra (nine samples) did not agree, however, a fourth spectrum of three samples was obtained, and the four averaged.
If one of the four spectra differed widely from the other three, statistical methods were employed to determine the validity of discarding the divergent results. If the deviation of one of the results from the mean of the other three was found to be greater than four times the mean deviation of the other three and greater than three standard deviations from the mean of the other three, the one result was considered to be trivial, on a weighted basis, and the result was discarded. By commonly accepted statistical principles, 68 percent of all results should be one standard deviation or less from the mean, 28 percent should be from one to two standard deviations from the mean, and the remaining 4 percent should fall not farther than three standard deviations from the mean.
Table 2--Results of analyses.
| Sample no. |
Germanium content of ash, percent | Statistical evaluation of spectrographic data |
Wet ash in coal, percent |
Germanium in coal, percent |
Germanium in coal, ounces per ton |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4* | Average | Md | Percent Md |
Standard deviation |
||||
| 53305 | 0.0177 | 0.0173 | 0.0185 | A | 0.0178 | 0.0004 | 2.2 | 0.00061 | 11.44 | 0.00204 | 0.65 |
| 53306 | 0.0088 | 0.0076 | 0.0095 | A | 0.0086 | 0.0007 | 8.1 | 0.00096 | 28.96 | 0.00249 | 0.80 |
| 53307 | 0.0308 | 0.0260 | 0.0270 | 0.0323 | 0.0290 | 0.0025 | 8.6 | 0.0030 | 14.30 | 0.00414 | 1.32 |
| 53308 | 0.0126 | 0.0126 | 0.0122 | A | 0.0125 | 0.0002 | 1.6 | 0.00023 | 15.03 | 0.00188 | 0.60 |
| 53309 | 0.0155 | 0.0142 | 0.0148 | B | 0.0148 | 0.0004 | 2.7 | 0.00065 | 15.54 | 0.00230 | 0.74 |
| 53310 | 0.0066 | 0.0069 | 0.0061 | B | 0.0065 | 0.0003 | 4.6 | 0.00041 | 19.78 | 0.00129 | 0.41 |
| 53311 | 0.0700 | 0.0670 | 0.0670 | B | 0.0680 | 0.0013 | 1.9 | 0.0017 | 7.06 | 0.00480 | 1.54 |
| 53312 | 0.0144 | 0.0128 | 0.0131 | A | 0.0134 | 0.0006 | 4.5 | 0.00085 | 16.66 | 0.00223 | 0.71 |
| 53313 | 0.0074 | 0.0080 | 0.0071 | B | 0.0075 | 0.0003 | 4.0 | 0.00046 | 20.80 | 0.00156 | 0.50 |
| 53314 | 0.0169 | 0.0200 | 0.0173 | 0.0194 | 0.0184 | 0.0013 | 7.1 | 0.0015 | 13.23 | 0.00243 | 0.78 |
| CR-8-M | 0.0048 | 0.0058 | 0.0056 | 0.0048 | 0.0052 | 0.00045 | 8.7 | 0.00053 | 15.16 | 0.00079 | 0.25 |
| CR-6-B | 0.0150 | 0.0152 | 0.0158 | A | 0.0153 | 0.0003 | 2.0 | 0.00042 | 15.54 | 0.00238 | 0.76 |
| CR-13-B | 0.0141 | 0.0137 | 0.0126 | A | 0.0135 | 0.0006 | 4.4 | 0.00078 | 17.40 | 0.00235 | 0.75 |
| CK-6-B | 0.0077 | 0.0094 | 0.0087 | A | 0.0086 | 0.0006 | 7.0 | 0.00085 | 20.31 | 0.00175 | 0.56 |
| CR-14-B | 0.0076 | 0.0074 | 0.0084 | A | 0.0078 | 0.0004 | 5.1 | 0.00053 | 18.81 | 0.00147 | 0.47 |
| BN-2-B | 0.0085 | 0.0107 | 0.0112 | A | 0.0101 | 0.0011 | 10.9 | 0.0014 | 18.32 | 0.00185 | 0.59 |
| CR-4-M | 0.0031 | 0.0041 | 0.0030 | 0.0040 | 0.0036 | 0.0005 | 13.9 | 0.00058 | 19.52 | 0.0007 | 0.22 |
| CK-4-M | 0.0040 | 0.0065 | 0.0043 | 0.0058 | 0.0052 | 0.0010 | 19.2 | 0.0012 | 13.24 | 0.00069 | 0.22 |
| BN-3-B | 0.0041 | 0.0051 | 0.0057 | 0.0044 | 0.0044 | 0.0006 | 12.5 | 0.00072 | 20.47 | 0.00098 | 0.31 |
| CR-9-B | 0.0152 | 0.0212 | 0.0205 | 0.0274 | 0.0274 | 0.0032 | 15.2 | 0.0050 | 11.52 | 0.00242 | 0.77 |
| CR-1-C | 0.0098 | 0.0080 | 0.0070 | 0.0107 | 0.0107 | 0.0014 | 15.7 | 0.0017 | 19.60 | 0.00174 | 0.56 |
| 53315 | 0.0078 | 0.0120 | 0.0082 | 0.0105 | 0.0105 | 0.0016 | 16.7 | 0.0020 | 18.26 | 0.00175 | 0.56 |
| 53142 | 0.0128 | 0.0123 | 0.0102 | B | 0.0118 | 0.0010 | 8.5 | 0.0014 | 14.98 | 0.00177 | 0.57 |
| 53143 | 0.0212 | 0.0195 | 0.0219 | B | 0.0209 | 0.0009 | 4.3 | 0.0012 | 7.50 | 0.00157 | 0.50 |
| * A, 4th determination discarded; B, 3 determinations deemed sufficient. | |||||||||||
The concentration of germanium in the 24 coal samples ranges from 0.0036 to 0.0680 percent in the ash, and from 0.00069 to 0.00480 percent in the total coal (Table 2). From a practical standpoint, the concentration of germanium in the coal ranges from 0.22 ounce per ton of coal in CR-4-M and CK-4-M to 1.54 ounces per ton of coal in sample 53311. On the basis of these results and considering the current price of germanium at $295 per pound as of February 27, 1954 (Engineering and Mining Journal, 1954) the germanium content of the coals examined is evaluated at $4.06 per ton of coal to $28.40 per ton as a maximum for the coals sampled. The ash of coal sample 53311, which contained 21.8 ounces (1.36 pounds) of germanium per ton of ash, would be valued at $401.20 per ton of ash, if no germanium were lost in the ignition. This seems to compare favorably with other sources, both domestic and foreign, although no exact figures are readily available.
Some investigators have adopted a pessimistic attitude toward the recovery of germanium from coal (Mining Engineering, 1953) because of low concentrations of germanium found in the thicker eastern coals. The analyses shown in this study however, represent the entire coal at the location sampled, and although the total eastern coals are said rarely to contain as much as 0.002 to 0.003 percent germanium, 10 of the 24 coal samples examined contained 0.002 percent or more and 2 contained substantially more than 0.004 percent. These higher values are attributed to the thinness of the Kansas coals, as thin coals, in general, seem to contain a higher concentration of germanium.
Aherns, L. H. (1950) Spectrochemical analysis: Addison-Wesley Press, Inc., Cambridge, Mass., pp. 1-330.
Engineering and Mining Journal (1954) Market prices, miscellaneous metals, ores, and minerals: vol. 155, no. 3, p. 102.
Goldschmidt, V. M., and Peters, C. L. (1933) Geochemistry of germanium: Nach. Ges. Wiss. Gottingen, Math.-Phys. Kl. vol. 3, pp. 141-166.
Hambleton, W. W. (1953) Petrographic study of southeastern Kansas coals: Kansas Geol Survey, Bull. 102, pt. 1, pp. 1-76. [available online]
Harrison, G. R. (1946) M.I.T. wavelength tables: John Wiley and Sons, Inc., New York, pp. 1-429.
Meyers, A. T., and Brunstetter, B. C. (1947) Magnetic rotation of the direct current arc in spectrographic analysis: Anal. Chemistry, vol. 19, no. 1, p. 71.
Rusanov, A. K. (1940) Use of a carbon arc in a direct quantitative study of the composition of minerals: (English translation) Bull. Acad. Sci., U.R.S.S. ser. Phys., vol 4, pp. 148-149.
Tucker, W. P., and Waring, C. L. (1954) Effect of ashing temperatures on the volatility of germanium in low-rank coal samples: Anal. Chemistry, vol. 26, no. 7, pp. 1198-1199.
Kansas Geological Survey, Geology
Placed on web April 21, 2009; originally published in Oct. 1954.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/109_8/index.html