Kansas Geological Survey, Open-file Report 83-7
KGS Open File Report 83-7
A Report for the Kansas Department of Health and Environment
March 1983
Read the PDF version (1.4 MB)
Saline water has been found in the domestic well of the parents of Jim Thomas located in the NE of the NE of the SE of Section 22, T. 30 S., R. 4 E., approximately 2 miles southeast of the town of Rock, northwest Cowley County. The chloride concentration of a sample collected from this well earlier in 1983 was approximately 1000 mg/L as determined by the Kansas Department of Health and Environment. The owner has stated to the Department that the well had previously not yielded saline water. A small oil field with two wells currently producing is located to the southwest of and in the same quarter section as the Thomas well. A tank battery for the separation of oil brine and oil from a producing well is located within a few hundred feet of the water well and in the same quarter-quarter-quarter section. According to the Department of Health and Environment, the oil brine is disposed in a well in the SW of the SW of the SE of Section 22. The concern of Mr. Thomas is that brine from the oil field is the source of the saltwater contamination of the well water.
Water samples were collected from the Thomas well and the nearby collection tank for oil brine from the Johnson Lease on February 15, 1983 by the Department of Health and Environment. The samples were sent to the Kansas Geological Survey for identification of the saltwater source by the procedures of Whittemore et al. (1981). These methods are especially effective for distinguishing oil-field brine from halite~solution brine sources contaminating waters. This report gives the results of the chemical identification of the water samples provided.
Bromide concentrations were measured by an automated phenol red method on a Technicon Autoanalyzer (Basel, et al., 1982). Argentometric titration was used to determine chloride content in the oil brine and an automated ferric thiocyanate method for chloride in the well water.
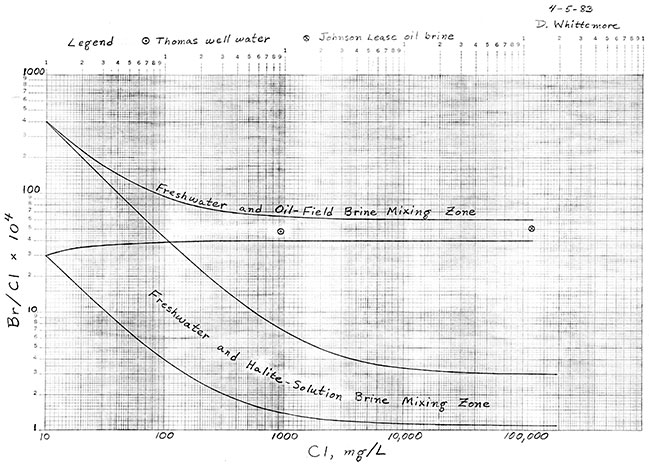
Dissolved chloride and bromide concentrations for the Thomas well water were 929 mg/L and 4.5 mg/L, respectively. Chloride and bromide contents of the oil brine from the nearby collection tank were 118,000 mg/L and 600 mg/L respectively. Bromide/chloride weight ratios are plotted versus chloride concentration for these samples in Figure 1.
Figure 1--Zones of mixing of fresh waters with oil-field and halite-solution brines.

The curves in Figure 1 are the boundary lines for the zone of mixing of fresh waters with halite-solution brines from Permian formations and the zone of mixing of fresh waters with the Johnson Lease oil-field brine. The location of the freshwater and halite-solution mixing zone is based on analytical data for a large number of fresh to saline groundwaters and subsurface brines, as well as samples prepared by dissolving different sections of cores of the Hutchinson Salt Member of the Wellington Formation. The range of bromide/chloride for the freshwater and oil-field brine mixing zone was selected to incorporate any possible variations in the Johnson Lease brine and analytical error. The boundary curves are theoretical lines for the mixing of various amounts of the freshwater (low Chloride) and brine (high chloride) endpoints.
The point on Figure 1 for the Thomas well water falls within the zone of mixing of fresh waters with the Johnson Lease oil brine. The bromide content of the water is about an order of magnitude greater than would be expected if evaporite (halite) solutions were responsible for the salinity.
The depth of the well and the hydrogeology of the area also rule out natural sources of saltwater. Mr. Thomas stated that the water well is 165 feet deep. The approximate elevation of the ground surface in the area of the well is 1230 feet as taken from the 7.5 minute topographic map of the Wilmot Quadrangle.
Elevations of the tops of the Barneston Limestone, Hatfield Shale, and Wreford Limestone of the Chase Group, Permian System, are 1215 feet, 1135 feet, and 1080 feet above sea level, respectively, as estimated from the geologic map and stratigraphy described in Bayne (1962). The bottom of the well is at an elevation of about 1065 feet, thus the water is probably derived from bedding planes, joints, and fractures in limestones of the Barneston Limestone, Hatfield Shale, and Wreford Limestone. Unpolluted groundwaters from wells in these units in central and northern Cowley County and southern Butler County east of the Walnut River contain from 26 to 190 mg/L dissolved sulfate and from 7 to 100 mg/L dissolved chloride (Baynes, 1962; Leonard, 1972).
A typical well water from the above strata in the region of the Thomas well has sulfate and chloride concentrations of about 90 mg/L and 60 mg/L, respectively.
The source of salt water contaminating the Thomas well water is oil-field brine with the same chemical characteristic as the brine from the nearby collection tank for the Johnson lease.
Basel, C.L., Defreese, J.D., and Whittemore, D.O., 1982, Interferences in automated phenol red method for determination of bromide in water: Analytical Chemistry 54, 2090-2094.
Bayne, C.K., 1962, Geology and ground-water resources of Cowley County, Kansas: Kansas Geological Survey, Bull. 158, 219 p. [available online]
Leonard, R.B., 1972, Chemical quality of water in the Walnut River Basin, South-Central Kansas: U.S. Geological Survey, Water-Supply Paper 1982, 113 p. [available online]
Whittemore, D.O., Basel, C.L., Galle, O.K., and Waugh, T.C., 1981, Geochemical identification of saltwater sources in the Smokey Hill River valley, McPherson, Saline, and Dickinson Counties, Kansas: Kansas Geological Survey, Open-File Rep. 81-6, 78 p. [available online]
Kansas Geological Survey
Placed online Feb. 20, 2017
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Hydro/Publications/1983/OFR83_7/index.htm