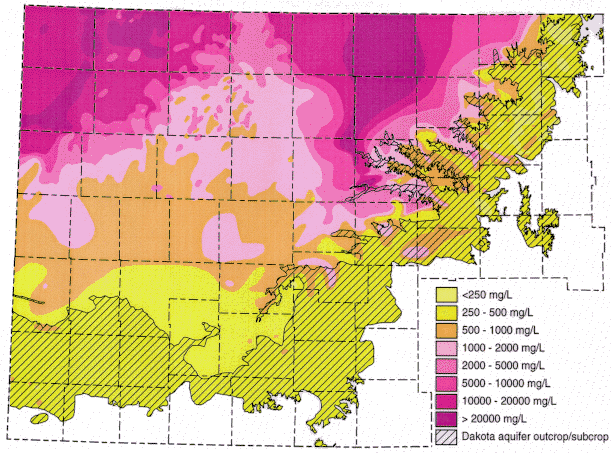
Ground water in the areas of the upper Dakota aquifer with high TDS or salinity (greater than 5,000 mg/L) shown in Figure 1 are of sodium-chloride type. Waters in the area of the confined aquifer with 500-2,000 mg/L TDS are generally soft (low calcium and magnesium content), sodium-bicarbonate in chemical type, and usually have elevated fluoride concentrations. Ground water with 2,000 to 5,000 mg/L TDS in the confined area is typically transitional between sodium-bicarbonate and sodium-chloride type. Waters in the outcrop and subcrop areas with less than 500 mg/L TDS are usually of calcium bicarbonate and sometimes of calcium, magnesium-bicarbonate type. Concentrations of TDS between 500 and 2,000 mg/L in waters in the outcrop/subcrop areas are often due to high calcium and sulfate levels such that the waters can be calcium-sulfate in type. Elevated sulfate concentrations can also produce sulfate type waters in less saline portions of the confined aquifer.
Figure 1. Distribution of total dissolved solids (TDS) concentrations in ground waters in the Dakota aquifer in western and central Kansas. Water less than 1000 mg/L TDS is defined as fresh. Water with 1000-2000 mg/L TDS is usable for many purposes but is less desireable than freshwater. A concentration of 10,000 mg/L TDS is defined in the state regulations of the Kansas Corporation Commission as the upper limit of usable water; above 10,000 mg/L a water is classified as unusable or mineralized.

Figures 2 and 3 show the change in the relative concentrations of selected constituents in waters from supply wells along a traverse from the recharge area in southeastern Colorado, through the confined Dakota aquifer in western Kansas to the discharge area in central Kansas. Although chloride is the predominant anion contributing to the large increase in TDS concentration in the Dakota aquifer in central Kansas (Figure 1), other anions are more important for TDS increases in western Kansas, as indicated by the relatively low chloride content ( < 100 mg/L) up to about 101 deg longitude (Figure 2). Sulfate and chloride contents of ground waters in the overlying High Plains aquifer and in the Dakota aquifer to the south near and within the unconfined areas tend to be substantially lower than in Dakota aquifer waters well within the confined aquifer area.
Figure 2. Regional profile of sodium, chloride, and sulfate concentrations in ground waters in the upper Dakota aquifer along a cross section from southeastern Colorado to central Kansas.
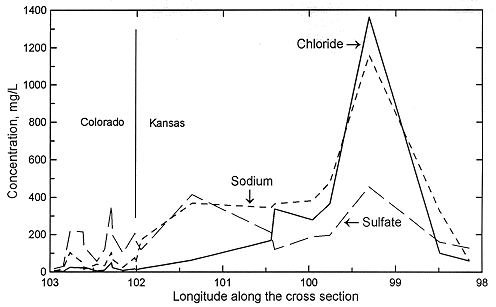
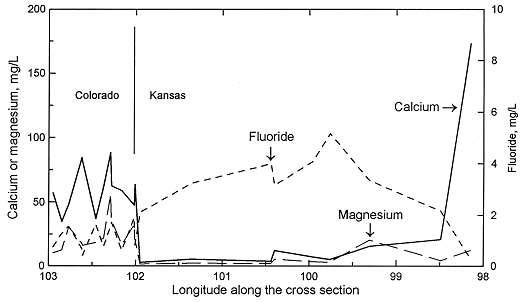
Sodium concentration in Dakota waters (Figure 2) follows a similar pattern as TDS along the cross section. The increase in sodium concentration along the flow path is derived from both softening (cation exchange with calcium and magnesium) and increased intrusion of saltwater from the underlying Permian as the waters approach central Kansas. In contrast, dissolved calcium and magnesium contents decrease appreciably from the local flow area of southeastern Colorado to the confined Dakota aquifer in western and central Kansas (Figure 3). Ground waters in southeastern Colorado derive calcium and magnesium from leaching of carbonate minerals concentrated in soils in an environment of greater evapotranspiration than precipitation, and also from carbonate minerals in the aquifer rocks. The aquifer is well flushed in this area, thus any previous saline water in the aquifer has been essentially all removed and sodium and chloride concentrations in the ground water are low. The flushing in the Dakota aquifer in southeastern Colorado has been extensive enough to also remove high sodium contents adsorbed on clays deposited in brackish or marine environments or subjected to later saltwater intrusion from underlying Permian strata. Thus, any former capacity to soften recharge waters has been largely removed and recharge retains its higher calcium plus magnesium than sodium content.
Figure 3. Regional profile of calcium, magnesium, and fluoride concentrations in ground waters in the upper Dakota aquifer along a cross section from southeastern Colorado to central Kansas.
When the calcium-bicarbonate to calcium-sulfate type waters flowing deep enough in the system reach the confined portion of the Dakota aquifer in western Kansas, exchange of calcium and magnesium for sodium becomes important. The aquifer in the confined area has not been as well flushed as in the local flow areas, leaving high sodium concentrations on marine clays or on clays subjected to saline waters derived from Permian saltwater intrusion in earlier geologic time. The high exchange capacity of most aquifer clays acts as a reservoir that must be changed by large volumes of interacting waters before the adsorbed cation concentrations approach ratios that are near equilibrium with the recharge waters and thus no longer appreciably change the inflow chemistry.
Calcium and magnesium concentrations can become as low as a few mg/L each in the confined Dakota aquifer along the cross section in western Kansas. In contrast, the calcium concentrations in overlying High Plains aquifer waters and in the Dakota water from the southern flow path are within the range of the Dakota aquifer waters in southeastern Colorado. The decrease in calcium and magnesium is abrupt near the state line. The calcium plus magnesium/sodium ratio remains low along the northern flow path until the confining layer thins in central Kansas. Flushing of the saline water that intrudes from the underlying Cedar Hills Sandstone and removal of the high adsorbed sodium content on clays by recharge in the local flow area in central Kansas allows the return of the water type to calcium bicarbonate. The calcium plus magnesium/sodium ratio in the High Plains and Dakota waters in the unconfined Dakota aquifer in southwestern Kansas is within the same range as for the Dakota ground waters in southeastern Colorado and in the local recharge-discharge area in central Kansas. Relative changes in fluoride concentrations along the flow path are generally inversely related to the calcium concentrations due to the dissolution of fluoride-containing calcium minerals (Figure 3).
Previous Page--Factors Controlling Water Quality ||
Next Page--Vertical Changes in Water Quality
Dakota Home ||
Water Quality Index