Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 258, part 3
Prev Page--Methodology || Next Page--Discussion
![]()
![]()
![]()
Kansas Geological Survey, Current Research in Earth Sciences, Bulletin 258, part 3
Prev Page--Methodology ||
Next Page--Discussion
![]()
The overall trend in the 2006-08 monthly samples from the Ozark and multi-aquifer wells collected during the study reflects spatial and temporal changes in water type across the Ozark transition zone described in Macfarlane and Hathaway (1987) from the 1979-1980 sampling events in the Tri-state region (tables 2, 4-13; fig. 6). Water samples from Pittsburg 8 and 10 and Crawford RWD 4 well 3 in the Ozark aquifer are a mixed cation-bicarbonate type with a TDS range of 478-551 mg/L. In contrast, water samples from the Cherokee County RWD 3 well 1 in the Ozark aquifer are a sodium-chloride type with a TDS range of 541-644 mg/L. In between these chemistries, water samples from Crawford Consolidated RWD 1 well 2 and Girard 3 fall into the mixed cation-mixed anion classification. TDS concentration ranges from 639-674 mg/L and 663-721 mg/L for the samples collected from Girard 3 and Crawford Consolidated RWD 1 well 2, respectively. The Crawford RWD 5 well 1 and the Weir city well are both multi-aquifer wells. Samples from Crawford RWD 5 well 1 are a mixed cation-bicarbonate type with a TDS range of 516-566 mg/L. Samples from the Weir city well are a mixed cation-bicarbonate type and range from more sodium rich to more calcium and magnesium rich with a TDS range of 396-497 mg/L. In contrast, the water type in the samples from another multi-aquifer well, Columbus 4, varies from a mixed cation-bicarbonate to a sodium-bicarbonate type with a TDS range of 466-617 mg/L. However, points on fig. 6 for the Columbus well 4 samples with higher bicarbonate and lower sulfate and chloride percentages fall within the ranges of bicarbonate and sulfate and chloride percentages for the other two multi-aquifer wells.
Figure 6--Plot of chemical data for the 2006-08 monthly series of water sampling events from the nine wells and for samples from downgradient wells with >500 mg/L chloride concentration and for upgradient and downgradient wells sampled in 1980 from Crawford and Cherokee counties on a modified Piper diagram. See Appendix 3 in Macfarlane and Hathaway (1987) for a complete tabulation of the sample analysis data from the upgradient and downgradient wells.
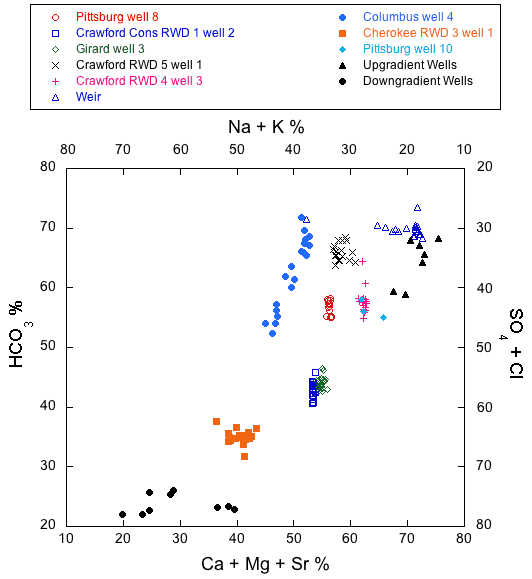
Some of the monthly data sets exhibit linear trends on the modified Piper diagram in fig. 6, while others do not. Vertical trends in the data on fig. 6 for Pittsburg well 8, Crawford Consolidated RWD 1 well 2, and Crawford RWD 4 well 3 indicate that fluctuations in water quality could be primarily attributed to changes in the relative proportions of bicarbonate to sulfate + chloride concentrations in the water from one monthly sampling to the next. The linear trend in the data for Columbus well 4 indicates fluctuations in the relative proportions of both cations and anions, and the horizontal trend in the data for the Weir city well indicates primarily variations in the relative proportions of calcium + magnesium to sodium + potassium. Calcium/magnesium equivalent ratios of waters from all the monitored wells range from about 1.2 to 1.4.
Six wells that were sampled for water quality in 1979-1980 (table 2) were included in the 2006-08 monthly sampling events (figs. 7-10). Comparison of the Crawford County water analyses from the 2006-08 events with those from 1979-1980 indicates significant changes in the quality and chloride concentrations of water produced by Pittsburg well 10 and Crawford Consolidated RWD No. 1 well 2. These wells are located within a regional cone of depression in the Ozark aquifer potentiometric surface (fig. 2; Macfarlane and Hathaway, 1987; Gillip et al., 2007).
The analyses of water from the Crawford County Consolidated RWD No. 1 well 2 and Pittsburg well 10 (fig. 7) reveal slight changes in the relative amounts of calcium + magnesium + strontium and sodium + potassium but an increase in the relative amount of sulfate + chloride that is more pronounced. In all cases the chloride generally increased more than the sulfate concentration (fig. 9). The average chloride concentration increased by about 50 mg/L from 1979-1980 to 2006-08 in water from both wells (from 40 to 90.7 mg/L for Pittsburg well 10 and 135 mg/L for a single sample from Crawford Consolidated RWD 1 well 2 in 1980 to 180 mg/L in 2006-08; fig. 9). However, this increase represented a doubling of chloride for the Pittsburg well but only about a 37% increase for the RWD well. The average chloride increased for Crawford RWD 5 well 1 by about 11 mg/L from 1979-1980 to 2006-08 (from an average of 59 mg/L in 1979-1980 to 68 mg/L in 2006-08; fig. 9). The average sulfate concentration apparently did not change from 1979-1980 to 2006-08 for Crawford RWD 5 well 1 and Pittsburg well 10 (from an average of 76 mg/L in 1979-1980 to 78 mg/L in 2006-08 in Pittsburg well 10 and from 25 mg/L to 27 mg/L in Crawford RWD 5 well 1; fig. 9). Sulfate increased by 9 mg/L in water from Crawford Consolidated RWD 1 well 2 from 1980 to 2006-08 (from 87 mg/L in a single sample from 1980 to an average of 96 mg/L in samples from 2006-08; fig. 9).
Figure 7--Plot of chemical data for the 1979-1980 and 2006-08 monthly series of water sampling events from Crawford County wells on a modified Piper diagram.
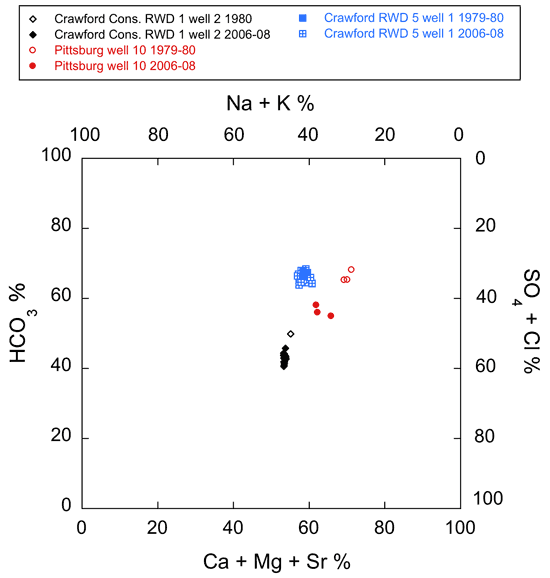
Figure 8--Plot of chemical data for the 1979-1980 and 2006-08 monthly series of water sampling events from Cherokee County wells on a modified Piper diagram.
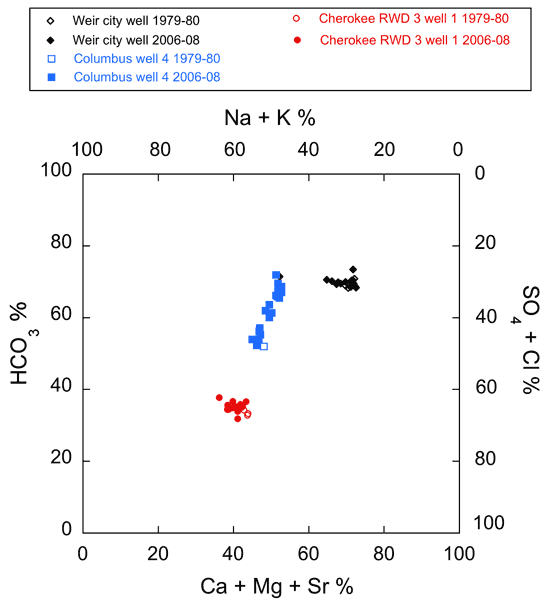
Figure 9--Box plot comparing the chloride, sulfate, and TDS concentrations of the 1979-1980 and 2006-08 water samples from wells in Crawford County.
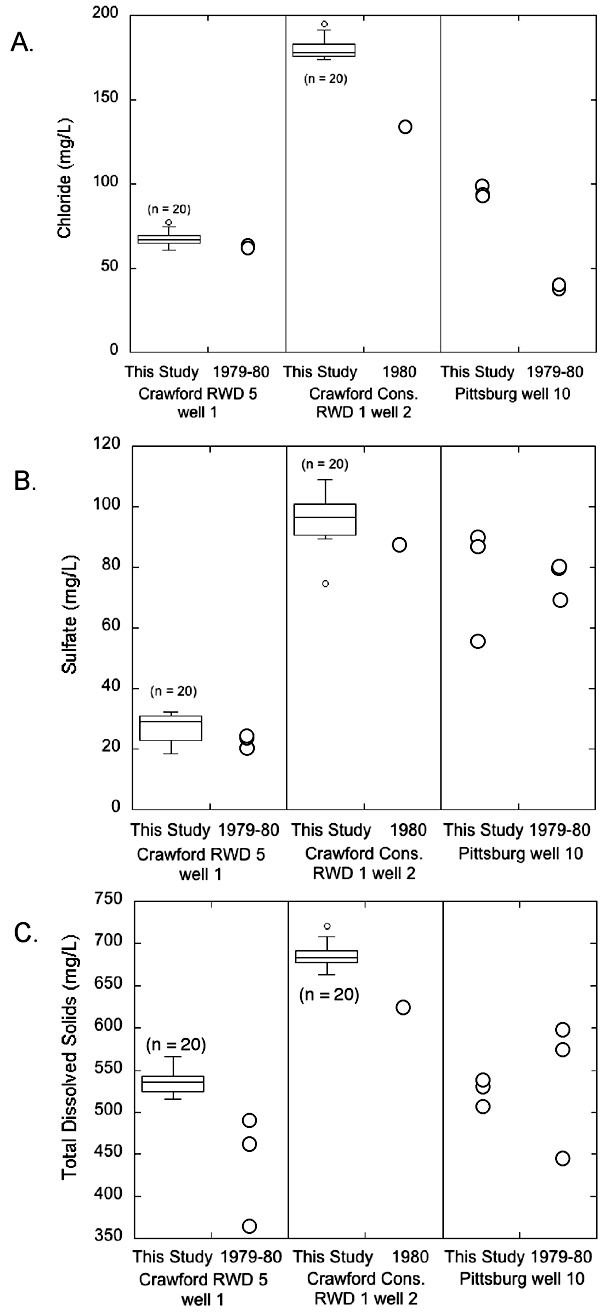
TDS concentration remained unchanged but the range was narrower for the 2006-08 water samples than for the 1979-1980 samples from Pittsburg well 10 (from 446-601 mg/L in 1979-1980 to 516-545 mg/L in 2006-08; fig. 9). The TDS content increased from 365-487 mg/L in 1979-1980 to 516-566 mg/L in 2006-08 in water from Crawford RWD 5 well 1; fig. 9). The average TDS concentration in water from Crawford Consolidated RWD 1 well 2 is almost 60 mg/L higher in the 2006-08 water samples than the single sample collected in 1980 (686 mg/L in 2006-08 and 625 mg/L in 1980 for the single sample; fig. 9).
In Cherokee County, the overall chemical quality of the water from the Weir city well and Cherokee RWD 3 well 1 apparently did not change significantly between 1979-1980 and 2006-08 (fig. 10). Assessment of change in the produced water from Columbus well 4 between 1979-1980 and 2006-08 is difficult because comparisons are being made between a single 1979-1980 analysis and many 2006-08 analyses that exhibit wide month-to-month variability in dissolved constituent composition. However, based on the average chloride concentration in 2006-08 for water samples from Columbus well 4, the chloride apparently decreased from the 1979-1980 concentration level (from 119 mg/L for a single sample in 1979 to an average value of 88.3 mg/L in 2006-08; fig. 10). The change in the average chloride concentration in water from the Weir city well is within analytical error (from 37.3 mg/L in 1979-1980 to 38.5 mg/L in 2006-08; fig. 10). The average chloride concentration in water from Cherokee RWD 3 well 1 increased slightly from 198 mg/L in 1979-1980 to 206 mg/L in 2006-08 (about 4%; fig. 10). The largest change in the average sulfate concentration occurred in Columbus well 4 where it decreased about 30% from 78 mg/L in a single sample in 1979 to an average concentration of 54.9 mg/L (fig. 10). The change in the average sulfate concentration in water from the Weir city well is within analytical error (from 60 mg/L in 1979-1980 to 61.4 mg/L in 2006-08; fig. 10). However, the average sulfate concentration in water from Cherokee RWD 3 well 1 increased from 35 mg/L in 1979-1980 to 42.2 mg/L in 2006-08 (about 20%; fig. 10).
Figure 10--Box plot comparing the chloride, sulfate, and TDS concentrations of the 1979-1980 and 2006-08 water samples from wells in Cherokee County.
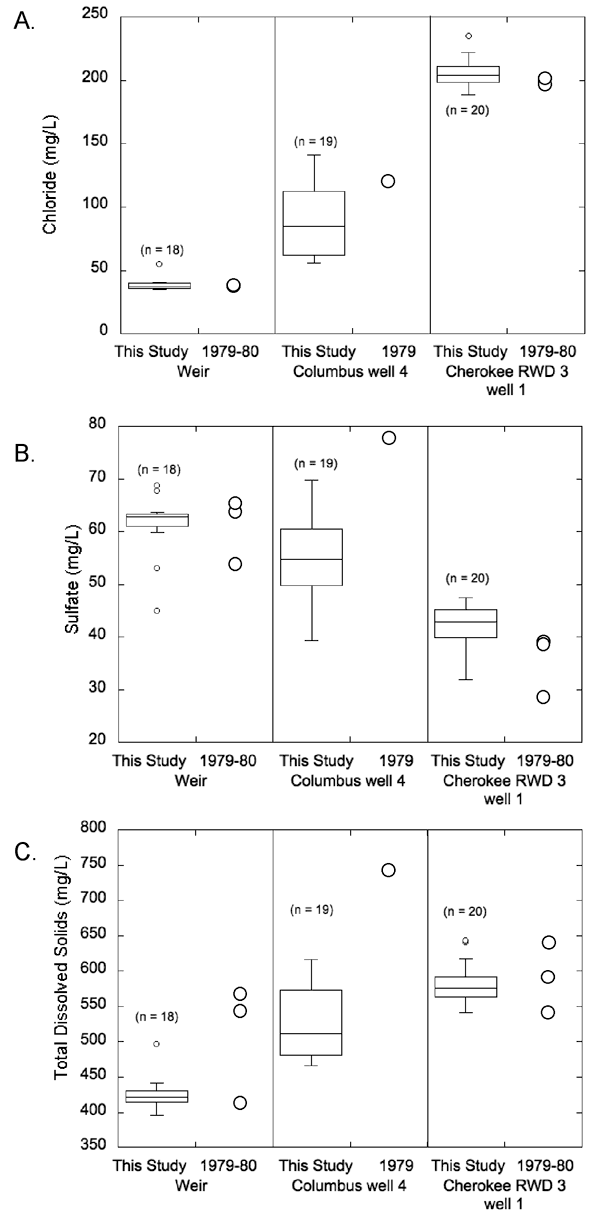
Average TDS concentrations decreased in water from all Cherokee County wells, with the largest average decrease of about 30% in Columbus well 4 (from 743 mg/L in a single sample collected in 1979 to an average concentration of 524 mg/L in the 2006-08 samples; fig. 10). The average TDS concentration in water from the Weir city well decreased by about 16% between the 1979-1980 and 2006-08 series of sampling events (from 510 mg/L in 1979-1980 to 426 mg/L in 2006-08; fig. 10). A much smaller decrease in TDS concentration occurred in water from Cherokee RWD 3 well 1 from 594 mg/L in 1979-1980 to 582 mg/L from 2006-08 (fig. 10).
In general, the temporal chemical variations in water from individual wells represented on figs. 6-8 are smaller than the spatial chemical differences in water from the various wells. The grouping of all of the Ozark aquifer wells in southeast Kansas for which points are plotted on these figures is substantially different from the grouping of the sodium-bicarbonate type waters for wells in the Springfield Plateaus aquifer and of the sodium-chloride brines just to the west. Figure 6 includes points for waters from the Ozark Plateaus aquifer in Crawford and Cherokee counties that contained >500 mg/L when sampled during 1980; these points illustrate the trend towards lower bicarbonate and combined divalent cation (calcium, magnesium, and strontium) percentages and higher chloride and sulfate and monovalent cation (sodium and potassium) percentages. The points for these saline waters, Cherokee RWD 3 well 1, and Columbus 4 form a trend representing the mixing of ground water substantially affected by the Na-Cl type brine with freshwater of mixed cation-bicarbonate type. The group of points for the other wells in fig. 6 sampled in 2006-08 form a general trend of mixed cation-bicarbonate type waters with generally decreasing sodium and chloride concentrations that parallels the trend for the saline ground waters to Cherokee RWD 3 well 1 and Columbus well 4 freshwaters, but that is shifted to higher divalent cation percentages.
Calcium, magnesium, sodium, and potassium concentrations decreased slowly with time in the June and September 2008 chemical-quality pumping tests for Pittsburg well 8 (fig. 11). In about the first 10 hours of both tests, the calcium/magnesium equivalent ratio fluctuated and then stabilized at approximately 1.34 until the end of the test. The bicarbonate concentration remained around 319 mg/L ± 6 mg/L and 318 mg/L ± 2 mg/L throughout the June and September tests, respectively. In the June test, the chloride and sulfate concentrations both generally decreased rapidly in the early part and then more gradually later in the test. In the September test, the chloride decreased during the first 10 hours of pumping and then leveled off at approximately 105 mg/L with some small fluctuation for the remainder of the test, while sulfate decreased slowly throughout the test. In both tests, the sulfate/chloride mass ratio trended towards lower values over time (fig. 12). In the June test, the sulfate/chloride ratio was in the range of 0.5 at the beginning of the test, but, in the September test, that ratio was somewhat less than 0.5 for most of the early part of that test. The bicarbonate/chloride mass ratio increased throughout the pumping period in the June test, but, in the September test, the ratio increased only during the first 10 hours and then was about 3 for remainder of the test (fig. 13). The TDS concentration decreased from 566 mg/L to 478 mg/L during the June test and from 543 mg/L to 484 mg/L during the September test.
Figure 11--Temporal variations in the concentrations of selected major and minor constituents in water samples during the chemical-quality pumping tests of Pittsburg well 8 in June and September 2008.
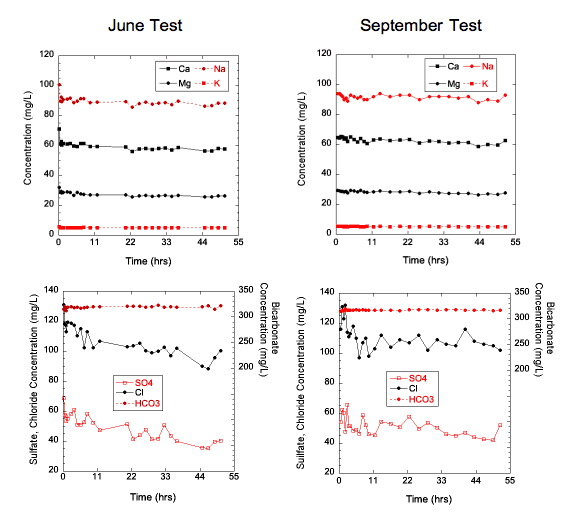
Figure 12--Temporal variations in the SO4/Cl mass ratio in water samples during the chemical-quality pumping tests of Pittsburg well 8 in June and September 2008.
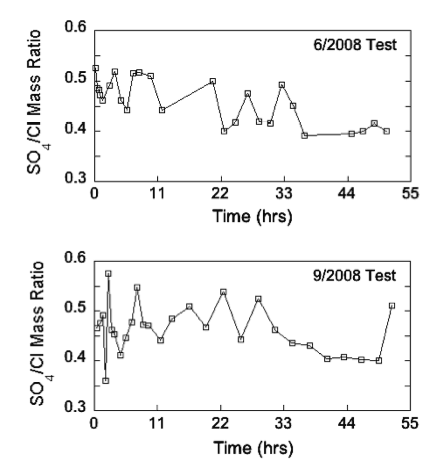
Figure 13--Temporal variations in the HCO3/Cl mass ratio in water samples during the chemical-quality pumping tests of Pittsburg well 8 in June and September 2008.
The bicarbonate/(calcium + magnesium + strontium - sulfate) equivalent ratio is a measure of the amount of bicarbonate relative to that required to satisfy the calcium, magnesium, and strontium in the water that match up with carbonate mineral sources. The sulfate equivalent concentration is subtracted from the cation equivalent concentration to remove cation sources that match up with sulfate minerals. The (sodium + potassium)/chloride equivalent ratio is a measure of the amount of sodium + potassium relative to that required to satisfy the chloride equivalent concentration in the water. Because both ratios exceeded 1, they are defined as the excess bicarbonate and excess sodium + potassium ratios, respectively. In the June test, the lowest value of the excess bicarbonate ratio was near 1.1 in the very first sample but fluctuated between about 1.2 and 1.3 for the remainder of the pumping period, while the excess sodium + potassium ratio varied but trended upward from 1.2 to around 1.4 to 1.5 (figs. 14 and 15). In the September test, the excess bicarbonate ratio fluctuated appreciably during the first 4 hours and then stabilized around 1.2. The excess sodium + potassium ratio also varied substantially in the first four hours of the test and then increased and fluctuated around a value of approximately 1.4 (fig. 15). The low excess bicarbonate and sodium indicated by the ratios suggest that a significant amount of sodium-bicarbonate water was not contributed from the Springfield Plateau aquifer to the Ozark aquifer while Pittsburg well 8 was pumping.
Figure 14--Temporal variations in the bicarbonate/(calcium + magnesium + strontium - sulfate ratio) in water samples during the chemical-quality pumping tests of Pittsburg well 8 in June and September 2008.
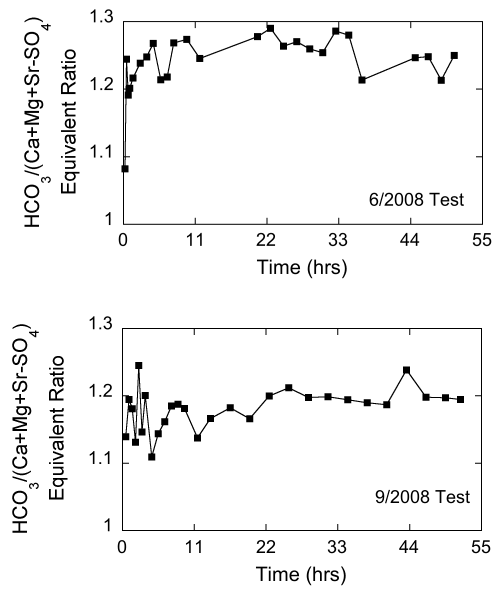
Figure 15--Temporal variations in the (sodium + potassium)/chloride ratio in water samples during the chemical-quality pumping tests of Pittsburg well 8 in June and September 2008.
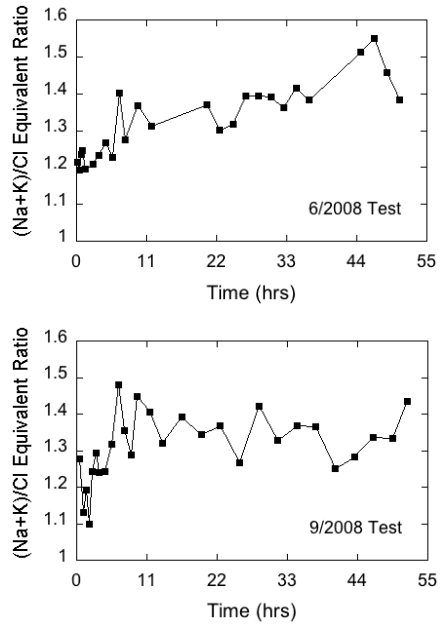
Overall, the produced waters from the wells monitored in this project are mixtures of ground waters from 1) sodium-chloride brine and calcium, magnesium-bicarbonate freshwater sources in the Ozark aquifer (fig. 1); 2) the Ozark and Springfield Plateau aquifers in multi-aquifer wells; and 3) zones within the Ozark aquifer, each with its own ground-water chemistry (Macfarlane and Hathaway, 1987). Downward leakage from the overlying Springfield Plateau aquifer, though probably minor, may also mix with Ozark aquifer ground water in areas where the confining layer is fractured, thin, or absent. The relative amount contributed by each of these sources depends on well location within the transition zone, well construction, intensity and duration of pumping, and thickness and degree of fracturing of the confining layer separating the Ozark from the Springfield Plateau aquifer where it has not been eroded.
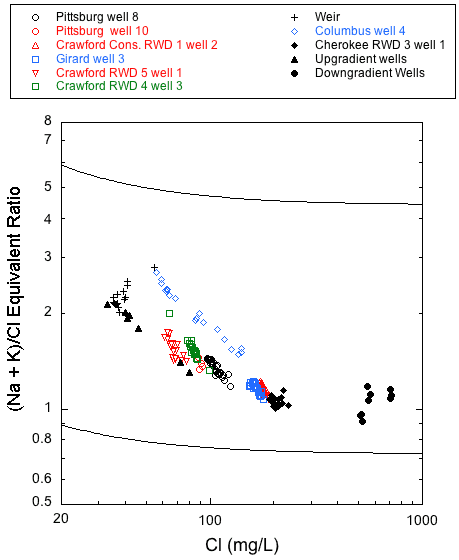
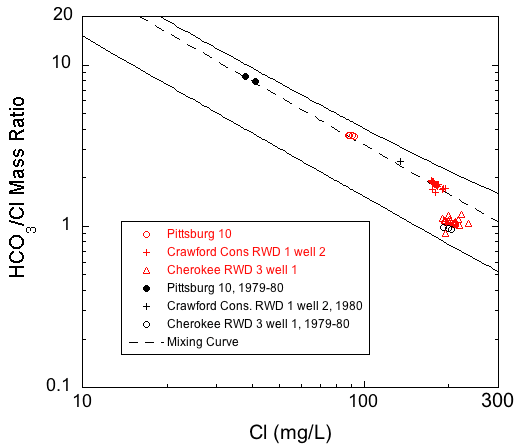
Produced water type generally becomes increasingly more sodium and chloride rich with increasing distance down the hydraulic gradient from the eastern, fresh-transition zone boundary (fig. 1). This relationship is most clearly illustrated in the plots of chloride versus the bicarbonate/chloride ratio and chloride versus the (sodium + potassium)/chloride ratio for the wells sampled in this project (figs. 16 and 17). Both plots show two outer mixing lines that bound regions where points plot for published and unpublished analyses of Ozark aquifer water samples collected in 1979-1980 from the Tri-state region (Macfarlane and Hathaway, 1987). Points for saline waters (with chloride concentration >500 mg/L) collected in 1980 from downgradient wells in the Ozark Plateau aquifer in Crawford and Cherokee counties and from low-chloride upgradient wells in southwestern Missouri are also plotted in figs. 16 and 17. The dashed line in fig. 16 represents a mixing curve fitted to the water-chemistry data from the upgradient and downgradient end members. This mixing curve plots within the region defined by the two bounding curves, and with the exception of Cherokee RWD 3 well 1, points for all the Ozark well samples fall along it. A decrease in the bicarbonate to chloride ratio with an increase in the chloride concentration from the Crawford RWD 4 well 3 and Pittsburg well 10 to Girard well 3 and Crawford Consolidated Rural Water District 1 well 2 demonstrates a progressive westward change in water type across southeastern Crawford County. The data from multi-aquifer wells plot very close to or directly on the dashed mixing curve line, which suggests that the relative contribution of water from the Springfield Plateau aquifer to these wells is minor. In fig. 17, the (sodium + potassium)/chloride equivalent ratio approaches 1 as the chloride concentration increases to 200 mg/L. However, for reasons discussed later in this section, many of the monthly data sets do not plot parallel to the bounding mixing curves for the 1979-1980 data.
Figure 16--Chloride concentration versus bicarbonate/chloride ratio for the monthly samples of 2006-08 and for samples from upgradient and downgradient wells sampled in 1979-1980 from Crawford and Cherokee counties and adjacent southwestern Missouri. The solid lines are curves that describe the mixing curves that bound where points would plot for the 1979-1980 sample data for the Tri-state region and the dashed line is a mixing curve based on a low-chloride end member for the freshest waters from the 2006-08 monthly data and a high-chloride end member for the middle of the group of the saline waters. The curves describe the mixing of various proportions of waters from the low TDS concentration, calcium, magnesium-bicarbonate province and the high TDS, sodium-chloride sources.
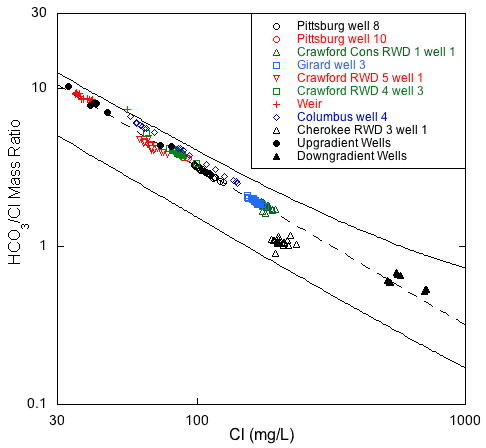
Figure 17--Chloride concentration versus sodium/chloride ratio values for the monthly samples of 2006-08 and for samples from upgradient and downgradient wells sampled in 1979-1980 from Crawford and Cherokee counties and adjacent southwestern Missouri. The solid lines are curves that describe the mixing curves that bound where points would plot for the 1979-1980 sample data for the Tri-State region. The curves describe the mixing in various proportions of waters from the low TDS concentration, calcium, magnesium-bicarbonate province and the high TDS, sodium-chloride province.
Addition of the historic data from the 1979-1980 period to the chloride versus bicarbonate/ratio plot of fig. 16 provides a means of evaluating changes in water chemistry over time in the context of the mixing of water within the Ozark aquifer transition zone (fig. 18). Differences among the sets of data collected from this project and the historic data indicate that the most significant changes in Ozark aquifer chemistry occurred in the waters produced from Pittsburg 10 and Crawford Consolidated RWD 1 well 2, with little if any change in the water pumped by Cherokee RWD 3 well 1 (fig. 18). For the Crawford RWD 5 well 1 multi-aquifer well, a slight shift in the monthly data toward higher chloride relative to the historic data suggests only a minor change in water quality with respect to chloride and bicarbonate. The quality of the produced water from the other multi-aquifer wells does not appear to have changed significantly between the two sampling periods.
Figure 18--Comparison of the 1979-1980 and 2006-08 chloride and bicarbonate/chloride ratios in samples from Pittsburg well 10, Crawford Consolidated RWD 1 well 2, and Crawford RWD 5 well 1.
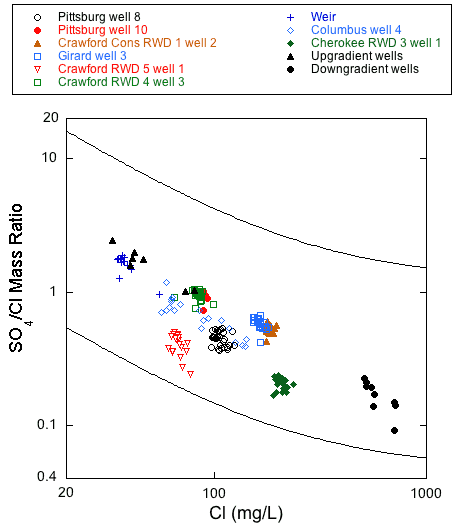
Within the transition zone, the sulfate/chloride ratio generally decreases with increasing chloride concentration (fig. 19) as mixing with sodium-chloride type water increases down the hydraulic gradient towards the Cherokee basin brines. In addition, the sulfate concentration generally decreases as hydrogen sulfide concentration increases (Macfarlane and Hathaway, 1987). This relationship is thought to reflect a lateral change within the Ozark aquifer toward more chemically reducing conditions. Although, in aggregate, the monthly sample data generally follow a pattern of decreasing sulfate/chloride ratio with increasing chloride concentration, only the Weir city well and Columbus well 4 monthly data had a substantial change in chloride concentration that followed the overall trend of points in fig. 19.
Figure 19--Chloride concentration versus sulfate/chloride ratio for the monthly samples of 2006-08 and for samples from upgradient and downgradient wells sampled in 1979-1980 from Crawford and Cherokee counties and adjacent southwestern Missouri. The solid lines are curves that describe the mixing curves that bound where points would plot for the 1979-1980 sample data for the Tri-state region. The curves describe the mixing of various proportions of water of low TDS concentration and calcium, magnesium-bicarbonate type with water of low to moderate sulfate and high TDS concentration and sodium-chloride type with very low sulfate.
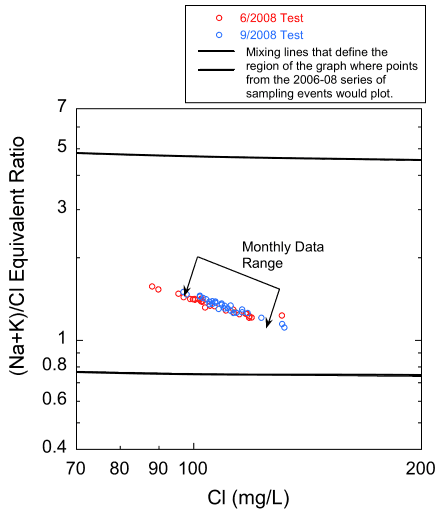
Data from the water-quality pumping tests also suggest that zones of differing water quality within the aquifer contribute water to a pumping well at different rates throughout a single pumping period. It is also possible that the quality of water produced by individual zones may change without a change in the relative amount contributed to the well during pumping. Figure 20 is a plot of the bicarbonate/chloride ratio versus chloride concentration for the samples from both pumping tests plotted with the same mixing curve as the dashed line on fig. 16. The plot shows an excellent fit between the data and the chloride-bicarbonate mixing curve. The trend of the plotted pumping test chloride and the (sodium + potassium)/chloride ratio values follows well the trend of the monthly data for Pittsburg 8 and many of the other supplies sampled, but not as well as the overall trend of the data from the 1979-1980 samplings (fig. 21). The ranges of the bicarbonate to chloride and sodium + potassium to chloride ratios and the chloride concentration values are greater for the samples from the pumping tests than for the Pittsburg well 8 monthly samples. The differences in trend between the regional 1979-1980 data sets and the monthly data and the high degree of variability of the sodium + potassium/chloride ratios over the pumping period are both consistent with time varying amounts of these constituents being contributed to the open borehole from different zones each with its own water chemistry.
Figure 20--Chloride concentration versus bicarbonate/chloride ratio for the samples from the June and September 2008 water-quality pumping tests of Pittsburg well 8. The dashed line is the same mixing curve as on fig. 16 that fits the monthly datasets for the nine project wells.
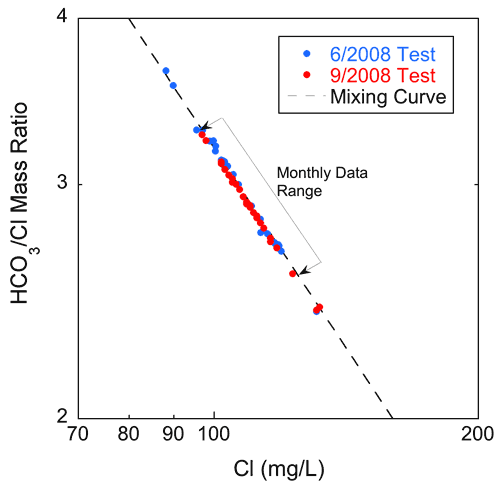
Figure 21--Chloride concentration versus sodium + potassium/chloride ratio values for the samples from the June and September pumping tests for Pittsburg well 8. The solid lines are mixing curves that bound the region where the points would plot for the data from the 1979-1980 sampling events.
Springfield Plateau aquifer wells located above the Ozark aquifer transition zone in southeast Kansas and southwest Missouri produce sodium-bicarbonate type water (Darr, 1978; Macfarlane and Hathaway, 1987). Macfarlane and Hathaway (1987) noted that water samples from some of the multi-aquifer wells within the bounds of the Ozark aquifer transition zone contained bicarbonate in excess of that required to satisfy the equivalent carbonate contribution of calcium, magnesium, and strontium in the sample. The excess bicarbonate ratio is about the same for both the Ozark and multi-aquifer wells, but the excess sodium + potassium ratios are generally higher for the multi-aquifer wells. Excess sodium + potassium values range from about 1.5 to almost 3 in samples from the multi-aquifer wells and 1 to about 1.5 for samples from the Ozark wells. The lower values for the Ozark well samples suggest that leakage across the confining unit is at most minor.
Prev Page--Methodology || Next Page--Discussion
Kansas Geological Survey
Placed online June 25, 2010
http://www.kgs.ku.edu/Current/2010/Macfarlane/04_results.html
email:webadmin@kgs.ku.edu