Prev Page--Geology || Next Page--Logs
Quality of Ground Water
The chemical character of waters from the test holes of this area is indicated by the analyses of 51 samples given in Table 4. The analyses were made by Howard Stoltenberg in the Water and Sewage Laboratory of the Kansas State Board of Health, using the methods outlined by Collins (1928). All the samples of water were collected by McFarland. Each sample was taken from the bailer when water first appeared in the hole after casing had been set.
Table 4--Analyses of waters from test holes in Russell County, Kansas Analyzed by Howard Stoltenberg. Parts per milliona and equivalents per millionb (in italics).
| Test Hole no. |
Location | Depth | Geologic source |
Date of collection |
Calcium (Ca) |
Magnesium (Mg) |
Sodium and Potassium (Na+K) c |
Bicarbonate (HCO3) |
Sulphate (SO4) |
Chloride (Cl) |
Fluoride (F) |
Dissolved solids c |
Hardness (Calculated as CaCO3) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Carbonate | Non- carbonate |
|||||||||||||
| 8 | T. 13 S., R. 15 W. NW SE sec. 35 |
710-732 | Permian | 11/1943 | 437 21.81 |
1,490 122.54 |
21,540 936.56 |
610 10.00 |
7,550 157.19 |
32,400 913.68 |
1.8 .09 |
63,750 | 7,214 | 500 | 6,714 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
60-65 | Greenhorn | 11/1943 | 374 18.66 |
84 6.91 |
714 31.04 |
132 2.16 |
769 16.01 |
1,325 37.36 |
1.0 .05 |
3,399 | 1,278 | 108 | 1,170 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
225-233 | Dakotae | 11/1943 | 42 2.10 |
48 3.95 |
2,136 92.87 |
512 8.39 |
531 11.06 |
2,810 79.24 |
4.5 .24 |
5,832 | 302 | 302f | 0 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
303 | Dakotad | 11/1943 | 51 2.54 |
50 4.11 |
2,250 97.83 |
546 8.95 |
555 11.56 |
2,970 83.75 |
4.0 .21 |
6,157 | 332 | 332g | 0 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
412-420 | Dakotad | 11/1943 | 129 6.44 |
132 10.86 |
4,132 179.66 |
600 9.83 |
1,019 21.22 |
5,875 165.68 |
3.6 .19 |
11,897 | 864 | 492 | 372 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
475-485 | Kiowa | 11/1943 | 500 24.95 |
688 56.58 |
14,740 640.90 |
986 16.16 |
4,260 88.69 |
21,900 617.58 |
1.4 .07 |
43,091 | 4,075 | 808 | 3,267 |
| 9 | T. 13 S., R. 14 W. NW SW sec. 32 |
625 | Permian | 12/1943 | 616 30.74 |
922 75.83 |
18,885 821.12 |
1,530 25.08 |
5,444 113.34 |
28,000 789.60 |
1.8 .09 |
55,399 | 5,326 | 1,254 | 4,072 |
| 10 | T. 13 S., R. 14 W. NW NE sec. 33 |
20 | Tertiary (?) | 12/1943 | 2,468 123.15 |
630 51.81 |
5,482 238.36 |
261 4.28 |
1,770 36.85 |
13,200 372.24 |
1.2 .06 |
23,812 | 8,747 | 214 | 8,533 |
| 10 | T. 13 S., R. 14 W. NW NE sec. 33 |
377 | Dakotad | 12/1943 | 284 14.17 |
223 18.34 |
5,770 250.88 |
351 5.75 |
1,348 28.07 |
8,850 249.57 |
2.4 .13 |
16,828 | 1,625 | 288 | 1,337 |
| 10 | T. 13 S., R. 14 W. NW NE sec. 33 |
560-570 | Permian | 12/1943 | 578 28.84 |
1,257 103.38 |
21,061 915.73 |
894 14.65 |
6,713 139.76 |
31,700 893.94 |
1.8 .09 |
62,205 | 6,608 | 733 | 5,875 |
| 11 | T. 14 S., R. 14 W. NE NW sec. 10 |
295-310 | Dakotad | 12/1943 | 211 10.53 |
268 22.04 |
8,400 365.23 |
996 16.32 |
2,479 51.61 |
11,700 329.94 |
2.6 .14 |
24,057 | 1,630 | 818 | 812 |
| 11 | T. 14 S., R. 14 W. NE NW sec. 10 |
400 | Kiowa | 12/1943 | 333 16.62 |
501 41.20 |
11,818 513.85 |
954 15.64 |
3,348 69.71 |
17,250 486.45 |
2.4 .13 |
34,206 | 2,890 | 782 | 2,108 |
| 11 | T. 14 S., R. 14 W. NE NW sec. 10 |
422-430 | Cheyenne | 12/1943 | 340 16.97 |
493 40.54 |
11,637 505.98 |
961 15.75 |
3,560 74.12 |
16,800 473.76 |
2.4 .13 |
33,793 | 2,874 | 788 | 2,086 |
| 12 | T. 14 S., R. 14 W. SE SW sec. 14 |
270-280 | Dakotad | 1/1944 | 269 13.42 |
203 16.69 |
6,036 262.45 |
798 13.08 |
1,100 22.90 |
9,100 256.62 |
1.6 .08 |
17,508 | 1,506 | 654 | 852 |
| 12 | T. 14 S., R. 14 W. SE SW sec. 14 |
330-340 | Dakotad | 1/1944 | 586 29.24 |
625 51.40 |
14,167 615.98 |
1,283 21.03 |
3,747 78.01 |
21,200 597.84 |
1.5 .08 |
41,610 | 4,031 | 1,052 | 2,979 |
| 12 | T. 14 S., R. 14 W. SE SW sec. 14 |
423-443 | Cheyenne | 1/1944 | 640 31.94 |
748 61.52 |
16,270 707.42 |
1,400 22.95 |
4,192 87.28 |
24,500 690.90 |
1.6 .08 |
47,752 | 4,671 | 1,148 | 3,523 |
| 12 | T. 14 S., R. 14 W. SE SW sec. 14 |
680-692 | Permian | 1/1944 | 688 34.33 |
1,796 147.70 |
23,652 1,028.39 |
217 3.56 |
8,550 178.01 |
36,500 1,029.30 |
2.0 .11 |
71,405 | 9,098 | 178 | 8,920 |
| 13 | T. 14 S., R. 14 W. SW SE sec. 10 |
270-275 | Dakotad | 1/1944 | 175 8.73 |
249 20.48 |
7,013 304.93 |
739 12.11 |
1,519 31.63 |
10,300 290.46 |
2.0 .11 |
19,997 | 1,460 | 606 | 854 |
| 13 | T. 14 S., R. 14 W. SW SE sec. 10 |
355-370 | Dakotad | 1/1944 | 227 11.33 |
233 19.16 |
7,175 311.97 |
732 12.00 |
1,417 29.50 |
10,675 301.04 |
1.4 .07 |
20,460 | 1,524 | 600 | 924 |
| 13 | T. 14 S., R. 14 W. SW SE sec. 10 |
412-432 | Kiowa | 1/1944 | 618 30.84 |
976 80.27 |
19,141 832.25 |
1,222 20.03 |
5,222 108.72 |
28,900 814.98 |
1.6 .08 |
56,081 | 5,554 | 1,002 | 4,552 |
| 13 | T. 14 S., R. 14 W. SW SE sec. 10 |
448-465 | Cheyenne | 1/1944 | 612 30.54 |
972 79.94 |
19,254 837.16 |
1,144 18.75 |
5,354 111.47 |
29,000 817.80 |
1.6 .08 |
56,338 | 5,522 | 938 | 4,584 |
| 16 | T. 13 S., R. 13 W. NW SW sec. 31 |
240-245 | Dakotae | 3/1944 | 40 2.00 |
43 3.54 |
2,303 100.13 |
849 13.92 |
616 12.83 |
2,800 78.96 |
6,651 | 276 | 276h | 0 | |
| 16 | T. 13 S., R. 13 W. NW SW sec. 31 |
355-368 | Dakotad | 3/1944 | 62 3.09 |
71 5.84 |
3,248 141.22 |
908 14.88 |
839 17.47 |
4,180 117.88 |
9,308 | 446 | 446i | 0 | |
| 16 | T. 13 S., R. 13 W. NW SW sec. 31 |
380-410 | Dakotad | 3/1944 | 380 18.96 |
398 32.73 |
10,687 464.67 |
1,069 17.52 |
2,843 59.19 |
15,600 439.92 |
30,977 | 2,584 | 876 | 1,708 | |
| 16 | T. 13 S., R. 13 W. NW SW sec. 31 |
435-440 | Kiowa | 3/1944 | 676 33.73 |
999 82.16 |
18,425 801.12 |
898 14.72 |
5,298 110.30 |
28,100 792.42 |
54,396 | 5,792 | 736 | 5,056 | |
| 16 | T. 13 S., R. 13 W. NW SW sec. 31 |
465-486 | Kiowa | 3/1944 | 700 34.93 |
1,090 89.64 |
19,158 832.99 |
1,078 17.67 |
5,479 114.07 |
29,300 826.26 |
56,805 | 6,226 | 884 | 5,342 | |
| 17 | T. 14 S., R. 14 W. NE NW sec. 25 |
110-125 | Dakotae | 3/1944 | 167 8.33 |
39 3.21 |
508 22.09 |
373 6.11 |
194 4.04 |
830 23.41 |
1.2 .06 |
2,112 | 577 | 306 | 271 |
| 17 | T. 14 S., R. 14 W. NE NW sec. 25 |
255-260 | Dakotad | 3/1944 | 582 29.04 |
664 54.61 |
15,790 686.55 |
1,566 25.67 |
4,290 89.32 |
23,250 655.65 |
1.4 .07 |
46,143 | 4,181 | 1,284 | 2,897 |
| 17 | T. 14 S., R. 14 W. NE NW sec. 25 |
370-380 | Cheyenne | 3/1944 | 768 38.32 |
918 75.50 |
21,530 936.12 |
917 15.03 |
5,039 104.91 |
33,000 930.60 |
1.4 .07 |
62,173 | 5,689 | 752 | 4,937 |
| 17 | T. 14 S., R. 14 W. NE NW sec. 25 |
420-433 | Permian | 3/1944 | 790 39.42 |
1,142 93.92 |
22,440 975.69 |
698 11.44 |
6,136 127.75 |
34,400 970.08 |
1.8 .09 |
65,608 | 6,664 | 572 | 6,092 |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
20-43 | Greenhorn | 5/1944 | 1,820 90.82 |
250 20.56 |
2,986 129.83 |
120 1.97 |
388 8.08 |
8,200 231.24 |
13,764 | 5,568 | 98 | 5,470 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
120-130 | Dakotae | 5/1944 | 46 2.30 |
27 2.22 |
1,510 65.65 |
659 10.80 |
441 9.18 |
1,780 50.20 |
4,463 | 226 | 226j | 0 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
186-190 | Dakotae | 5/1944 | 62 3.09 |
36 2.96 |
1,307 65.52 |
490 8.03 |
398 8.29 |
1,960 55.27 |
4,453 | 302 | 302k | 0 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
275-300 | Dakotad | 5/1944 | 460 22.95 |
549 45.15 |
12,500 543.50 |
1,093 17.91 |
3,213 66.89 |
18,700 527.34 |
36,515 | 3,404 | 896 | 2,508 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
320-335 | Dakotad | 5/1944 | 336 16.77 |
374 30.76 |
9,662 420.10 |
1,152 18.88 |
2,600 54.13 |
14,000 394.80 |
28,124 | 2,376 | 944 | 1,432 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
384-391 | Kiowa | 5/1944 | 672 33.53 |
908 74.67 |
19,640 853.95 |
1,576 25.83 |
5,163 107.49 |
29,400 829.08 |
57,359 | 5,408 | 1,292 | 4,116 | |
| 18 | T. 14 S., R. 14 W. NE NW sec. 30 |
420-460 | Cheyenne and Permian |
5/1944 | 644 32.14 |
916 75.33 |
19,725 857.64 |
1,425 23.36 |
5,162 107.47 |
29,600 834.72 |
57,472 | 5,372 | 1,168 | 4,204 | |
| 19 | T. 14 S., R. 14 W. SE SW sec. 21 |
235-250 | Dakotad | 6/1944 | 192 9.58 |
96 7.90 |
2,186 95.05 |
730 11.96 |
429 8.93 |
3,250 91.65 |
6,883 | 874 | 596 | 278 | |
| 19 | T. 14 S., R. 14 W. SE SW sec. 21 |
265-290 | Dakotad | 6/1944 | 192 9.58 |
225 18.50 |
6,383 277.53 |
683 11.19 |
1,687 35.12 |
9,200 259.44 |
18,370 | 1,404 | 560 | 844 | |
| 19 | T. 14 S., R. 14 W. SE SW sec. 21 |
320-350 | Dakotad | 6/1944 | 714 35.63 |
551 45.31 |
11,550 502.19 |
1,064 17.44 |
3,344 69.62 |
17,600 496.32 |
34,823 | 4,046 | 872 | 3,174 | |
| 19 | T. 14 S., R. 14 W. SE SW sec. 21 |
440-475 | Cheyenne | 6/1944 | 648 32.34 |
878 72.21 |
20,064 872.38 |
925 15.16 |
5,041 104.95 |
30,400 857.28 |
57,956 | 5,226 | 758 | 4,468 | |
| 20 | T. 14 S., R. 14 W. NE SE sec. 27 |
78-85 | Pleistocene | 6/1944 | 74 3.69 |
12 0.99 |
97 4.22 |
405 6.64 |
14 0.29 |
70 1.97 |
672 | 234 | 234l | 0 | |
| 20 | T. 14 S., R. 14 W. NE SE sec. 27 |
160-170 | Dakotae | 6/1944 | 21 1.05 |
14 1.15 |
1,104 48.00 |
568 9.31 |
183 3.81 |
1,315 37.08 |
3,205 | 110 | 110m | 0 | |
| 20 | T. 14 S., R. 14 W. NE SE sec. 27 |
215-225 | Dakotae | 6/1944 | 142 7.09 |
113 9.29 |
2,791 121.35 |
581 9.52 |
810 16.86 |
3,950 111.39 |
8,387 | 819 | 476 | 343 | |
| 20 | T. 14 S., R. 14 W. NE SE sec. 27 |
345-360 | Dakotad | 6/1944 | 672 33.53 |
910 74.84 |
21,252 924.04 |
837 13.72 |
5,203 108.33 |
32,300 910.86 |
61,174 | 5,416 | 686 | 4,730 | |
| 20 | T. 14 S., R. 14 W. NE SE sec. 27 |
400-405 | Kiowa | 6/1944 | 684 34.13 |
864 71.06 |
20,706 900.30 |
581 9.52 |
4,855 101.08 |
31,750 895.35 |
59,440 | 5,258 | 476 | 4,782 | |
| 21 | T. 13 S., R. 15 W. NW SW sec. 33 |
200-220 | Dakotae | 7/1944 | 118 5.89 |
62 5.10 |
1,866 81.13 |
425 6.97 |
611 12.72 |
2,570 72.47 |
5,652 | 550 | 348 | 202 | |
| 21 | T. 13 S., R. 15 W. NW SW sec. 33 |
330-375 | Dakotad | 7/1944 | 176 8.78 |
213 17.52 |
5,037 219.01 |
845 13.85 |
1,438 29.94 |
7,150 201.63 |
14,859 | 1,314 | 693 | 621 | |
| 21 | T. 13 S., R. 15 W. NW SW sec. 33 |
425-435 | Kiowa | 7/1944 | 410 20.46 |
554 45.56 |
11,264 489.76 |
1,532 25.11 |
3,424 71.29 |
16,300 459.66 |
33,484 | 3,300 | 1,256 | 2,044 | |
| 21 | T. 13 S., R. 15 W. NW SW sec. 33 |
495 | Cheyenne | 7/1944 | 538 26.85 |
803 66.04 |
16,450 715.25 |
1,879 30.80 |
4,712 98.10 |
24,100 679.62 |
48,482 | 4,643 | 1,540 | 3,103 | |
| 21 | T. 13 S., R. 15 W. NW SW sec. 33 |
545-550 | Permian | 7/1944 | 368 18.36 |
491 40.38 |
10,561 459.19 |
1,386 22.72 |
3,074 64.00 |
15,300 431.46 |
31,180 | 2,936 | 1,136 | 1,800 | |
| a One part per million is equivalent to one pound of substance per million pounds of water or 8.33 pounds per million gallons of water. b An equivalent per million (e.p.m.) is a unit chemical equivalent weight of solute per million unit weights of solution. Concentration in equivalents per million is calculated by dividing concentration in parts per million by the chemical combining weight of the substance or ion. c Calculated. d Lower one half of formation. e Upper one half of formation. f Total alkalinity, 420 parts per million, excess alkalinity, 118 parts per million. g Total alkalinity, 448 parts per million, excess alkalinity, 116 parts ver million. h Total alkalinity, 696 parts per million, excess alkalinity, 420 parts per million. i Total alkalinity, 744 parts per million, excess alkalinity, 298 parts per million. j Total alkalinity, 540 parts per million, excess alkalinity, 314 parts per million. k Total alkalinity, 402 parts per million, excess alkalinity, 100 parts per million l Total alkalinity, 332 parts per million, excess alkalinity, 98 parts per million. m Total alkalinity, 466 parts per million, excess alkalinity, 356 parts per million. |
|||||||||||||||
General Character of Waters
The following discussion is adapted from publications of the United States Geological Survey and the State Geological Survey of Kansas.
Dissolved solids
The residue left after a natural water has evaporated consists of rock materials, with which may be included some organic material and a small amount of water of crystallization. Water containing less than 500 parts per million of dissolved solids generally is entirely satisfactory for domestic use, except for difficulties resulting from its hardness, and, in some areas, because of excessive iron or corrosiveness. Water having more than 1,000 parts per million is likely to contain enough of certain constituents to produce a noticeable taste or to make the water unsuitable in some other respects.
The dissolved solids in samples of water collected from test holes in this area ranged from 672 to 71,405 parts per million; hence none of the waters sampled is suitable for most ordinary purposes. Only one sample contained between 500 and 1,000 parts per million, and all except six samples contained more than 5,000 parts per million.
Hardness
The hardness of water, which is the property that generally receives the most attention, is most commonly recognized by its effect when soap is used with the water in washing. Calcium and magnesium cause almost all the hardness of ordinary water. These constituents are also the active agents in the formation of the greater part of all the scale formed in steam boilers and in other vessels in which water is heated or evaporated.
In addition to the total hardness, the table of analyses indicates the carbonate hardness and the noncarbonate hardness. The carbonate hardness is that due to the presence of calcium and magnesium bicarbonate. It is largely removed by boiling. In some reports this type of hardness has been called temporary hardness. The noncarbonate hardness is due to the presence of sulphates or chlorides of calcium and magnesium, but it cannot be removed by boiling and has sometimes been called permanent hardness. With reference to use with soaps, there is no difference between the carbonate and noncarbonate hardness. In general, the noncarbonate hardness forms harder scale in steam boilers.
Water having a hardness less than 50 parts per million is generally rated as soft, and its treatment for removal of hardness under ordinary circumstances is not necessary. Hardness between 50 and 150 parts per million does not seriously interfere with the use of water for most purposes, but it does slightly increase the consumption of soap; its removal by a softening process is profitable for laundries or other industries using large quantities of soap. Water in the upper part of this range of hardness will cause much scale in steam boilers. Hardness exceeding 150 parts per million can be noticed by anyone; if the hardness is 200 or 300 parts per million it is common practice to soften water for household use or to install a cistern to collect soft rainwater. Where municipal water supplies are softened, an attempt is generally made to reduce the hardness to 60 or 80 parts per million. The additional improvement from further softening of a whole public supply is not deemed worth the increase in cost.
The hardness of samples of water collected from the test holes in this area ranged from 110 to 9,098 parts per million. The softest water analyzed was from a sandstone near the top of the Dakota formation encountered in test hole 20, and the hardest water was obtained from Permian redbeds in test hole 12. Only 1 sample had a hardness between 100 and 200 parts per million, 3 had a hardness between 200 and 300 parts, 3 had a hardness between 300 and 400 parts, 6 had a hardness between 400 and 1,000 parts, and 38 had a hardness of more than 1,000 parts. All except one of the samples having a hardness of less than 1,000 parts per million were obtained from sands in the Dakota formation. The other was obtained from Pleistocene deposits in test hole 20.
Iron
Next to hardness, iron (Fe) is the constituent of natural waters that receives the most attention. The quantity of iron in ground waters may differ greatly from place to place, even though the waters are from the same formation. If a water contains much more than 0.1 part per million of iron, the excess may separate out and settle as a reddish sediment. Iron, which may be present in sufficient quantity to give a disagreeable taste and to stain cooking utensils, may be removed from most waters by simple aeration and filtration, but a few waters require the addition of lime or some other substance. Because the samples of water collected from the bailer in drilling the test holes contained sufficient silt and other rock cuttings to make them cloudy, it was not practicable to determine the iron content.
Fluoride
Although determinable quantities of fluoride (F) are not as common as fairly large quantities of other constituents of natural waters, it is desirable to know the amount of fluoride present in water that is likely to be used by children. Fluoride in water has been shown to be associated with the dental defect known as mottled enamel, which may appear on the teeth of children who drink water containing excessive quantities of fluoride during the period of formation of the permanent teeth. It has been stated that waters containing 1 part per million or more of fluoride are likely to produce mottled enamel, although the effect of 1 part per million is not usually very serious (Dean, 1936). If the water contains as much as 4 parts per million of fluoride, 90 percent of the children exposed are likely to have mottled enamel and 35 percent or more of the cases will be classed as moderate or worse.
No samples of water collected in this area in which fluoride was determined contained less than 1 part per million of fluoride, and one sample obtained from a sandstone in the upper part of the Dakota formation in test hole 9 contained 4.5 parts per million of fluoride. The water is generally not potable and therefore is not likely to be used by children.
Calcium
Calcium (Ca) is taken into solution as the bicarbonate by the reaction of natural waters containing carbonic or organic acids with calcium carbonate, which is the principal constituent of limestone and an important constituent of dolomite. It is also dissolved in large quantities from gypsum (calcium sulphate).
Calcium is the least abundant metallic element in all except eight of the samples here considered; its concentration ranges from 21 parts per million in water from a sandstone in the Dakota in test hole 20 to 2,468 parts in water from Tertiary (?) deposits in test hole 10.
Magnesium
Magnesium (Mg) is dissolved from practically all rocks, but mainly from dolomite and dolomitic limestones, by reactions similar to those for calcium. In most natural waters magnesium is much less abundant than calcium, but in this area the relative abundance of the two constituents is reversed. Magnesium is the only element besides calcium that causes any appreciable amount of hardness in most natural waters.
The concentration of magnesium ranges from 12 parts per million in water from Pleistocene rocks in test hole 20 to 1,796 parts per million in water from the Permian redbeds penetrated by test hole 12.
Sodium and potassium
Sodium (Na) and potassium (K) are dissolved from practically all rocks, and they are present in large quantities in most of the samples of water from this area. The two elements were not determined separately in any of the analyses. Their concentration ranged from 97 parts per million in water from the Pleistocene deposits tapped by test hole 20 to 23,652 parts per million in water from the Permian redbeds encountered by test hole 12. Twenty-four (nearly half) of the samples contained a larger amount of sodium and potassium than is found in average sea water.
Moderate quantities of sodium have little effect on the suitability of water for ordinary use, but if the quantity is much more than 100 parts per million, foaming in steam boilers may result unless special precautions are taken. Some natural waters contain such large quantities of sodium salts that they are injurious to vegetation. Most of the waters from the test holes in this area would injure vegetation and would foam in steam boilers.
Carbonate and bicarbonate
Carbonate (CO3) and bicarbonate (HCO3) in natural waters result from solution of carbonate rocks (such as limestone, dolomite, and calcareous shale) through the action of carbonic acid in the waters. Carbonate is not generally present in appreciable quantities in natural waters but it is found in some treated waters. In most of the analyses here considered there is less bicarbonate than any other negative ion; it ranges in concentration from 120 parts per million in water from the Greenhorn limestone encountered in test hole 18 to 1,879 parts per million in water from the Cheyenne sandstone encountered in test hole 21. The bicarbonate as such has little effect on the use of a water.
Sulphate
Sulphate (SO4) in ground waters is derived principally from gypsum (calcium sulphate) associated with limestone, from the oxidation of pyrite (FeS2) and other sulphides, or from connate waters. The concentration of sulphate here ranges from 14 to 8,550 parts per million, but its relative abundance with respect to the other constituents remains fairly constant.
Sulphate itself has little effect on the general use of a water. Magnesium sulphate and sodium sulphate, if present in sufficient quantity, impart a bitter taste. Sulphate in a hard water may increase the cost of softening and form a hard scale in steam boilers which is difficult to remove.
ChlorideChloride (Cl) is an abundant constituent of sea water and is dissolved in small quantities from rock materials or in some localities comes from sewage. However, the sources of chloride are many; therefore its presence in large quantities cannot be taken as a definite indication of pollution. The chloride content of samples from this area ranges from 70 to 36,500 parts per million. In most of the waters analyzed it is the most abundant constituent by weight.
Chloride has little effect on the suitability of water for ordinary use, unless there is enough to impart a salty taste. There is enough chloride in 20 of these samples to impart a taste saltier than that of sea water. Waters high in chloride may be corrosive if used in steam boilers.
NitrateNitrate (NO3) in otherwise potable water is generally considered a final oxidation product of nitrogenous organic material. Therefore a large quantity of nitrate in ground water suggests the possible presence of harmful bacteria derived from privies, cesspools, barnyards, cultivated fields, or other places where oxidized nitrogenous matter is common.
The nitrate content, in samples in which it was determined, ranges from 4.0 parts per million in two samples from sandstones of the Dakota formation to 66 parts per million in the Greenhorn limestone (test hole 9). A sample from the Permian redbeds from a depth of 710 feet in test hole 8 contained 28 parts per million of nitrate. This does not necessarily indicate the presence of bacteria, for a certain amount of nitrate is to be expected in waters in which the total concentration of dissolved solids is large. Values for nitrate and fluoride have been omitted from most of the analyses because analysis of these two ions is difficult and time-consuming when the total concentration is high, and the information is not necessary unless the water is potentially potable. As nitrate was determined in only six samples, it was omitted from Table 4.
Range in Quantity of Dissolved Solids
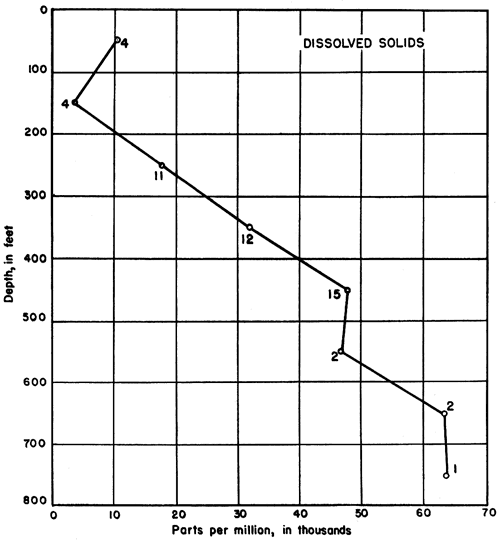
Except in near-surface samples, the amount of dissolved solids was found to increase with increasing depth of sampling in the test holes. This relationship is shown in Figure 6, where each point represents the average concentration for samples within that particular 100-foot interval. The small numerals at each point refer to the number of samples from which each average was computed. The value for the top 100 feet is meaningless, for variation near the surface is extremely great. The number of parts per million of dissolved solids is plotted separately for each analysis from beneath the Greenhorn limestone in Figure 7A, where a general trend toward higher concentrations at greater depths is easily discernible. In this diagram the position of each point in relation to the vertical axis refers to the middle of the interval from which the sample was taken.
Figure 6--Graph showing the relationship between the depth from which water samples were obtained and the concentration of dissolved solids. The numerals at each point refer to the number of analyses from which the average was computed. Each point represents the mean concentration for samples within that particular 100-foot interval,
If the data are plotted with respect to geologic formations, however, the points become less scattered and the trend more decisive (Fig. 7B). The thickness of each formation represented on the vertical axis is a mean value obtained by averaging the thicknesses in all the holes from which samples were obtained from that formation. The point for each sample is spotted according to its relative position with respect to the boundaries of the formation from which it came. Thus, if a sample from a particular test hole came from a position nine-tenths the distance from the top of the Dakota formation, it is plotted nine-tenths of the distance from the top of the interval shown on the graph.
Figure 7--A, Scatter diagram showing the relationship between the concentration of dissolved solids and the depths from which water samples were obtained. B, Scatter diagram showing the relationship between the concentration of the dissolved solids and stratigraphic zones. Each circle represents one analysis.
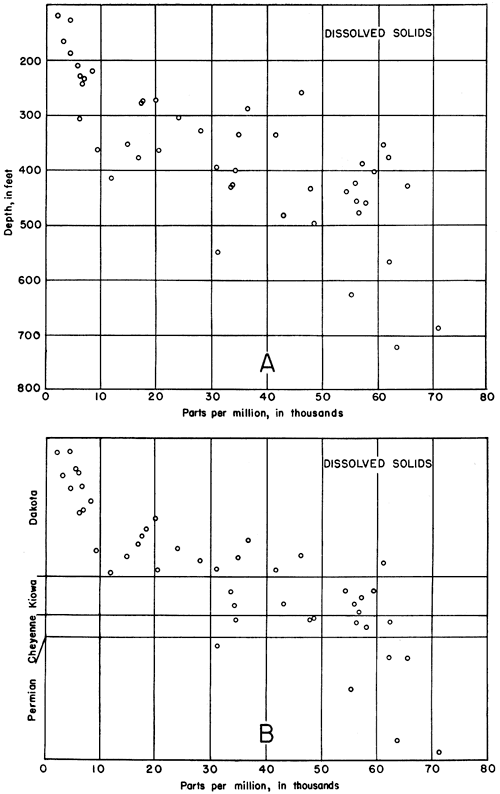
It may be seen from the graph (Fig. 7B) that the concentration of dissolved solids in all the samples from approximately the upper half of the Dakota formation is less than 9,000 parts per million, whereas the concentration in samples from the lower half of the Dakota ranges from 9,000 to 61,000 parts and in more than half of the analyses is between 10,000 and 30,000 parts. Thus the ranges in dissolved solids for the two approximate halves of the Dakota formation are mutually exclusive. The halfway mark has no relation to the boundary between the Terra Cotta clay and Janssen clay members, which is much nearer the top of the formation. The concentration of dissolved solids in waters from the Kiowa and Cheyenne formations ranges from 33,000 to 62,000 parts per million. There is no appreciable difference in concentration of dissolved solids between waters from the two formations, although the average concentration in the samples from the Kiowa shale (49,358 parts per million) is slightly less than that in samples from the Cheyenne sandstone (51,082 parts per million). The sharp increase in concentration between waters of the Dakota and the Kiowa is noteworthy. The concentration of dissolved solids is greater than 47,000 parts per million in all except one of the samples from the Permian redbeds, and the range is from 31,000 to 71,000 parts.
Relative Concentrations of Constituents
The discussion that follows is based on the results given in equivalents per million in Table 4. Percentages of equivalents per million are used for convenience in comparing different waters. Equivalents per million are the units in which the bar diagrams of Figures 8 and 9 are plotted. Percentages of equivalents make possible the direct comparison of two or more waters of different concentrations of dissolved solids.
Four samples of water were obtained from post-Dakota formations, and these are extremely variable in character. One sample from the Greenhorn limestone (test hole 9, dissolved solids 3,399 parts per million) contained 14 percent sulphate and 17 percent calcium, while another from the Greenhorn (test hole 18, dissolved solids 13,764 parts per million) contained about the same relative amount of calcium (19 percent) but only 1.7 percent sulphate. This latter water has some of the characteristics of brines from oil wells in the area. A water from the Pleistocene rocks (test hole 20, total solids 672 parts per million) contained 37 percent bicarbonate, 23 percent sodium, and only 1.6 percent sulphate. Water from the Tertiary (?) (test hole 10, total solids 23,812 parts per million) contained 4.5 percent sulphate, 29 percent sodium, and only 0.5 percent bicarbonate.
All the other water samples came from the Dakota, Kiowa, and Cheyenne formations and the Permian redbeds; they exhibit remarkable uniformity in percentages of their constituents (Fig. 8). The percentage concentrations of calcium range from 0.9 to 12.4 percent; all except one are between 0.9 and 4.3 percent. The concentration of magnesium ranges from 1.1 to 6.1 percent, and that of sodium from 42.2 to 47.8 percent. The same narrow range may be observed in the variation of the negative ions. The concentrations of bicarbonate range from 0.1 to 9.3 percent, those of sulphate from 3.8 to 7.4 percent, and those of chloride from 34.8 to 44.5 percent.
Figure 8--Analyses of typical waters from the Dakota, Kiowa, and Cheyenne formations, and Permian redbeds in a part of Russell County, Kansas.
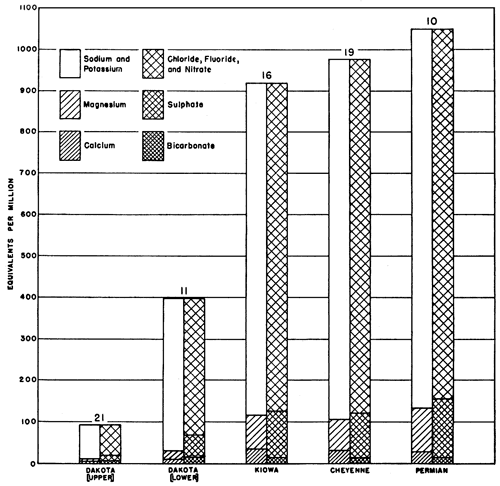
There is a consistent difference between waters of the upper half of the Dakota formation and waters of the Kiowa shale, Cheyenne sandstone, and Permian redbeds, however. The character of water from the lower half of the Dakota is variable. All samples of water from below the Dakota except one sample from test hole 21 contained less bicarbonate than any other constituent. All samples of water from the upper half of the Dakota except one sample from test hole 17, which may be affected by water from the overlying Pleistocene sands and gravels, contained more bicarbonate than calcium, and most samples contained more bicarbonate than calcium and magnesium together. This may have resulted from base exchange of sodium for calcium and magnesium caused by clay minerals of the Dakota formation (Latta, 1944, pp. 136, 137).
Approximately half the samples of water from the lower part of the Dakota formation contained the same relative proportions of constituents as those from the upper part; the rest contained an excess of calcium over bicarbonate. The former samples came from test holes outside or on the edges of oil-field areas as they existed at the close of 1942 (Frye and Brazil, 1943, pl. 1); the latter came from test holes which were, as a rule, well within the oil-field areas. These relationships seemingly can result from one or more of at least three situations. (1) The chemical composition of the water may be related to Cretaceous structures which reflect oil-pool structures in the underlying Paleozoic rocks and somehow control the concentration of the constituents; (2) brine from disposal wells may pollute the lower part of the Dakota; and (3) unplugged holes or pressure from shallow disposal wells may cause the mixing of water from pre-Dakota beds with water from the lower part of the Dakota.
(1) Lower Cretaceous structure on the top of the Kiowa shale does not seem to have much relationship to the oil-field areas or to the test holes in which the waters in the lower part of the Dakota formation differ chemically from those in the upper part of the Dakota; therefore, the first suggestion probably may be eliminated.
(2) Brine which has been pumped into shallow disposal wells in Russell County was derived from the Kansas City-Lansing, Arbuckle, and Gorham producing zones. Analyses of these brines by R. Q. Brewster and Calvin Vander Werf have been published in a report by Schoewe (1943), and the analyses of two typical oil-field brines, one from the Arbuckle and one from the Kansas City-Lansing, are given in Figure 9. The following relationships are characteristic of the published analyses: calcium exceeds magnesium, in the general proportion of 2 to 1; the amount of chloride is at least 5 percent greater than that of sodium, and in some brines is as much as 14 percent greater; the sulphate does not exceed 1 percent and the bicarbonate does not exceed 1.4 percent; the amount of calcium is at least 5 times that of bicarbonate.
Figure 9--Analyses of waters from the test holes and of brines from Russell County, and an analysis of a hypothetical mixture of brine with water from the lower part of Dakota formation. A, Typical water from the Kansas City-Lansing oil-producing horizon1. B, Typical water from the Arbuckle horizon1. C, Water from the lower part of the Dakota formation having characteristics of waters outside oil-field areas2. D, Hypothetical mixture of 5 percent B and 95 percent C, showing similarities to E. E, Water from lower part of the Dakota formation in an oil-field area2. F, Water from the middle part of the Dakota having characteristics of oil-field brines2 (Frye and Brazil, 1943). 1Analysis by R. Q. Brewster and Calvin Vander Werf (Schoewe, 1943). 2Analysis by Howard Stoltenberg.
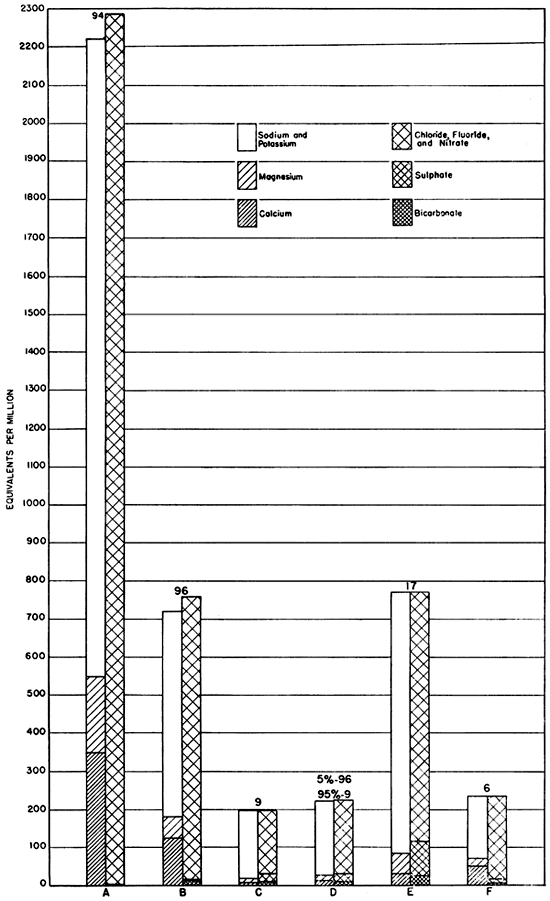
It is possible to postulate a mixture of water from the Dakota formation with oil-field brine which will give the approximate proportions of constituents observed in waters from the lower part of the Dakota in the oil-field areas. Thus a mixture of 95 percent of the lowest sample of water from the Dakota in test hole 9 and 5 percent of water sample 96 from the Arbuckle of the North Trapp pool (Brewster and Vander Werf in Schoewe, 1943, pp. 58, 59) will produce the hypothetical water shown in Figure 9, which has almost the chemical characteristics of the waters from the lower part of the Dakota in the oil-field areas. In most analyses, however, the percentage of sulphate in the supposedly affected waters from the lower part of the Dakota is as high as that in waters from the upper part of the formation. The concentration of dissolved solids in the Paleozoic brines of Russell County, particularly in that from the Kansas City-Lansing producing zone which averages 132,690 parts per million in the analyses by Brewster and Vander Werf, is much greater than that in the Dakota formation; and an admixture of these brines large enough to reverse the calcium-bicarbonate ratio should also produce an excess of calcium over magnesium and materially lower the percentage of sulphate. However, such is not the case in samples from test holes drilled for the present study. Addition of more than a very small amount (ca. 5 percent) of brine to the water from the Dakota would also raise the chloride content to more than that of the sodium, so that the possible range of admixture to produce the required result is very small, being limited in one direction by the calcium-bicarbonate ratio and in the other by the sodium-chloride ratio, amount of sulphate, and relative abundance of calcium and magnesium.
Waters from test holes 6 and 7 (Frye and Brazil, 1943, p. 67), however, do have the characteristics of Paleozoic brines, including high chloride content (greater than sodium), high bicarbonate content with respect to calcium, more calcium than magnesium, and low content of sulphate (Fig. 9). Test hole 6 was put down in an area in which there were several shallow disposal wells (Frye and Brazil, 1943, pl. 1), and test hole 7 was drilled 1 mile to the southeast and in the direction of the hydraulic gradient from no. 6.
(3) There remains the third alternative, for analyses of samples other than those from test holes 6 and 7, that pressure from shallow disposal wells or conditions resulting from drilling operations such as unplugged holes may have caused the entrance of water from the Kiowa shale, Cheyenne sandstone, or Permian redbeds into the lower part of the Dakota formation. This is supported by the close similarity between the waters of the lower part of the Dakota in the oil-field areas (Fig. 9) and waters from sands in the Kiowa and Cheyenne formations and the Permian redbeds (Fig. 8).
None of the samples of water collected from the upper part of the Dakota formation for the present investigation was potable, although it is believed that waters from this zone formerly were potable. Two problems, then, are involved: (1) What is the source of the water that was introduced into the upper part of the Dakota; and (2) why does this water differ from water in the lower part of the Dakota in the oil-field areas?
The most likely sources are the oil-field brines from the Paleozoic rocks and the waters from the Lower Cretaceous and Permian rocks, as in the case of affected waters in the lower part of the Dakota formation. The brines from the Paleozoic rocks are not adequate as a source because of their small percentage of sulphate and their calcium-magnesium ratio. The waters from the Lower Cretaceous and Permian rocks differ from the water in the upper part of the Dakota in the proportion of calcium to bicarbonate, but they may have been modified by base exchange in the clay of the Dakota. Base exchange may have been a more important factor in this zone in the modification of introduced waters from the Lower Cretaceous and Permian, either because their total concentration is lower than in waters in the lower part of the Dakota or because they have been in the upper part of the Dakota for a longer period of time. The high degree of uniformity in the waters from the upper part of the Dakota formation favors the latter suggestion.
Summary
Waters sampled from the test holes put down in the area had high concentrations of total solids and were not potable. The concentration of total dissolved solids increases with depth and with increasing age of the deposits. Waters from the Dakota and deeper formations were characterized by high percentages of sodium and chloride, moderately low percentages of magnesium and sulphate, and very low percentages of calcium and bicarbonate. Waters from the upper part of the Dakota and some waters from the lower part of the Dakota differed from the rest in their lower percentages of calcium and magnesium, resulting probably from base exchange. It is suggested that waters have entered the Dakota from the Lower Cretaceous and Permian rocks as a result of drilling operations, and, according to Ogden S. Jones, geologist of the Kansas State Board of Health (personal communication), it would seem imperative to use extreme care in setting up adequate cementing and casing programs in these zones.
Prev Page--Geology || Next Page--Logs
Kansas Geological Survey, Geology
Placed on web March 5, 2009; originally published Dec 31, 1945.
Comments to webadmin@kgs.ku.edu
The URL for this page is http://www.kgs.ku.edu/Publications/Bulletins/60_4/04_qual.html